Abstract
It is well known that the human skull achieves adult size through a superior–inferior gradient of maturation. Because the basicranium matures in size before the face, it has been suggested that the form of the basicranium might have ontogenetic knock-on effects on that of the face. However, although sequential spatially organized maturation of size is well described in the cranium, the maturation of skull shape is not. Knowledge of the maturation of shape is important, nevertheless, because it is claimed that the early determination of the spatial configuration of basicranial components, where the facial skeleton attaches, is relevant in the spatio-temporal ontogenetic cascade from basicranium to face. This paper examines the ontogeny of various components of the human skull in 28 individuals from the longitudinal Denver Growth Study. Sixty-six landmarks and semilandmarks were digitized on 228 X-rays and analysed using geometric morphometric methods. Bootstrapped confidence intervals for centroid size support previous studies suggesting a supero-inferior gradient of growth maturation (size over time), while developmental maturation (shape over time) is more complex. A sequence of shape maturation is described, in which the earliest structure to mature in shape was the midline cranial base (7–8 years), followed by the lateral cranial floor (11–12), midline neurocranium (9–10) and facial and mandibular structures (15–16). The absolute ages of shape maturation of the latter three depended on the criterion of maturity used, which was not the case for the basicranial components. Additionally, ontogenetic dissociations were found between the maturation of size and shape of the midline cranial base and lateral floor, possibly underlining its role as structural ‘interface’ between brain and facial ontogeny. These findings imply potential for bidirectional developmental influences between the lateral cranial floor and the face until about 11–12 years. The findings are discussed with regard to their relevance for palaeoanthropology and especially the evolutionary and developmental bases of skull morphological variation.
Keywords: basicranium, cranial base, craniofacial ontogeny, Denver Growth Study, modularity, Procrustes, sliding semilandmarks
Introduction
Among the most challenging endeavours in current palaeoanthropology is the unravelling of the morphological growth interactions between skull components that underpin the evolution of a large brain, and small face in modern humans (Lieberman, 1998; Spoor et al. 1999; Lieberman et al. 2002). The aim is to understand how shifts in the spatial and temporal organization, magnitudes of change in size and shape, and interactions between the modules that comprise the skull have led to the evolutionary changes observed in the hominin radiation. Such understanding has the potential to improve our ability to make phylogenetic interpretations of craniofacial variations among fossils. Traditionally, these issues are addressed through two complementary approaches. The first uses functional models to relate the biomechanics of the facial skeleton, often in isolation from the cranial base, to its ontogeny (Rak, 1986; Trinkaus, 1987, 2003; Spencer & Demes, 1993). The second is the structural approach in which developmental and spatial hypotheses are tested. An example of the latter is found in the attempt by various workers to link the evolution of the brain with changes in basicranial morphology on the one hand and of the face on the other (Enlow & Azuma, 1975; Bromage, 1992; Ross & Ravosa, 1993; Ross & Henneberg, 1995; Spoor, 1997; Lieberman, 1998; Lieberman et al. 2000a, 2002; Rosas, 2001; Jeffery & Spoor, 2002; Jeffery, 2003; Bastir & Rosas, 2004b; Rosas & Bastir, 2004; Ross et al. 2004). A current aim of research in this area is to understand how and to what extent structural or functional factors interact to produce a given facial morphology.
Mechanical and spatial growth interactions between the various components of the human skull during ontogeny are important in relation to structural hypotheses of skull ontogeny (Enlow & Hans, 1996; Lieberman, 1998; Spoor et al. 1999; Lieberman et al. 2000a, 2004; O’Higgins, 2001; Bastir et al. 2002, 2004; Bastir & Rosas, 2005, 2006). There exists a temporal sequence of craniofacial skeletal maturation, with different components achieving morphological adulthood at different times (Enlow, 1968; Bastir & Rosas, 2004a) (Fig. 1). Within a given temporal sequence, slight developmental modifications of maturation of one cranial component can have marked effects on the development of other adjacent or more distant components that mature later (Atchley & Hall, 1991; Lieberman et al. 2004).
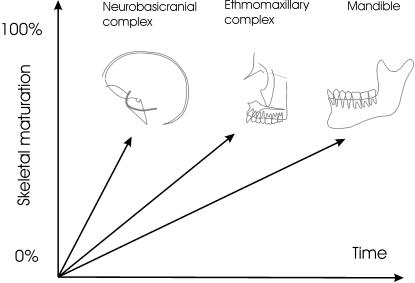
Fig. 1.
Craniofacial levels of skull development. The lines connecting the origin schematically indicate the hypothesized (Buschang et al. 1983; Enlow & Hans, 1996) differences in maturation of the various craniofacial levels; they do not imply ontogenetic shape trajectories, which would be multivariate and curvilinear. The temporal sequence of morphological maturation shows that the neurobasicranial complex reaches adult shape earlier than the ethmomaxillary complex and the mandible.
Because of the intermediate position of the basicranium between the brain and the face and the temporal sequence of skeletal maturation of the various components of the human skull, the earlier maturing basicranium is frequently hypothesized to set spatial conditions that can influence the slower and longer growing facial skeleton (Enlow et al. 1969; Buschang et al. 1983; Bhatia & Leighton, 1993; Enlow & Hans, 1996) (Fig. 1). This structural concept has a long history (Dabelow, 1931; De Beer, 1937; Biegert, 1957; Hofer, 1965), and it is encapsulated in what Enlow has termed ‘craniofacial levels’ (Enlow & Hans, 1996, p. 14). This idea posits a ‘descending cause-and-effect stratigraphic arrangement of structural levels in the design of the face’ (Enlow & Hans, 1996, p. 14). The notion of craniofacial levels implies a cascade of causal, developmental mechanisms and has found widespread application in orthodontics through counterpart analysis (Enlow, 1968; Enlow et al. 1969; Bhat & Enlow, 1985). Recently, it has been increasingly employed in palaeoanthropology (Enlow & Azuma, 1975; Shea, 1985; Bromage, 1992; Ravosa & Shea, 1994; Lieberman, 1998; McCarthy & Lieberman, 2001; Bastir et al. 2002, 2004).
The temporal sequence of maturation can also potentially influence the extent to which morphological integration is present among diverse skull components (Fig. 1). Recent investigations have identified a low level of integration between the midline (i.e. midsagittal) cranial base and the bilateral middle cranial fossae, possibly because of differences in the timing of maturation (Bastir et al. 2004; Bastir & Rosas, 2005, 2006). These interpretations are in line with more general earlier suggestions by Dabelow (1931), Enlow et al. (1971) and Lieberman et al. (2000a). The temporal sequencing of maturation closely relates to the emergence of modularity and its diagnosis at the morphological level (Riedl, 1975; Wagner, 1996; Von Dassow & Munro, 1999; Klingenberg et al. 2003; Bastir & Rosas, 2005, 2006). However, despite the obvious significance of ‘craniofacial levels’ in understanding craniofacial development and evolution there remain some unresolved issues.
Baughan et al. (1979) have shown three different ontogenetic schedules of maturation: cranial, facial and general skeletal patterns. Similarly, a growth gradient of maturation has been identified for linear measurements of the growing human skull by Buschang et al. (1983). These authors showed that the size of structures close to the basicranium reaches adult percentages earlier in ontogeny than do maxillary and mandibular components. These results support Enlow’s model. However, it is unclear how the developing shape as well as the size of the earlier maturing basicranium relates (through ontogenetic interactions) to the shape and size (form) and spatial relations of other, later maturing facial structures. This is because of the limitations of incomplete sets of linear measurements in preserving information about form (Rohlf & Marcus, 1993; Zelditch et al. 2004). Geometric morphometric analysis of basicranial and facial landmark configurations (Bookstein, 1991; Rohlf & Marcus, 1993; O’Higgins, 2001) can potentially contribute to the clarification of this issue.
A further issue in relation to craniofacial levels is that the concept principally relates to the maturation of midline structures as represented in lateral cephalograms. However, recent investigations have shown that lateral parts of the basicranium are better integrated with some facial structures (Bastir et al. 2002, 2004; Bastir & Rosas, 2005, 2006) than are midline structures, but little is known about their ontogenetic shape changes and maturation patterns.
Among the most important descriptors of basicranial morphology is the basicranial angle, between the spheno-occipital clivus and the sphenoidal plane (Landzert’s angle, CBA 4) (Landzert, 1866; Lieberman & McCarthy, 1999; McCarthy, 2001; Bastir, 2004). Although Lieberman & McCarthy (1999) found a relatively early developmental maturation of this angle of basicranial flexure in humans at approximately 2 years of age, it should be noted that not only the maturation of the angle, but also of the pre- and post-sellar proportions and of the spatial relations of the sella (neither captured by angular measurements) are important in the context of craniofacial levels, even though they are not currently well described. Thus, a detailed geometric morphometric analysis of the postnatal ontogeny of the midline basicranium is required to clarify spatio-temporal patterns of maturation in size and shape.
Regarding the lateral cranial floor it is thought that the anterior cranial fossae reach maturity at around 5–6 years of age, together with the frontal lobes (Enlow & Hans, 1996; Sgouros et al. 1999; Goodrich, 2005). By contrast, little seems to be known of the schedule of maturation of the middle cranial fossae (which are influenced by the development of the temporal lobes). Thus, Enlow & Hans (1996, p. 107) indicate only that they ‘continue to grow several more years’. Sgouros et al. (1999) have shown that some linear distances of the middle cranial fossae indicate maturation between 12 and 15 years, leaving open further details of the chronology of maturation and, more importantly, the associated changes in shape that occur during maturation. This uncertainty is probably related to the difficulties inherent in the quantitative assessment of the size or shape of curved structures, such as the anterior or middle cranial fossae.
Despite these difficulties we ought to know more detail of the maturation of these structures. Enlow’s observations and those of others distinguish embryologically and ontogenetically between the midline cranial base (‘os tribasilare’ of Virchow) and the anterior cranial floor (Hofer, 1960; Kummer, 1952; Starck & Kummer, 1962; McCarthy, 2001), and the lateral cranial fossae (Bastir et al. 2004; Bastir & Rosas, 2005). However, recently discovered and potentially important integrative relationships between lateral cranial floor structures and facial shape have emerged from recent studies (Bastir et al. 2004; Bastir & Rosas, 2005) that also indicate a degree of developmental independence between basicranial structures. Thus, to understand fully how the structures of the midline cranial base and lateral cranial floor integrate temporally and spatially and with other parts of the skull it is now necessary to incorporate all of these into a reanalysis of Enlow’s hypothesized ‘craniofacial levels’ (Enlow & Hans, 1996).
These considerations lead us to re-examine the maturation of size and shape in the human skull with the aim of adding detail to and testing Enlow’s notion of craniofacial levels. The ontogeny of size over time is termed ‘growth’, and that of shape is termed ‘development’ (Ponce de León & Zollikofer, 2001). Our aim is to characterize the temporal and spatial sequence of growth and development of components of the skull, taking account of off-midline structures and of subdivisions of the neurobasicranial complex. The first part of the present study addresses Enlow’s hypothesis of craniofacial levels with regard to the whole skull. The second part addresses the maturation patterns of different regions in the neurobasicranial complex, i.e. the midline, the non-midline basicranial structures and the midline vault. Thus, the hypotheses of Enlow’s craniofacial levels together with those of the previously discussed traditional morphometric studies lead us to expect that significant differences in size and shape between adults and non-adults disappear for the midline cranial base at around 2 years (Lieberman and McCarthy), the neurocranium around 8 years (Buschang et al. 1983), the lateral cranial floor (anterior and middle cranial fossae) between 12 and 15 years (Sgouros et al. 1999), followed sequentially by the ethmomaxillary complex and the mandible after 15.5 years (Baughan et al. 1979; Buschang et al. 1983; Enlow & Hans, 1996).
Materials and methods
The data of this investigation consist of 66 two-dimensional landmarks (Table 1) of 228 lateral radiographs of 14 females and males of the longitudinal Denver Growth Study (Maresh & Washburn, 1938; Maresh, 1948; Lieberman & McCarthy, 1999; Lieberman et al. 2001). Participants in this study are males and females of European descent, who were radiographed between the years of 1931 and 1966. A detailed description and additional technical information can be found in Lieberman & McCarthy (1999), Lieberman et al. (2001) and references therein. The ages of the sample range from newborn to adulthood, although in some cases data are missing for particular ages and in several of the youngest infants the radiographs are not adequately aligned or detail is obscured by the restraining hand of a parent or technician. For the present geometric morphometric study we used X-rays from 2 years of age to adulthood (18 or more years) at 2-year intervals (Table 2).
Table 1.
Landmarks and semilandmarks (sml = semilandmark). Compare with Fig. 2
| Landmark no. | Name | Landmark no. | Name |
|---|---|---|---|
| 1 | fronto-orbital junction | 34 | slm |
| 2 | posterior sphenoid | 35 | slm |
| 3 | sml (halfway between lm1 and lm2) | 36 | slm |
| 4 | anterior sphenoid | 37 | slm |
| 5 | anterior cribriform plate point | 38 | slm |
| 6 | dorsum sellae basis point | 39 | slm |
| 7 | basion | 40 | slm |
| 8 | intersection MCF–ACF | 41 | slm |
| 9 | slm | 42 | opisthion |
| 10 | slm | 43 | condylion |
| 11 | slm | 44 | slm |
| 12 | slm | 45 | slm |
| 13 | slm | 46 | slm |
| 14 | slm | 47 | slm |
| 15 | slm | 48 | slm |
| 16 | slm | 49 | slm |
| 17 | posterior inferior point at petrosal temporal | 50 | slm |
| 18 | nasion | 51 | slm |
| 19 | slm | 52 | gnathion |
| 20 | slm | 53 | mention |
| 21 | slm | 54 | B-point |
| 22 | slm | 55 | incision |
| 23 | slm | 56 | mental foramen |
| 24 | slm | 57 | mandibular foramen |
| 25 | slm | 58 | prosthion |
| 26 | slm | 59 | A-point |
| 27 | slm | 60 | ant. nasal spine |
| 28 | slm | 61 | post. nasal spine |
| 29 | slm | 62 | slm |
| 30 | slm | 63 | slm |
| 31 | slm | 64 | slm |
| 32 | slm | 65 | internal prosthion |
| 33 | slm | 66 | orbitale |
Table 2.
Age groups: definitions and numbers of individuals of the sample
| Age groups (mean age, years) | Definition | No. of females | No. of males | Total |
|---|---|---|---|---|
| G1 (2.1) | 1.75–3.5 years | 8 | 8 | 16 |
| G2 (3.9) | > G1, ≤ 5,5 years | 12 | 13 | 25 |
| G3 (5.7) | > G2, ≤ 7,5 years | 14 | 14 | 28 |
| G4 (7.7) | > G3, ≤ 9,5 years | 14 | 14 | 28 |
| G5 (9.9) | > G4, ≤ 11,5 years | 14 | 14 | 28 |
| G6 (11.4) | > G5, ≤ 13,5 years | 14 | 13 | 27 |
| G7 (13.3) | > G6, ≤ 15,5 years | 11 | 13 | 24 |
| G8 (15.8) | > G7, ≤ 17,5 years | 12 | 14 | 26 |
| G9 (19.1) | > G8 | 11 | 14 | 25 |
An examination of data concerning the velocities of growth in stature (Leigh, 1996) of these individuals indicated on average for this sample a velocity of zero is present by 19 years. This result suggests that 19 years (G9, Table 2) is a reasonable chronological estimate of biological maturity and adulthood for these individuals.
Procrustes-based geometric morphometrics (Rohlf & Slice, 1990; Bookstein, 1991) are used to analyse the geometric properties of anatomical structures addressing the spatial configurations of landmark coordinates. Information that is unrelated to the shape of the objects, such as absolute position, orientation and scale is extracted during the Procrustes superimposition and the remaining shape variables (Procrustes residuals) are analysed using thin plate spline (TPS) techniques, partial warps and uniform component scores by multivariate statistical procedures (Bookstein, 1991, 1996a; Rohlf, 1996).
We employ a combination of landmarks and slid semilandmarks (in the past these have also been called ‘quasilandmarks’–Bookstein, 1996b, 1997; Bookstein et al. 1999) that are distributed along outlines of the skull (Table 1). Landmarks on lateral structures were digitized by tpsDIG software (Rohlf, 1997) using common radiographic averaging methods (Enlow & Hans, 1996; Bastir & Rosas, 2005).
The use of slid semilandmarks permits quantification of curved morphological structures for analysis within a geometric morphometric framework when no Type I or Type II landmarks are available (Bookstein, 1991). Furthermore, they offer the possibility of powerful visualizations (detailed diagrams) representing the results of these analyses (Bookstein, 1991, 1997; Bookstein et al. 2002, 2003; Mitteroecker et al. 2004; Sheets et al. 2004; Gunz et al. 2005).
Whereas real landmarks contain shape information in all directions of the coordinate system, slid semilandmarks are uninformative with respect to their exact location along a curve or outline, and hence they have reduced degrees of freedom (Bookstein, 1996b; Zelditch et al. 2004). The estimation of slid semilandmarks usually starts with more or less evenly spaced (equidistant or equiangular) semilandmarks placed along outlines or curves. Their eventual position along the curve (which is taken to be due to error) is then determined such that it minimizes bending energy between specimens in relation to the ‘true’ landmarks. Two principal approaches to sliding of semilandmarks are currently described (Zelditch et al. 2004). One of these is the minimization of bending energy, also called relaxation (Bookstein, 1997; Bookstein et al. 1999, 2003), and the other is minimization of Procrustes distance from the mean shape (Martinón-Torres et al. 2006; F. J. Rohlf, personal communication). The first criterion results in the minimization of localized shape differences with consequent smoothing of the TPS-grid and is of particular interest when features of the spline are being interpreted as morphological information (Bookstein, 1991, 1997; Gunz et al. 2005). It applies to the non-affine part of the TPS-transformation (Bookstein, 1991) and is the approach taken here. The second criterion, i.e. minimization of Procrustes distance, minimizes variation of the affine and non-affine part of the shape transformations. It is of particular interest in systematic approaches to morphological variation relying on phenetic distances or other narrowly systematic quantities (Gunz et al. 2005).
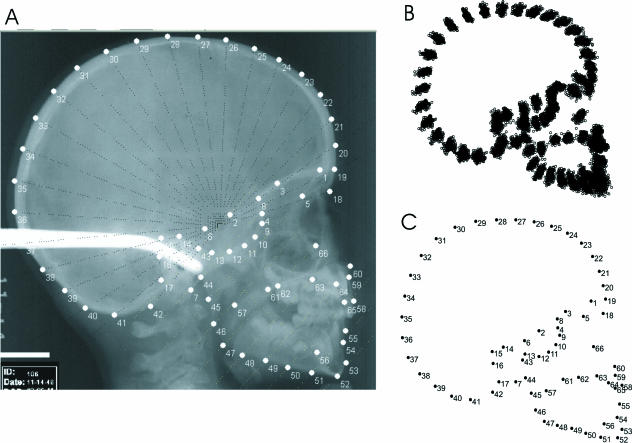
In our study, equidistant and equiangular semilandmarks were digitized using MakeFan6 (Sheets, 2001), which draws reproducible fans on image files, enabling the ready digitization of semilandmarks that result from the intersection of the fan lines and the outlines of the investigated anatomical structures (Fig. 2). The tpsDIG software (Rohlf, 1997) was used to digitize the landmarks and semilandmarks (Fig. 2). TpsUTIL (Rohlf, 1998) was used to define ‘slider-files’ to distinguish between real landmarks and semilandmarks. This file determines in tpsRELW software (Rohlf, 1997) which points will slide until the selected minimization criterion is fulfilled. The full landmark sets of the digitized specimens were divided into the configurations that represented several anatomical subsets of the skull.
Fig. 2.
(A) Lateral radiograph indicating the equiangular fan at the neurocranium and the equidistant combs across the basicranium and the face. The intersections of the fan/comb lines with the anatomical structures define semilandmarks. (B) Procrustes fit and slid semilandmarks. (C) Consensus configuration and landmark numbers.
In the first analysis the skull was divided into the neurobasicranial complex [basi- and neurocranium, landmarks (lm) 1–42], the ethmomaxillary complex (lm 58–66, 4, 5 and 18) and the mandible (lm 43–57). This part of the study addressed the analysis of morphological maturation of the whole skull, according to Enlow’s hypothesis of craniofacial levels (neurobasicranial complex, ethmomaxillary complex, mandible). In the second analysis the temporal sequence of maturation of the neurobasicranial complex was analysed in more detail. It was divided into the midline cranial base (lm 2, 4, 6, 7), the lateral cranial floor (anterior and middle cranial fossae) (lm 1, 2, 3, 5; 8–17) and the midline neurocranium (lm 18–42). Because of methodological reasons related to the use of sliding semilandmarks, nasion (a ‘true’ landmark needed to fix one endpoint of the midline series of slid neurocranial semilandmarks) was included in the neurobasicranial complex and the midline neurocranium even though from a developmental perspective nasion and glabella belong to the upper face. The effect of including glabella will be to give the impression of lengthened maturation schedules for the neurobasicranial complex and the midline neurocranium.
After the division of the landmarks sets into anatomical subsets, a Procrustes superimposition was carried out separately for each subset and the semilandmarks were accordingly re-slid. These aligned and relaxed data were then divided into nine age groups (Table 2). Next, the sizes and shapes of the anatomical substructures of the non-adult age groups were compared with the morphology of the corresponding structure of the adult sample.
It is known that bone shape becomes modified by remodelling throughout the entire life of an individual (e.g. because of aging, occlusional adjustments, etc.) and the same is true of growth (size increase) (Tanner, 1978; Enlow & Hans, 1996). However, it is also known that both processes slow in rate considerably as chronological age increases (Tanner, 1978; Enlow & Hans, 1996). These facts add some arbitrariness to the determination of maturity. To account for this, maturation of size and shape was assessed using two approaches. First, we examined the differences in size and shape between the oldest group, G9 (mean age 19.1 years), and each of the younger groups. Differences in mean shape between G9 and each non-adult ontogenetic stage were assessed by Goodall’s F-test using bootstrapping methods (Bookstein, 1997; Sheets, 2001; Gunz et al. 2005) and 95% confidence intervals of significantly different Procrustes distance were assessed by TwoGroup6 software (Sheets, 2001). Similarly, size variation was analysed by comparing the bootstrapped 95% confidence intervals of the G9 mean values with those of the younger age groups.
We also used a second approach to assessing maturation because shape trajectories might be curvilinear to some degree and Procrustes distances from each age class to the adults could even increase during ontogeny. Within the bounds of measurement error it was not possible to exclude curvilinearity, and so we also used cumulative Procrustes distances (i.e. shape trajectory length) between the youngest and subsequent age groups to the oldest group and their 95% confidence limits assessed by bootstrap analysis. In most cases both methods gave the same results; exceptions are described below. For reasons of comparability we used an analogous cumulative computation of centroid size between age classes and adults to assess maturation of this quantity. Identical results were obtained using either approach. Although the results may depend to some degree of sample size it is important to note that our sample sizes show only very small variation, expect for the youngest group (Table 2). Additionally, problems of sample sizes can be neglected when comparing maturation of different anatomical systems (e.g. the midline cranial base and lateral cranial floor) within the same samples. This is particularly relevant for the purpose of this study.
Measurement error was assessed by digitizing the real landmarks (see Table 1) of one individual at all ages on three different days. These data were then analysed by principal components analysis (PCA), and matrix correlations between the Procrustes distance matrices. The matrix correlations between day 1, day 2 and day 3 ranged from 0.87 to 0.89. Additionally, a descriptive analysis (PCA) of isolated individuals revealed that endocranial landmarks on the midline cranial base were more difficult to digitize accurately than other landmarks. This problem has been noted before (Björk, 1955; Grossman & Zuckerman, 1955; Buschang et al. 1983). Because of this error we divided the data into age classes and treated them as if they were cross-sectional rather than carry out longitudinal comparisons of individual maturation patterns. Our study therefore addresses mean tendencies of age groups, which have the further advantage that statistical assessment of maturity by age class is possible. We approached these assessments of differences between age classes and adults using permutation methods.
The first part of the study (examining Enlow’s model) involved comparison of the neurobasicranial, the ethmomaxillary complex and the mandible of G9 (the oldest age group) with each of the younger age groups. The ontogenetic maturation of size was assessed by comparing the mean centroid size of G9 (the oldest age group) and bootstrap estimates of its 95% confidence limits with each mean of the younger age groups as well as cumulative centroid size. For the analysis of shape maturation we used bootstrap versions of Goodall’s F-test and 95% confidence limits of Procrustes (approach 1) as well as the 95% confidence limits of shape trajectory length (approach 2).
The second part of the study used the same statistical protocol but it addressed in detail the ontogenetic maturation of the components of the neurobasicranial complex only, i.e. the midline cranial base, the lateral cranial floor and the midline neurocranial vault. In both cases we were concerned to discover if some components show maturation in either size or shape at earlier or later ages than expected according to traditional morphometric findings.
Results
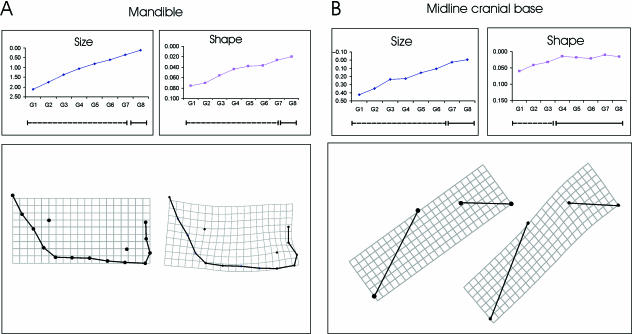
Tables 3 and 4 and Figs 3–5 show the results of the study of growth (size) maturation. The mean values indicate a continuous increment over all developmental stages. Table 3 shows that the size of the neurobasicranial complex becomes indistinguishable from adults in G6 (11.4 years) (Fig. 3A). The ethmomaxillary complex (Fig. 3B) and the mandible (Fig. 4A) continue to grow and achieve adult size at age group G8 (15.8 years, Table 3). This supports Enlow’s hypothesis of craniofacial levels in terms of size. Table 4 shows the increments of centroid size for the neurobasicranial components only. The midline cranial base (Fig. 4B, Table 4) becomes indistinguishable from the adult size range in G7 (13.3 years) while the cranial lateral floor (Fig. 5A, Table 4) increases in size until G8 (15.8 years). This is later than predicted (Sgouros et al. 1999). Adult size of the midline neurocranial vault (Fig. 5B, Table 4) is achieved at G6 (11.4 years), also later than predicted. In summary, these results support the view that neurocranial structures achieve adult size earlier than facial structures (Fig. 1).
Table 3.
Craniofacial components. Mean centroid sizes of the age classes and their 95% confidence intervals
| Neurobasicranial complex | Ethmomaxillary complex | Mandible | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group | Years | mean | 95%– | 95%+ | mean | 95%– | 95%+ | mean | 95%– | 95%+ |
| G1 | 2–3 years | 17.25 | 16.86 | 17.60 | 2.92 | 2.82 | 3.00 | 4.12 | 3.98 | 4.24 |
| G2 | 4–5 years | 17.80 | 17.64 | 17.97 | 3.21 | 3.17 | 3.26 | 4.47 | 4.39 | 4.55 |
| G3 | 6–7 years | 18.24 | 18.08 | 18.41 | 3.43 | 3.39 | 3.48 | 4.86 | 4.79 | 4.94 |
| G4 | 8–9 years | 18.42 | 18.27 | 18.59 | 3.59 | 3.55 | 3.63 | 5.17 | 5.11 | 5.23 |
| G5 | 10–11 years | 18.66 | 18.50 | 18.82 | 3.78 | 3.72 | 3.83 | 5.43 | 5.35 | 5.52 |
| G6 | 12–13 years | 18.76 | 18.59 | 18.94 | 3.92 | 3.87 | 3.98 | 5.63 | 5.55 | 5.70 |
| G7 | 14–15 years | 18.88 | 18.67 | 19.07 | 4.07 | 4.02 | 4.11 | 5.88 | 5.80 | 5.95 |
| G8 | 16–17 years | 19.03 | 18.83 | 19.23 | 4.19 | 4.13 | 4.24 | 6.12 | 6.03 | 6.21 |
| G9 | 18 and more | 19.11 | 18.89 | 19.30 | 4.26 | 4.19 | 4.32 | 6.23 | 6.11 | 6.35 |
Table 4.
Neuro- and basicranial components. Mean centroid sizes of the age classes and their 95% confidence intervals
| Midline cranial base | lateral cranial floor | neurocranium | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group | Years | mean | 95%– | 95%+ | mean | 95%– | 95%+ | mean | 95%– | 95%+ |
| G1 | 2–3 years | 1.50 | 1.46 | 1.54 | 4.37 | 4.29 | 4.45 | 15.09 | 14.74 | 15.39 |
| G2 | 4–5 years | 1.58 | 1.55 | 1.60 | 4.62 | 4.57 | 4.67 | 15.55 | 15.39 | 15.70 |
| G3 | 6–7 years | 1.69 | 1.66 | 1.71 | 4.71 | 4.67 | 4.76 | 15.93 | 15.79 | 16.07 |
| G4 | 8–9 years | 1.70 | 1.66 | 1.73 | 4.72 | 4.67 | 4.77 | 16.10 | 15.96 | 16.24 |
| G5 | 10–11 years | 1.77 | 1.74 | 1.79 | 4.84 | 4.79 | 4.89 | 16.31 | 16.16 | 16.47 |
| G6 | 12–13 years | 1.82 | 1.79 | 1.84 | 4.91 | 4.87 | 4.95 | 16.41 | 16.24 | 16.57 |
| G7 | 14–15 years | 1.90 | 1.87 | 1.93 | 4.97 | 4.93 | 5.02 | 16.51 | 16.32 | 16.68 |
| G8 | 16–17 years | 1.93 | 1.90 | 1.96 | 5.04 | 4.98 | 5.09 | 16.64 | 16.47 | 16.81 |
| G9 | 18 and more | 1.92 | 1.88 | 1.97 | 5.09 | 5.04 | 5.14 | 16.73 | 16.54 | 16.91 |
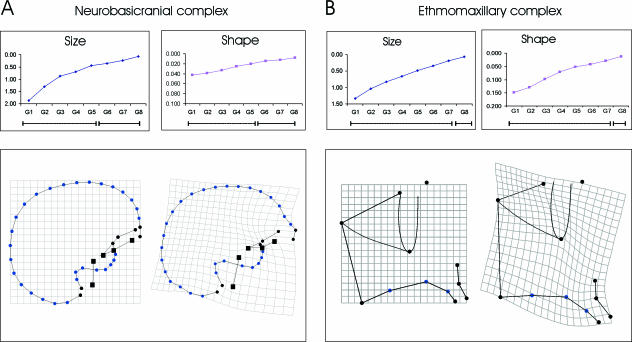
Fig. 3.
Maturation patterns of (A) the neurobasicranial and (B) the ethmomaxillary complex. In the inset graphs centroid size and shape (Procrustes) distances to adults are plotted. The significances of the differences in size and shape between the subadults and adults are shown on the bars beneath these graphs, in which dashed lines indicate significant differences from adult. These indicate the immature portions of the ontogenetic trajectories, solid lines the mature ones (absence of significant differences). The reference and transformation grids for the relevant landmark configurations are shown beneath each graph. Squares indicate midline basicranial structures while filled dots indicate lateral basicranial structures.
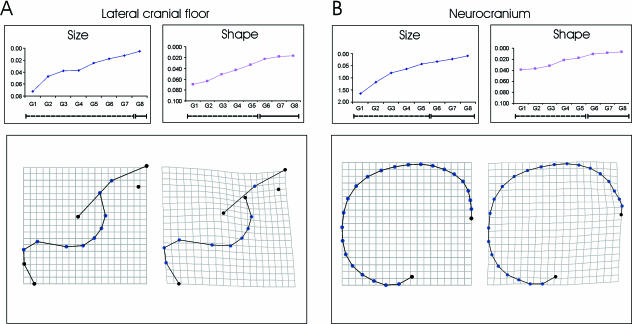
Fig. 5.
Maturation patterns of (A) the lateral cranial floor and (B) the midline neurocranium. In the inset graphs centroid size and shape (Procrustes) distances to adults are plotted. The significances of the differences in size and shape between the subadults and adults are shown on the bars beneath these graphs in which dashed lines indicate significant differences from adult. These indicate the immature portions of the ontogenetic trajectories, solid lines the mature ones (absence of significant differences). The reference and transformation grids for the relevant landmark configurations are shown beneath each graph. Note the ontogenetic dissociation in the lateral cranial floor between the achievement of adult size (in G8) and adult shape (in G6).
Fig. 4.
Maturation patterns of (A) the mandible and (B) the midline cranial base. The differences in size and shape between the subadults and the adults are given. In the inset graphs centroid size and shape (Procrustes) distances to adults are plotted. The significances of the differences in size and shape between the subadults and adults are shown on the bars beneath these graphs in which dashed lines indicate significant differences from adult. These indicate the immature portions of the ontogenetic trajectories, solid lines the mature ones (absence of significant differences). The reference and transformation grids for the relevant landmark configurations are shown beneath each graph. Note the ontogenetic dissociation in the midline cranial base between the achievement of adult size (by G7) and adult shape (by G4).
Developmental (shape) maturation is shown in Tables 5 and 6 and Figs 3–5. Table 5 indicates that the differences in shape of the neurobasicranial complex between non-adults and adults disappear at G6 (11.4 years). The multiple morphological changes are shown in Fig. 3 and consist of relative anterior protrusion of the glabella, a re-orientation and positioning of the anterior and middle cranial fossae, together with an increase in basicranial flexion at the midline basicranium (Fig. 3A).
Table 5.
Craniofacial components, shape differences from adults measured as Procrustes distance d, 95% confidence intervals for significant distances, Goodall’s F-test and P values
| Neurobasicranial complex | Ethmomaxillary complex | Mandible | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group | Years | d | 95%– | 95%+ | Goodall’s F | P | d | 95%– | 95%+ | Goodall’s F | P | d | 95%– | 95%+ | Goodall’s F | P |
| G1 | 2–3 years | 0.042 | 0.030 | 0.050 | 11.810 | 0.000 | 0.148 | 0.134 | 0.164 | 42.23 | 0.001 | 0.076 | 0.062 | 0.087 | 18.81 | 0.000 |
| G2 | 4–5 years | 0.039 | 0.035 | 0.045 | 14.120 | 0.000 | 0.129 | 0.120 | 0.142 | 44.59 | 0.001 | 0.070 | 0.060 | 0.080 | 20.77 | 0.000 |
| G3 | 6–7 years | 0.033 | 0.030 | 0.039 | 9.980 | 0.000 | 0.097 | 0.087 | 0.108 | 27.28 | 0.001 | 0.056 | 0.049 | 0.064 | 18.81 | 0.000 |
| G4 | 8–9 years | 0.026 | 0.023 | 0.032 | 5.690 | 0.000 | 0.070 | 0.061 | 0.085 | 11.15 | 0.000 | 0.044 | 0.037 | 0.055 | 9.23 | 0.000 |
| G5 | 10–11 years | 0.020 | 0.018 | 0.028 | 3.580 | 0.010 | 0.051 | 0.043 | 0.067 | 7.38 | 0.001 | 0.038 | 0.032 | 0.050 | 7.51 | 0.001 |
| G6 | 12–13 years | 0.014 | 1.770 | 0.070 | 0.042 | 0.035 | 0.057 | 4.84 | 0.001 | 0.037 | 0.028 | 0.050 | 7.31 | 0.001 | ||
| G7 | 14–15 years | 0.012 | 1.240 | 0.231 | 0.028 | 0.023 | 0.044 | 2.15 | 0.023 | 0.027 | 0.019 | 0.042 | 3.20 | 0.008 | ||
| G8 | 16–17 years | 0.008 | 0.490 | 0.909 | 0.013 | 0.41 | 0.939 | 0.020 | 1.70 | 0.123 | ||||||
Table 6.
Neuro- and basicranial components. Shape differences from adults measured as Procrustes distance d, 95% confidence intervals for significant distances, Goodall’s F-test and P values
| Midline cranial base | Lateral cranial floor | Neurocranium | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group | Years | d | 95%– | 95%+ | Goodall’s F | P | d | 95%– | 95%+ | Goodall’s F | P | d | 95%– | 95%+ | Goodall’s F | P |
| G1 | 2–3 years | 0.060 | 0.043 | 0.081 | 10.620 | 0.000 | 0.070 | 0.064 | 0.084 | 10.66 | 0.000 | 0.039 | 0.033 | 0.046 | 16.46 | 0.000 |
| G2 | 4–5 years | 0.041 | 0.029 | 0.058 | 7.930 | 0.000 | 0.063 | 0.057 | 0.074 | 12.19 | 0.000 | 0.037 | 0.032 | 0.042 | 20.56 | 0.000 |
| G3 | 6–7 years | 0.032 | 0.019 | 0.051 | 3.600 | 0.011 | 0.051 | 0.044 | 0.062 | 8.91 | 0.000 | 0.032 | 0.026 | 0.039 | 14.78 | 0.000 |
| G4 | 8–9 years | 0.015 | 0.810 | 0.508 | 0.043 | 0.036 | 0.055 | 5.28 | 0.000 | 0.021 | 0.017 | 0.028 | 6.43 | 0.000 | ||
| G5 | 10–11 years | 0.018 | 1.260 | 0.310 | 0.033 | 0.028 | 0.048 | 3.18 | 0.001 | 0.017 | 0.015 | 0.025 | 4.24 | 0.002 | ||
| G6 | 12–13 years | 0.021 | 1.860 | 0.118 | 0.023 | 1.37 | 0.213 | 0.011 | 1.76 | 0.128 | ||||||
| G7 | 14–15 years | 0.010 | 0.430 | 0.756 | 0.018 | 0.84 | 0.553 | 0.009 | 1.13 | 0.341 | ||||||
| G8 | 16–17 years | 0.016 | 0.850 | 0.437 | 0.016 | 0.64 | 0.717 | 0.006 | 0.55 | 0.725 | ||||||
The face (i.e. the ethmomaxillary complex and the mandible) achieves adult shape at G8 (15.7 years, Table 5) and the morphological changes are shown in Figs 3B and 4A. The ethmomaxillary complex (Fig. 3B) becomes relatively vertically taller through a strong vertical expansion of the midfacial region and relative forward and upward rotation and projection of the anterior alveolar process. There is a relative contraction within the cribriform plate, indicating that this area has grown relatively little. This is accompanied by an expansion of the anterior upper facial region (between anterior cribriform and nasion). Figure 4A shows mandibular development, which consists of a strong vertical elongation of the ramus and rotation of the corpus towards the ramus, increasing the angulation. Additionally, the chin becomes pronounced.
The adult shape of the midline cranial base is achieved by G4 (7.7 years, Table 6), much later than predicted. Figure 4B shows the morphological modifications. The pre-sellar part becomes relatively elevated, while the post-sellar part becomes elongated inferiorly and slightly inferiorly rotated. Both processes produce basicranial flexion.
The lateral cranial floor attains adult shape by G6 (11.4 years, Table 6), earlier than expected, and the morphological changes (Fig. 5A) consist of a relative retraction of the anterior cranial fossae, a relative forward projection of the middle cranial fossae and a slight lowering of their anterior poles. The petrosal part of the middle cranial fossa becomes moderately expanded posteriorly at landmarks 12–15. Figures 4 and 5 show that, interestingly, both basicranial components achieve their adult shape before adult size.
Finally, the adult shape of the midline vault (Fig. 5B) is achieved by G6 (11.4 years), later than expected. Ontogenetic changes include relative anterior projection of the glabellar region, a slight relative lowering of the vertex between the bregmatic and lambdoid region, and a slight relative elevation of the nuchal area.
The second approach using shape trajectory length revealed a very similar picture of sequential shape maturation. However, whereas the ages for shape maturation of the neurobasicranial complex (G6), the midline cranial base (G4) and the lateral cranial floor (G6) did not change, both facial components (G7) and the neurocranial outline (G5) reached 95% of shape trajectory length one age group earlier than when using bootstrapped Goodall’s F-test, and Procrustes distance from adults. Together, the results indicate that some aspects of the initial expectations hold, whereas others do not. These details are discussed in the following section.
Discussion
The aim of the present study was to investigate the spatio-temporal patterning of maturation of human skull size and shape in order to assess the potential for basicranial influence on facial shape (Enlow & Hans, 1996). Twenty-eight individuals of the longitudinal Denver Growth Study (Maresh & Washburn, 1938; Lieberman et al. 2001) were analysed between 2 years of age and adulthood (19 years) and the timing of the achievement of adult size and shape was studied.
The notion of a spatio-temporal ontogenetic gradient passing from the basi- and neurocranium to the ethmomaxillary complex and the mandible (Baughan et al. 1979; Buschang et al. 1983; Enlow & Hans, 1996) is examined in two steps. In the first we address the maturation of the whole skull, and in the second, the maturation of various components of the neurobasicranial complex only.
The maturation of size
Several studies have shown that craniofacial measurements approach adult sizes along a spatially ordered gradient, in which basicranial measurements achieve maturity earlier than facial measurements (Baughan et al. 1979; Baume et al. 1983; Buschang et al. 1983). Baughan et al. (1979) established three different categories, a neurobasicranial, a facial and a skeletal pattern of ontogenetic maturation. In contrast to these authors, Buschang et al. (1983) observed in a descriptive study a somewhat continuous gradient of increasing linear measurements until adulthood. The first structures to achieve adult size were head height and basicranial length, while the last structures were vertical measurements of the maxilla and the mandible (Buschang et al. 1983). Additionally, some overlap of maturation patterns between different anatomical structures was observed. Thus, the authors suggested a growth gradient rather than discrete categories of growth patterns.
Although in our data (Tables 3 and 4) similar tendencies to those of Buschang et al. (1983) were found, statistical tests of adult (G9) and non-adult mean values revealed similar temporal patterns of size maturation for the maxilla and the mandible. No significant difference was observed between developmental stage G8 and adults in the size of the mandible and maxilla (Table 3, Fig. 4A). This leads us to conclude that both parts of the facial skeleton achieve adult size at a similar time, around the age of 16 years. This contrasts with the results of Buschang et al. (1983), which indicate the mandible achieves adult morphology later than the maxilla. However, in line with previous findings (Biegert, 1957; Baughan et al. 1979; Enlow & Hans, 1996), distinct neural and facial patterns of size maturation have been identified.
Interestingly, within the cranium another gradient of size maturation has been detected. The first structure to achieve adult size is the neurocranium (G6), then the midline cranial base (G7) and finally the lateral cranial floor (G8) together with the facial compartments. As the neurocranium is directly related to the growth of the brain (Moss & Young, 1960; Enlow & Hans, 1996) it is not surprising that it achieves adulthood at a similar ontogenetic age. The midline cranial base reaches mature size at G7 (13.3 years), which is roughly when the spheno-occipital suture ceases growth activity (Enlow & Hans, 1996; Scheuer & Black, 2000).
The lateral cranial floor seems to manifest similar growth dynamics to the face. These components achieve mature size at G8 (15.8 years; Table 4). This finding can be interpreted as a further indication of highly integrated growth between lateral basicranial and facial structures (Bastir et al. 2004; Bastir & Rosas, 2005, 2006).
With respect to the anterior cranial floor (Table 4, Fig. 5) it seems that the ethmoid relates strongly to the growth of the face rather than that of the brain, in which growth has ceased much earlier. The inclusion of the ethmoid in the face on comparative embryological grounds has been suggested earlier by several workers (Kummer, 1952; Biegert, 1957; Hofer, 1965; Enlow, 1968; McCarthy, 2001; Bastir, 2004). The anterior cranial floor might therefore be considered as an ‘interface’ establishing a structural connection between the facial and the basicranial structures, which accommodates morphological modifications of the organ systems on either side.
In addition, it is interesting to note that the midline and lateral basicranial structures follow different growth dynamics. This has been suggested in other studies (Sgouros et al. 1999; Goodrich, 2005). With regard to these findings some caution might be required when referring to the neurobasicranial complex as a morphologically consistent unit. Although in the analysis of adult variation patterns the notion of a neurobasicranial complex (Lieberman et al. 2000b) may be justified, in comparative ontogenetic studies no morphologically or developmentally integrated ‘unit’ can be expected.
At first sight, it seems surprising that the neurobasicranial complex as a whole (Table 3, Fig, 3) achieves size maturity earlier (G6) than some of its components, i.e. the midline cranial base (G7) and the lateral cranial floor (G8) (Table 4, Figs 4B and 5A,B). However, the landmarks of the whole neurobasicranial complex span several anatomical structures. As mentioned before, these landmarks include structures that share close relationships to the brain, such as most of the semilandmarks of the neurocranial vault outline, some landmarks which are part of the upper face (nasion, glabella), and some landmarks of the midline cranial base and lateral cranial floor with possible intermediate relationships to the brain and the face. Thus, a pooled analysis of these structures effectively presents a developmentally ‘blurred’ picture, while analysis of several of the components in isolation may give a more detailed picture that better differentiates the various influences on craniofacial spatio-temporal relationships.
The maturation of shape
The analyses of shape data also lend support to the concept of craniofacial levels with spatio-temporally ordered maturation. This finding is independent of the approach we use to assess maturity. The shape of neurobasicranial structures (Tables 5 and 6) achieves adult morphology much earlier than the facial components of the skull. Again, and similar to the maturation of size, within the face no evidence has been found that the ethmomaxillary complex matures earlier in shape than the mandible. However, using shape trajectory length it should be noted that the mandible at G7 is much closer to the lower 95% confidence limit of shape trajectory length in the oldest group than is the ethmomaxillary complex. Although this might suggest a longer developmental trajectory for the mandible, both achieve 95% shape maturity in stage G7.
It is possible that the pooling of males and females may influence the results of maturation of facial structures. It is known that facial dimorphism in humans mainly relates to slightly prolonged growth and development of males compared with females (Bhatia & Leighton, 1993; Enlow & Hans, 1996; Rosas & Bastir, 2002). Therefore, it is likely that females are increasingly represented in the lower range of the 95% confidence limits with males in the higher. Our results thus give the average age ranges of skull maturation. However, in this study, owing to the fact that there are slightly more males at G7 and G8 (Table 2), there may be a slight bias towards the male maturation pattern. An ontogenetic study addressing maturation of facial sexual dimorphism should consider this.
As mentioned above it is difficult to determine which criterion of shape maturity should be used. Although the confidence intervals of Procrustes distance to the oldest group depend on sample size, the shape trajectory length depends on sample composition; the inclusion of more specimens would improve the reliability of the confidence intervals of the oldest group whereas inclusion of younger or older specimens would increase shape trajectory length by increasing the variation within the sample. Shape trajectory length accounts for curvilinearity, but, by contrast, it is more sensitive to the influence of cumulative measurement error (a problem when working with X-ray data) than are the confidence intervals of the oldest group. Certainly, a geometric morphometric analysis of longitudinal three-dimensional data (MRI, CT reconstructions) with less measurement error would be desirable in confirming our findings. However, despite these problems and the fact that the issue of facial maturity in terms of absolute years cannot be resolved here, the key aspect of this study, i.e. the elucidation of the spatio-temporal pattern of skull maturation, is not affected by the arbitrariness of the criterion of maturity nor does it affect our assessment of the maturation sequence of the analysed anatomical structures.
Within the cranium, a gradient of shape maturation has been identified (Figs 4B and 5). The midline cranial base first displays adult shape at 7.7 years whereas the lateral cranial floor and the neurocranial outline mature later: the lateral cranial floor at 11.7 years and the neurocranial vault either at 11.7 (assessment approach 1) or at 9.9 years (assessment approach 2). Thus, a key finding of this study is that the two basicranial components, the midline cranial base and the lateral cranial floor, show a temporal dissociation from each other in the maturation of shape irrespective of the maturation criterion used. Our results show that the midline cranial base attains adult shape at about the time at which Enlow (1968) and Ranly (1988) indicate that the frontal lobes mature. We also show that the lateral cranial floor and midline neurocranial vault mature when the growth of the complete brain is nearly completed (Ranly, 1988). This is close to the time of cessation of medio-lateral growth of the middle cranial fossa (Sgouros et al., 1999; Goodrich, 2005) and may point to the lateral cranial floor being influenced by the ontogeny of the temporal lobes. It should be borne in mind that in the case of the neurobasicranial complex as well as the neurocranial vault the maturation pattern we observe might be affected (prolonged) by the inclusion of upper facial parts such as nasion and glabella.
The establishment of adult shape of the midline basicranium by 7.7 years is interesting. It is known that the basicranial angle attains mature values at around 2 years (Lieberman & McCarthy, 1999; Lieberman et al. 2004). Hence, this discrepancy between angular and shape data points to complex morphological modifications, which are not completely reflected by the midline basicranial angle (O’Higgins, 2001; Bastir, 2004). It may be fruitful in future studies to concentrate on the size and shape of this key basicranial structure using more complete morphological descriptions than the angle. Although Fig. 4B shows that morphological changes in midline cranial base ontogeny do involve modifications of its angle, it can also be seen that these modifications are produced by changing the spatial position of the posterior sphenoid (lm 2), which becomes elevated with respect to the spheno-occipital clivus. This is in line with earlier observations (Kummer, 1952; Björk, 1955; Starck & Kummer, 1962). Additionally, the spheno-occipital clivus becomes elongated in a downward direction by growth at the spheno-occipital synchondrosis (Enlow, 1968; Sperber, 1989; Enlow & Hans, 1996; Scheuer & Black, 2000). When analysed in terms of angles the ontogeny of the midline cranial base might be wrongly imagined as comprising a hinge-like rotation of pre-sellar and post-sellar parts of the midline cranial base about a common centre (Lieberman et al. 2000a; McCarthy & Lieberman, 2001). The TPS grid (Fig. 4B), nevertheless, indicates additional positional shifts of these parts, which do not share a common centre of rotation, as would be seen in a hinge. That the morphological details of changing position of the ‘rotation centres’ are not captured by the midline basicranial angle has caused some dispute in questions of heterochrony in human evolution (Kummer, 1952; Starck & Kummer, 1962; contra Gould, 1977). While angular values can be identical, the spatial relationships of structures producing these angles can be different (Bastir, 2004). Figure 4B shows a shearing of the TPS grid that indicates configurational changes of basicranial elements producing the flexion classically measured by angles (for a review see Lieberman & McCarthy, 1999).
Additionally, it seems that important modifications occur at landmark 2, which is posterior to the attachment of the facial block (Lieberman et al. 2000a). It remains to be determined to what degree the vertical elevation of landmark 2 affects the position of the face. However, as landmark 2 is on the cerebral surface of the midline cranial base and the face is attached at its external part, this important question should be examined in a more detailed investigation using three-dimensional landmarks from both sides of the cranial base and floor.
With respect to potential base–face interactions, changes of the midline cranial base (Fig. 4B) have to be considered together with developmental modifications of the lateral cranial floor, which develops over a longer period of ontogeny (Table 5, Fig. 5A) (Enlow & Hans, 1996; Sgouros et al. 1999; Goodrich, 2005). In both components ontogenetic shape change produces a vertical increase of the posterior face and the nasopharyngeal space (Rosas & Bastir, 2002), accompanied by an increase of the height of the posterior face at the maxilla and the mandible (Bastir & Rosas, 2004b) (Figs 3B and 4A).
The present findings indicate that the definitive adult shape of the whole basicranium (midline cranial base and lateral cranial floor) is attained at around 12 years (Tables 3 and 4; Figs 4B and 5A). It is at this final stage that the morphological configuration is attained that has been suggested to set specific spatial conditions in the area where the facial block (McCarthy & Lieberman, 2001) attaches. Therefore, the superior and posterior dimensions of the facial block of necessity become fixed at that age. This temporal information taken together with the hypothetical spatial growth boundaries suggested in relation to counterparts (Enlow et al. 1969; Bhat & Enlow, 1985) and which define the anterior limits of growth fields (Enlow & Azuma, 1975; Bromage, 1992) support the hypothesis that from this age onwards ongoing growth of facial organ systems (Baume et al. 1983; Buschang et al. 1983) can only proceed in an inferior direction in humans.
Implications for development and evolution
Hallgrimsson et al. (2002, p. 133) have discussed how developmental systems ‘structure the production of phenotypic variation’. From the present data it seems that the temporal sequence of ontogenetic maturation relates in specific ways to the hierarchical nature of morphological variation and integration that can be identified in adults. A recent study has found that the midline base and the middle cranial fossae are characterized by low levels of shape covariation (Bastir & Rosas, 2005). This low level of morphological integration was hypothesized to be, at least in part, a result of differential maturation of these structures. The exact timing of these temporal developmental dissociations has not been completely clear (Enlow, 1968; Enlow & Hans, 1996; Sgouros et al. 1999; Goodrich, 2005). This study shows that while the midline cranial base has finished development at 7.7 years (G4), the lateral middle cranial fossae still proceed for 3–4 years later (G6) with anterior expansion and lowering of their position with respect to the anterior cranial fossae (Figs 4 and 5). Any co-ordinated (or integrated) development between midline cranial base and the lateral cranial floor structures must thus occur before the midline cranial base achieves adult shape, i.e. prior to 8 years of age. In other words, both structures share approximately half of the developmental time prior to adulthood and their divergent maturation patterns may explain their surprisingly low level of morphological covariation (Bastir & Rosas, 2005).
Such an interpretation adds a temporal aspect to the investigation of modularity (Wagner, 1996; Von Dassow & Munro, 1999; Klingenberg et al. 2003). Thus, structures that appear morphologically integrated are characterized by their own, specific temporal patterns of ontogeny, and possibly evolution. These possibilities were recently discussed with regard to the basicranial evolution of Pleistocene hominids from Java (Baba et al. 2003), and different patterns of evolution of the midline cranial base and lateral cranial floor structures have been suggested (Baba et al. 2003; Bastir, 2004; Bastir et al. 2006). The present findings of dissociated ontogeny of size and shape (Tables 3–6) together with those of decreased levels of morphological integration (Bastir & Rosas, 2005) provide developmental support for the evolutionary hypothesis of Baba et al. (2003). A preliminary study using two-dimensional data from fossils seems to support this view (Bastir et al. 2006) but more investigation in fossil hominids is needed. However, a temporal mosaic of development theoretically facilitates a morphological mosaic in evolution.
Basicranial–facial interactions
As mentioned in the introduction, Enlow & Hans (1996, p. 14) have suggested a ‘descending cause-and-effect stratigraphic arrangement of structural levels in the design of the face’ (emphasis added). Our findings imply that the ‘descending cause-and-effect’ relation may only occur once basicranial morphology has settled on its adult form, which is rather late in ontogeny. Prior to 12–13 years, both stratigraphic levels are still prone to morphological modifications. Thus, bidirectional (i.e. descending as well as ascending) developmental interactions between the face and the basicranium cannot be ruled out during these earlier ontogenetic periods. Regarding ascending influences, functional, biomechanical approaches to explaining facial morphology (Preuschoft, 1989; Spencer & Demes, 1993; Kupczik et al. 2006) are relevant here although the potential for the direct influence of biomechanical forces in the masticatory apparatus and face on lateral cranial floor structures needs to be investigated in further detail. However, experimental work in rats suggests that removal of temporal muscles significantly influences the external height, length and the width of the braincase (Moore, 1959), which points to the possibility of facial functional influence on basicranial variation, and especially of the anterior and lateral cranial floor, given the temporal sequences of maturation identified in the current study.
Summary
The present findings support the existence of craniofacial developmental levels (Enlow & Hans, 1996). In addition, our results specify the age ranges at which each of the skull compartments attains mature morphology (size and shape) and add a spatial dimension (midline vs. off-midline) to basicranial morphological maturation. Procrustes distance to the oldest age group (G9) (approach 1) as well shape trajectory length (approach 2) were both used to assess maturation. The latter was more robust if shape trajectories are curvilinear but was less robust in terms of cumulative measurement error. The skeletal maturation of skull shape follows a gradient that starts at the midline cranial base (7.7 years), extends to the lateral cranial floor and neurocranial outline (11.7 years), and then to the face, which matures at 15.7 years. Using approach 2 the neurocranial outline and both facial structures appear to mature one age group earlier. The maturation of size again shows a supero-inferior gradient, known from previous studies (Buschang et al. 1983). Within the cranium the neurocranial outline in the midline is the first structure to attain adult size (11.4 years), followed by the midline cranial base (13.6 years), and the lateral cranial floor together with the face at 15.7 years. The study indicates that the size and shape of basicranial elements can become dissociated during ontogeny. Future investigations should address the temporal and morphological pattern of shape maturation in non-human primates and fossil hominids. These findings will complement the results and interpretations of the present investigation and add to our understanding of the organization of morphological variation in the human skull.
Acknowledgments
We are grateful to Dan Lieberman and Chris Dean for help with the data of the Denver Growth Study. We thank James Rohlf, Dave Sheets, Phillip Gunz, Phillip Mitteroecker and Luis Maria Carrascal for help and discussion of some methodological aspects, and Kornelius Kupczik, Andrea Cardini and Sam Cobb for discussion. Chris Ruff and Roger Siervogel provided data from the Denver Growth Study on stature. Associate Editor Dan Lieberman and anonymous reviewers provided very helpful and important suggestions for the improvement on the manuscript. This study was supported by project BOS-2003-0153 of the Dirección General de Investigación of the Spanish government. M.B. is funded by a postdoctoral fellowship of the Spanish Ministry of Science and Education (MEC).
References
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Baba H, Aziz F, Kaifu Y, Suwa G, Kono RT, Jacob T. Homo erectus Calvarium from the Pleistocene of Java. Science. 2003;299:1384–1388. doi: 10.1126/science.1081676. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Kuroe K. Morphogenetic determinants of mandibular ramus breadth. A test in modern human populations. Am J Phys Anthropol. 2002;S115:40–41. [Google Scholar]
- Bastir M. PhD thesis. Department of Anthropology, Autonoma University of Madrid; 2004. A geometric morphometric analysis of integrative morphology and variation in human skulls with implications for the Atapuerca-SH hominids and the evolution of Neandertals. Structural and systemic factors of morphology in the hominid craniofacial system. [Google Scholar]
- Bastir M, Rosas A. Comparative ontogeny in humans and chimpanzees: Similarities, differences and paradoxes in postnatal growth and development of the skull. Ann Anat. 2004a;186:503–509. doi: 10.1016/S0940-9602(04)80096-7. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Facial heights: evolutionary relevance of postnatal ontogeny for facial orientation and skull morphology in humans and chimpanzees. J Hum Evol. 2004b;47:359–381. doi: 10.1016/j.jhevol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Kuroe K. Petrosal orientation and mandibular ramus breadth: evidence of a developmental integrated petroso-mandibular unit. Am J Phys Anthropol. 2004;123:340–350. doi: 10.1002/ajpa.10313. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. The hierarchical nature of morphological integration and modularity in the human posterior face. Am J Phys Anthropol. 2005;128:26–34. doi: 10.1002/ajpa.20191. [DOI] [PubMed] [Google Scholar]
- Bastir M, O’Higgins P, Rosas A. Human evolution: relationships between the basicranium and the face. Ann Hum Biol. 2006;32:790. [Google Scholar]
- Bastir M, Rosas A. Correlated variation between the lateral basicranium and the face: a geometric morphometric study in different human groups. Arch Or Biol. 2006;51:814–824. doi: 10.1016/j.archoralbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Baughan B, Demirjian A, Levesque GY, Lapalme-Chaput L. The pattern of facial growth before and during puberty, as shown by French-Canadian girls. Ann Hum Biol. 1979;6:59–76. doi: 10.1080/03014467900003371. [DOI] [PubMed] [Google Scholar]
- Baume R, Buschang P, Weinstein S. Stature, head height, and growth of the vertical face. Angle Orthod. 1983;83:477–484. [PubMed] [Google Scholar]
- Bhat M, Enlow DH. Facial variations related to headform type. Angle Orthod. 1985;55:269–280. doi: 10.1043/0003-3219(1985)055<0269:FVRTHT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Leighton BC. A Manual of Facial Growth. A Computer Analysis of Longitudinal Cephalometric Growth Data. Oxford: Oxford University Press; 1993. [Google Scholar]
- Biegert J. Der Formwandel des Primatenschädels und seine Beziehungen zur ontogenetischen Entwicklung und den phylogenetischen Spezialisationen der Kopforgane. Ggbrs Morph Jahrb. 1957;98:77–199. [Google Scholar]
- Björk A. Cranial base development. Am J Orthod. 1995;41:198–225. [Google Scholar]
- Bookstein FL. Morphometric Tools for Landmark Data. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Bookstein FL. Combining the tools of geometric morphometrics. In: Marcus LF, editor. Advances in Morphometrics. New York: Plenum Press; 1996a. pp. 131–151. [Google Scholar]
- Bookstein FL. Applying landmark methods to biological outline data. In: Mardia KV, Gill CA, Dryden IL, editors. Image Fusion and Shape Variability Techniques. Leeds: Leeds University Press; 1996b. pp. 79–87. [Google Scholar]
- Bookstein FL. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Im Anal. 1997;1:225–243. doi: 10.1016/s1361-8415(97)85012-8. [DOI] [PubMed] [Google Scholar]
- Bookstein F, Schäfer K, Prossinger H, et al. Comparing frontal cranial profiles in archaic and modern Homo by morphometric analysis. Anat Rec. 1999;257:217–224. doi: 10.1002/(SICI)1097-0185(19991215)257:6<217::AID-AR7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec. 2002;269B:162–174. doi: 10.1002/ar.10110. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Gunz P, Mitteroecker P, Prossinger H, Schaefer K, Seidler H. Cranial integration in Homo: singular warps analysis of the midsagittal plane in ontogeny and evolution. J Hum Evol. 2003;44:167–187. doi: 10.1016/s0047-2484(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Bromage TG. The ontogeny of Pan troglodytes craniofacial architectural relationships and implications for early hominids. J Hum Evol. 1992;23:235–251. [Google Scholar]
- Buschang P, Baume R, Nass G. A craniofacial growth maturity gradient for males and females between 4 and 16 years of age. Am J Phys Anthropol. 1983;61:373–381. doi: 10.1002/ajpa.1330610312. [DOI] [PubMed] [Google Scholar]
- Dabelow A. Über Korrelationen in der phylogenetischen Entwicklung der Schädelform II. Die Beziehungen zwischen Gehirn und Schädelbasisform bei den Mammaliern. Ggbrs Morph Jahrb. 1931;67:84–133. [Google Scholar]
- De Beer GR. The Development of the Vertebrate Skull. Oxford: Clarendon Press; 1937. [Google Scholar]
- Enlow DH. The Human Face: An Account of the Postnatal Growth and Development of the Craniofacial Skeleton. New York: Harpers & Row; 1968. [Google Scholar]
- Enlow DH, Moyers RE, Hunter WS, McNamara JA., Jr A procedure for the analysis of intrinsic facial form and growth. Am J Orthod. 1969;56:6–23. doi: 10.1016/0002-9416(69)90254-1. [DOI] [PubMed] [Google Scholar]
- Enlow DH, Kuroda T, Lewis AB. The morphological and morphogenetical basis for craniofacial form and pattern. Angle Orthodontist. 1971;41:161–188. doi: 10.1043/0003-3219(1971)041<0161:TMAMBF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Enlow DH, Azuma M. Functional growth boundaries in the human and mammalian face. In: Bergsma D, Langman J, Paul NW, editors. Morphogenesis and Malformation of the Face and the Brain. New York: Alan R. Riss; 1975. pp. 217–230. [Google Scholar]
- Enlow DH, Hans MG. Essentials of Facial Growth. Philadelphia: W.B. Saunders Company; 1996. [Google Scholar]
- Goodrich JT. Skull base growth in craniosynostosis. Child’s Nervous System. 2005. in press. [DOI] [PubMed]
- Gould SJ. Ontogeny and Phylogeny. Cambridge, MA: Harvard University Press; 1977. [Google Scholar]
- Grossman JW, Zuckerman S. An X-ray study of growth changes in the base of the skull. Am J Phys Anthropol. 1955;13:515–519. doi: 10.1002/ajpa.1330130310. [DOI] [PubMed] [Google Scholar]
- Gunz P, Mitteroecker P, Bookstein FL. Semilandmarks in three dimensions. In: Slice D, editor. Modern Morphometrics in Physical Anthropology. New York: Kluwer Academic/Plenum Press; 2005. [Google Scholar]
- Hallgrimsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological integration in primate limbs. Yrb Phys Anthropol. 2002;45:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H. Studien zum Problem des Gestaltwandels des Schädels der Säugetiere insbesondere der Primaten, I. Die medianen Krümmungen des Schädels und ihre Erfassung nach der Methode von Landzert. Zschrft Morph Anthropol. 1960;50:299–316. [Google Scholar]
- Hofer H. Die morphologische Analyse des Schädels des Menschen. In: Heberer G, editor. Menschliche Abstammungslehre, Fortschritte der Anthropogenie, 1863–1964. Stuttgart: Gustav Fischer Verlag; 1965. pp. 145–226. [Google Scholar]
- Jeffery N, Spoor F. Brain size and the human cranial base. Am J Phys Anthropol. 2002;118:324–340. doi: 10.1002/ajpa.10040. [DOI] [PubMed] [Google Scholar]
- Jeffery N. Brain expansion and comparative prenatal ontogeny of the non-hominoid cranial base. J Hum Evol. 2003;45:263–284. doi: 10.1016/j.jhevol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Mebus K, Auffray J-C. Developmental integration in a complex morphological structure: how distinct are the modules in the mouse mandible? Evol Dev. 2003;5:522–531. doi: 10.1046/j.1525-142x.2003.03057.x. [DOI] [PubMed] [Google Scholar]
- Kummer B. Untersuchungen über die ontogenetische Entwicklung des menschlichen Schädelbasiswinkels. Z Morph Anthropol. 1952;43:331–360. [Google Scholar]
- Kupczik K, Dobson CA, O’Higgins P, Fagan MJ, Crompton RH, Oxnard CE. Relating strain to craniofacial morphology in Macaca fascicularis: modelling and validation. Ann Hum Biol. 2006;32:791. [Google Scholar]
- Landzert T. Der Sattelwinkel und sein Verhaeltnis zur Prognathie. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft. 1866;6 [Google Scholar]
- Leigh SL. Evolution of human growth spurts. Am J Phys Anthropol. 1996;101:455–474. doi: 10.1002/(SICI)1096-8644(199612)101:4<455::AID-AJPA2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Lieberman DE. Sphenoid shortening and the evolution of modern human cranial shape. Nature. 1998;393:158–162. doi: 10.1038/30227. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McCarthy RC. The ontogeny of cranial base angulation in humans and chimpanzees and its implication for reconstructing pharyngeal dimensions. J Hum Evol. 1999;36:487–517. doi: 10.1006/jhev.1998.0287. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Ross C, Ravosa MJ. the primate cranial base: ontogeny, function, and integration. Yrbk Phys Anthropol. 2000a;43:117–169. doi: 10.1002/1096-8644(2000)43:31+<117::aid-ajpa5>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Pearson OM, Mowbray KM. Basicranial influence on overall cranial shape. J Hum Evol. 2000b;38:291–315. doi: 10.1006/jhev.1999.0335. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McCarthy RC, Hiiemae KM, Palmer JB. Ontogeny of postnatal hyoid larynx descent in humans. Arch Oral Biol. 2001;46:117–128. doi: 10.1016/s0003-9969(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McBratney BM, Krovitz G. The evolution and development of cranial form in Homo sapiens. Proc Natl Acad Sci USA. 2002;99:1134–1139. doi: 10.1073/pnas.022440799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DE, Krovitz GE, McBratney-Owen B. Testing hypotheses about tinkering in the fossil record: the case of the human skull. JExpZool. 2004;302:284–301. doi: 10.1002/jez.b.21004. [DOI] [PubMed] [Google Scholar]
- Maresh MM, Washburn AH. Size of the heart in healthy normal children. Am J Dis Child. 1938;56:33–60. [Google Scholar]
- Maresh MM. Growth of the heart related to bodily growth during childhood and adolescence. Pediatrics. 1948;2:382–402. [PubMed] [Google Scholar]
- Martinón-Torres M, Bastir M, Bermúdez de Castro JM, Gómez A, Sarmiento S, Muela A. Geometric morphometric analysis of hominin lower second premolars: evolutionary implications. J Hum Evol. 2006. in press. [DOI] [PubMed]
- McCarthy R. Anthropoid cranial base architecture and scaling relationships. J Hum Evol. 2001;40:41–66. doi: 10.1006/jhev.2000.0446. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Lieberman DE. Posterior maxillary (PM) plane and anterior cranial architecture in primates. Anat Rec. 2001;264:247–260. doi: 10.1002/ar.1167. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P, Gunz P, Bernhard M, Schaefer K, Bookstein FL. Comparison of cranial ontogenetic trajectories among great apes and humans. J Hum Evol. 2004;46:679–698. doi: 10.1016/j.jhevol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Moore WJ. PhD thesis. AL: Faculty of Medicine, University of Birmingham; 1959. The influence of muscle function on the growth of the rat skull. [Google Scholar]
- Moss M, Young RW. A functional approach to craniology. Am J Phys Anthrop. 1960;45:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- O’Higgins P. The study of morphological variation in the hominid fossil record: biology, landmarks and geometry. J Anat. 2001;197:103–120. doi: 10.1046/j.1469-7580.2000.19710103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de León M, Zollikofer C. Neandertal cranial ontogeny and its implications for late hominid diversity. Nature. 2001;412:534–538. doi: 10.1038/35087573. [DOI] [PubMed] [Google Scholar]
- Preuschoft H. Splechtna H, Hilgers H. Trends in Vertebrate Moprhology. New York: Gustav Fischer Verlag; 1989. Biomechanical approach to the evolution of the facial skeleton of hominoid primates; pp. 421–431. [Google Scholar]
- Rak Y. The Neandertal: a new look at an old face. J Hum Evol. 1986;15:151–164. [Google Scholar]
- Ranly DM. A Synopsis of Craniofacial Growth. Norwalk, CT/San Mateo, CA: Appleton & Lange; 1988. [Google Scholar]
- Ravosa MJ, Shea BT. Pattern in craniofacial biology: evidence from the Old World Monkeys (Cercopithecidae) Int J Primatol. 1994;15:801–822. [Google Scholar]
- Riedl R. Die Ordnung des LebendigenSystembedingungen der Evolution. Hamburg: Paul Parey Verlag; 1975. [Google Scholar]
- Rohlf FJ, Slice D. Extensions of the procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- Rohlf FJ, Marcus L F. A revolution in morphometrics. Tree. 1993;8:129–132. doi: 10.1016/0169-5347(93)90024-J. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ. Morphometric spaces, shape components and the effects of linear transformations. In: Marcus LF, editor. Advances in Morphometrics. New York: Plenum Press; 1996. pp. 117–128. [Google Scholar]
- Rohlf F. 1.08. New York: Department of Ecology and Evolution, State University, Stony Brook; 1997. tpsDIG. [Google Scholar]
- Rohlf FJ. 1.19. New York: Department of Ecology and Evolution, State University, Stony Brook; 1998. tpsUTIL. [Google Scholar]
- Rosas A. Occurrence of Neanderthal features in mandibles from the Atapuerca-SH site. Am J Phys Anthropol. 2001;114:74–91. doi: 10.1002/1096-8644(200101)114:1<74::AID-AJPA1007>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Rosas A, Bastir M. Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am J Phys Anthropol. 2002;117:236–245. doi: 10.1002/ajpa.10023. [DOI] [PubMed] [Google Scholar]
- Rosas A, Bastir M. Geometric morphometric analysis of allometric variation in the mandibular morphology from the hominids of Atapuerca, Sima de los Huesos Site. Anat Rec Part A. 2004;278A:551–560. doi: 10.1002/ar.a.20049. [DOI] [PubMed] [Google Scholar]
- Ross CF, Ravosa MJ. Basicranial flexion, relative brain size, and facial kyphosis ind nonhuman primates. Am J Phys Anthropol. 1993;91:305–324. doi: 10.1002/ajpa.1330910306. [DOI] [PubMed] [Google Scholar]
- Ross C, Henneberg M. Basicranial flexion, relative brain size, and facial kyphosis in Homo sapiens and some fossil hominids. Am J Phys Anthropol. 1995;98:575–593. doi: 10.1002/ajpa.1330980413. [DOI] [PubMed] [Google Scholar]
- Ross CF, Henneberg M, Ravosa MJ, Richard S. Curvilinear, geometric and phylogenetic modeling of basicranial flexion: is it adaptive, is it constrained? J Hum Evol. 2004;46:185–213. doi: 10.1016/j.jhevol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Scheuer L, Black S. Developmental Juvenile Osteology. New York: Academic Press; 2000. [Google Scholar]
- Sgouros S, Natarajan K, Hockley A, Goldin JH, Wake M. Skull base growth in childhood. Ped Neurol. 1999;31:259–268. doi: 10.1159/000028873. [DOI] [PubMed] [Google Scholar]
- Shea BT. On aspects of skull form in African apes and orangutans, with implication for hominoid evolution. Am J Phys Anthropol. 1985;68:329–342. doi: 10.1002/ajpa.1330680304. [DOI] [PubMed] [Google Scholar]
- Sheets HD. IMP, Integrated Morphometric Package. 2001 http://www.canisius.edu/~sheets/morphsoft.html.
- Sheets DH, Kim K, Mitchel CE. A combined landmark and outline-based approach to ontogenetic shape change in the Ordovician trilobite Triarthrus becki. In: Elewa A, editor. Morphometrics in Paleontology. Berlin: Springer; 2004. pp. 67–82. [Google Scholar]
- Spencer MA, Demes B. Biomechanical analysis of masticatory system configuration in Neandertals and Inuits. Am J Phys Anthropol. 1993;91:1–20. doi: 10.1002/ajpa.1330910102. [DOI] [PubMed] [Google Scholar]
- Sperber GH. Craniofacial Embryology. London: Wright; 1989. [Google Scholar]
- Spoor F. Basicranial architecture and relative brain size of Sts5 (Australopithecus africanus) and other Plio-Pleistocene hominids. S Afr J Sci. 1997;93:182–186. [Google Scholar]
- Spoor F, O’Higgins P, Dean C, Lieberman DE. Anterior sphenoid in modern humans. Nature. 1999;397:572. doi: 10.1038/17505. [DOI] [PubMed] [Google Scholar]
- Starck D, Kummer B. Zur Ontogenese des Schimpansenschädels (mit Bemerkungen zur Fetalisationshypothese) Anthr Anz. 1962;25:204–215. [Google Scholar]
- Tanner JM. Foetus into ManPhysical Growth from Conception to Naturity. Cambridge, MA: Harvard Univerity Press; 1978. [Google Scholar]
- Trinkaus E. The Neandertal face: evolutionary and functional perspectives on a recent hominid face. J Hum Evol. 1987;16:429–443. [Google Scholar]
- Trinkaus E. Neandertal faces were not long; modern human faces are short. Proc Natl Acad Sci USA. 2003;100:8142–8145. doi: 10.1073/pnas.1433023100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Dassow G, Munro E. Modularity in animal development and evolution: elements of a conceptual framework for evodevo. J Exp Zool. 1999;285:307–325. [PubMed] [Google Scholar]
- Wagner G. Homologues, natural kinds and the evolution of modularity. Am Zool. 1996;36:36–43. [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets HD, Fink WL. Geometric Morphometrics for Biologists. A Primer. San Diego: Elsevier Academic Press; 2004. [Google Scholar]