Abstract
Arguably, the gold standard of biological repair of articular cartilage lesions is autologous chondrocyte transplantation. Although the clinical outcomes appear to range between good and excellent in most cases, there are, nevertheless, both clinical and biological challenges that remain to improve rehabilitation and clinical outcome. One of the major biological problems relates to tissue integration of the reparative tissue into the host tissue at a predictable level. Often within a single lesion, varying degrees of integration can be observed from total integration through to non-integration as one passes through the defect. Here we briefly review some of the literature relating to this problem and include some of our own data drawn from questions we have posed about the biological nature of cartilage/cartilage integration. The nature and status of the tissue that comprises the wound lesion edge is central to tissue integration, and controlling aspects of trauma and free-radical-induced cell death together with matrix synthesis are identified as two components that require further investigation. Interestingly, there appears to be a limited ability of chondrocytes to be able to infiltrate existing cartilage matrices and even to occupy empty chondrocyte lacunae. Proliferation as a result of blunt and sharp trauma shows differential responses. As expected, blunt trauma induces a greater proliferative burst than sharp trauma and is more widespread from the lesion edge. However, in the case of sharp trauma, the basal cells enter proliferation before surface zone chondrocytes, which is not the case in blunt wounds. Regulation of these and associated processes will be necessary in order to devise strategies that can predict successful integration in biological repair procedures.
Keywords: cartilage, chondrocyte, osteoarthritis, repair, tissue integration, tissue
Introduction
The poor reparative potential of articular cartilage has led to many strategies, both scientific and surgical, to augment this potential and provide solutions to the treatment of localized defects that result from joint trauma or disease such as osteochondritis dissecans. The two main procedures that have evolved are osteochondral mosaicplasty and autologous chondrocyte implantation (ACI) (for a review see Hunziker, 2002). In the former case, osteochondral plugs are removed from areas of the joint that are least loaded and implanted into holes made in the subchondral plate. Because the plugs are slightly larger than the holes and are sunk into place, they are held firm until bone integration takes place. This technique is used for the treatment of both small cartilage defects and medium osteoarthritic lesions (Gross, 2003). ACI, by contrast, relies on the harvesting of cartilage around the periphery of the joint, releasing the resident chondrocytes, and expanding them in monolayer cultures, which are then transferred into the defect and held in place by periosteal or a collagenous flap sutured or adhered over the defect. Depending on the literature that one consults, both techniques provide good clinical outcomes. However, in the case of ACI, in which chondral defects alone may be treated, there is an added issue of integration between the new tissue that is formed from the implanted chondrocytes and the existing host tissue. Furthermore, there is a lack of blinded trials to evaluate the true efficacy of the treatments.
The question of whether two pieces of cartilage are able to fuse with each other was addressed many years ago in relation to the differing developmental origins of cartilage in terms of mesodermal and neural-crest-derived chondrocytes. Thus, when somtaopleurally derived mesenchyme of stage 18 (Hamburger & Hamilton, 1951) was mixed with stage 13 somatic mesoderm, the two populations segregated from each other, and this also occurred if the same populations were mixed both from stage 18 embryos (Zwilling, 1968). However, if stage 22 chick limb mesenchyme was mixed with 12-day mouse limb mesenchyme, no sorting took place, emphasizing the importance of differentiative status over species differences. More recently, Johnson et al. (2004) tested the ability of articular, costal and auricular chondrocytes embedded within a fibrin glue and held between two discs of articular cartilage to form an integrated matrix between them. They reported that all combinations formed similar matrices that had integrated with the articular cartilage discs. However, if similar experiments are carried out with the matrix intact and the data are analysed in terms of ability to fuse rather than segregate or sort, the result is quite different. Consequently, Chiakulus (1957) studied different cartilages from the larval salamander Ambystoma maculatum (spotted salamander) and after transecting them, transplanted them in apposition into the tail region. Such combinations contained humerus/humerus (homotypic, somatopleural) and femur/Meckel's cartilage (heterotypic, somatopleural/neural crest). He found that when the cartilage was from the same developmental origin (i.e. somatopleural or neural crest) the cartilages fused, but when the cartilages were from different origins they did not fuse (Fig. 1). As Hall (2005) pointed out, although Chiakulus explained his observations on heterogeneity of developmental origin, other explanations are also viable, such as the ability to hypertrophy and even on intrinsic matrix differences. Corroborating data were obtained by Fyfe & Hall (1979), who cultured chick tibial cartilage with Meckel's cartilage in opposition and again found that fusion occurred when two cartilages were from the same origin but not when the origins differed. It would be interesting to test whether species differences also existed in addition to those discussed above. Further insights could also be gained by testing whether a similar result is obtained if one of the cartilages was devitalized, thus indicating whether cell viability played a role in the fusion process.
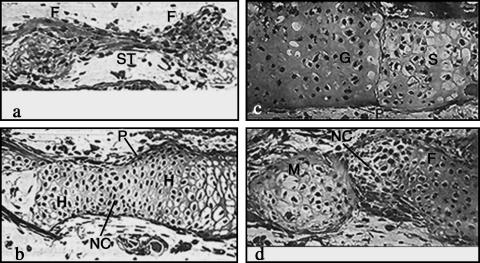
Fig. 1.
Frontal sections through the tail fin of Ambystoma maculatum showing implanted cartilage elements that had been transected and placed in opposition to judge fusion ability between cartilages of differing developmental origin. (a) Femur (F) and femur, and (b) humerus (H) and humerus. Note complete fusion with evidence of neocartilage (NC) comprising the area between the original transects in these homotypic implants. (P), perichondrium. (c) Gill bar (G) and scapula (S). (d) Meckel's cartilage (M) and femur (F) showing that in heterotypic implants (mesodermal and neural crest) no fusion takes place with cells occupying the boundaries between the opposed elements. (After Chiakulus, 1957.)
These data are useful because they suggest caution for applied tissue engineering with cartilage say from the nose to repair cartilage of the knee. It is certainly worth considering repeating some of these experiments with mammalian species, including humans.
Most recent studies on integrative repair in articular cartilage have focused both on structural and on biochemical aspects, with the former incorporating mechanical testing of the integration. It has been appreciated for some time that small proteoglycans are inhibitory to integration of tissue generated within chondral defects and that treatment of the defects with chondroitinase ABC promotes integration (Quinn & Hunziker, 2002). More recently, it has been reported that lubricin [surface zone proteoglycan (SZP); proteoglycan 4 (PRG-4)], which is present in synovial fluid, is also inhibitory to integration of cartilage explants and constructs in vitro yet hyaluronan, a molecule occupying a large amount of space, had no effect (Schaefer et al. 2004; Englert et al. 2005). Clearly, it seems the responses are not solely attributable to the stearic effects of the proteoglycans and there appears to be some unknown specificity.
There have been many studies in a variety of model systems both in vitro and in vivo where the strength of the integration has been measured by a variety of mechanical means. For example, in an equine model of ACI, the tensile modulus of the repair tissue (0.6 MPa) was much lower than that of intact controls (5.2 MPa). Furthermore, the integration strength to failure was less than half of that for intact controls (1.2 vs. 2.7 MPa for intact tissue) and that the addition of insulin-like growth factor (IGF) to the chondrocytes at implantation had no effects on these values (Gratz et al. 2006). In a model where natural (agarose, fibrin) and synthesized (polyglycolic acid) scaffolds were used as cell-seeded constructs and implanted into annular cartilage constructs that were cultured for up to 40 days, it was found that significant differences existed between the construct types. However, neither failure stress nor energy to failure correlated with biochemical content of the construct, and adhesion strength was always significantly lower than native tissue as might be expected. It has also been reported using another in vitro push-out model that pretreatment with low dosages of hyaluronidase and collagenase improved the integrative viability, matrix integrity and push-out strength (1.32 MPa) as compared with non-treated controls (0.84 MPa), thus lending support for the studies mentioned above (van de Breevaart Bravenboer et al. 2004). Clearly, there is still much work to be done if we wish to define the conditions that will ensure that any given reparative procedure can predict good tissue integration.
Against the above work, we have asked a number of questions regarding the nature of cartilage integration and have carried out a number of experiments addressing these issues at a fundamental level.
Understanding the cartilage wound margin is essential to integration
Implicitly, in terms of whether transplanted cells or a tissue construct will fuse functionally to the host tissue is dependent on the tissue interface and whether the host tissue is receptive to integration in terms of viability, matrix metabolism and content as discussed above? A matrix turning over slowly is likely to take a considerable time to fuse. A viable tissue may fuse with a devitalized matrix but in the absence of colonization of that matrix, it will ultimately fail through mechanical fatigue as a result of lack of turnover.
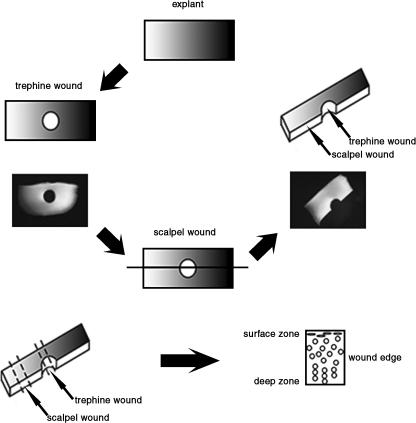
In order to investigate some of these aspects further, we devised an in vitro model system that used articular cartilage from both 7-day and 18-month bovine cartilage from the metcarpophalangeal joint. Briefly, a full-depth 1-cm2 (approx.) explant was removed by scalpel and following excision, explants were incubated at 37 °C in 5% CO2 for 24 h to equilibrate the tissue prior to wounding. The explants were wounded centrally with a trephine of 1.7 mm internal diameter, from the articular surface downwards removing a core of tissue. A scalpel cut bisecting the trephine wound was then made with a no. 23 scalpel blade to create a scalpel wound on the same explant (Fig. 2). Wounded explants and unwounded controls were incubated for up to 10 days with media changed every 48 h. Thus, the trephine wound was considered blunt and representative of many orthopaedic instruments used in joint procedures whilst the scalpel wound represented sharp incision. Cell viability in response to wounding was assessed using the live/dead cell kit (Molecular Probes, Cambridge, UK) and matrix synthesis was assessed both autoradiographically and quantitatively by scintillation counting after labelling with 3H-proline (collagen) and 35S (glycosaminoglycans) (Tew et al. 2000, 2001; Redman et al. 2004).
Fig. 2.
Diagrammatic representation of the trephine and scalpel wound created in articular cartilage full-depth explants. Serial cross-sections were taken for analysis through the scalpel wound then the trephine wound.
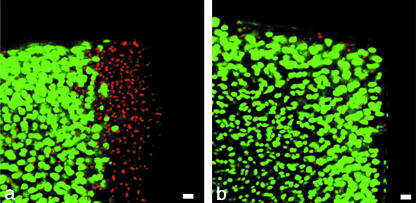
We found that in trephine wounds, there was a band of cell death of between 100 and 150 µm from the lesion edge into the tissue depth and that this band was wider in the surface zone as compared with deeper layers (Tew et al. 2000; Redman et al. 2004). Furthermore, previous work has shown this death to be the result of a combination of apoptosis and necrosis and that the apoptosis was progressive for up to 10 days (Tew et al. 2000). By contrast, in scalpel-made wounds, there was little evidence of cell death and, of that present, most was at the lesion edge (Fig. 3).
Fig. 3.
Live (green)/dead (red) labelling of cross-sections, showing the wound edge in the surface zone and mid zone of articular cartilage 2 days after wounding with a trephine (a) and scalpel (b). Scale bar, 20 µm.
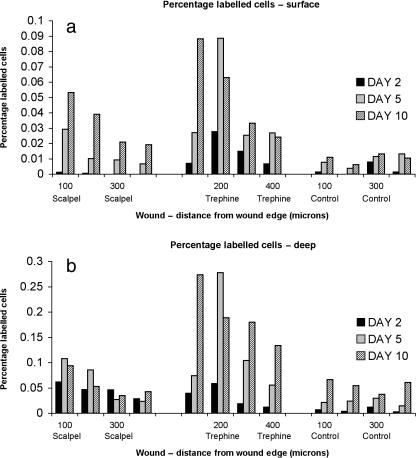
We then investigated the occurrence of proliferation as a result of the two differing traumas by thymidine incorporation. Wounded explants were pulse labelled overnight and harvested on days 2, 5 and 10. Autoradiographs were prepared and the labelled cells counted as a function of lacunae. The explanation for this method was that because of the progressive nature of the apoptosis, only late-stage apoptotic cells could be detected in the histological sections, and we were not able to detect those entering the apoptotic cycle that could not divide. Our negative controls comprised explants that did not contain a trephined or scalpel wound and we counted the relevant area within the centre of the explant. Figure 4 shows a histogram plot of the percentage of labelled cells in the uppermost and lowermost 400 µm of the tissue depth. In general, there was a greater degree of proliferation in the lower tissue depth compared with the upper. Additionally, with scalpel wounds, there was no proliferation detectable in the upper tissue depth at 2 days but this was detectable within the deep zone. This result is intriguing as it suggests that cells in the deep zone that are more differentiated than surface cells and may even be terminally differentiated can enter the cell cycle more rapidly than surface cells (or at least enter S-phase more rapidly). Hence, in scalpel-made wounds the response differs between surface and deep layers. By contrast, with trephine-made wounds, the pattern of response is very similar between the two regions. The data suggest that the degree of mechanical disruption to the matrix is a key regulator of proliferation and previous work by us has shown that biochemical extraction of cartilage explants with hyaluronidase had no effect on proliferation but that collagenase was a potent promoter of cells to enter the division cycle (Lee et al. 1994). More recently, Vincent et al. (2002) showed that trauma-induced proliferation of chondrocytes was due to the release of bFGF, presumably from the matrix that activated the extracellular regulated kinase pathway (ERK).
Fig. 4.
Histograms showing the mean (n = 36) percentage of 3H-thymidine-labelled cells 0–100 µm, 101–200 µm, 201–300 µm and 301–400 µm from the wound edge in scalpel-wounded, trephine-wounded and unwounded control tissue at days 2, 5 and 10 post-wounding within the surface zone (a) and deep zone (b) regions.
By contrast, there was much greater amount of label incorporation as a result of wounding with a trephine as compared with both scalpel-made wounds and controls. Again, the fraction of labelled cells was significantly higher in the lower tissue depth compared with upper depth significant differences determined by anova. However, it is clear that in the trephine-wounded cartilage, the greatest degree of label incorporation occurs behind the zone of cell death. Given that, as stated above, the apoptosis is progressive, analysis of 10-day sections shows that in trephine-wounded explants the few cells that have survived within the band of apoptosis/necrosis are either labelled from the pulse or exist as doublets within the lacunae (Fig. 5). Hence, it seems that any chondrocytes that do survive within this zone enter the cell cycle.
Fig. 5.
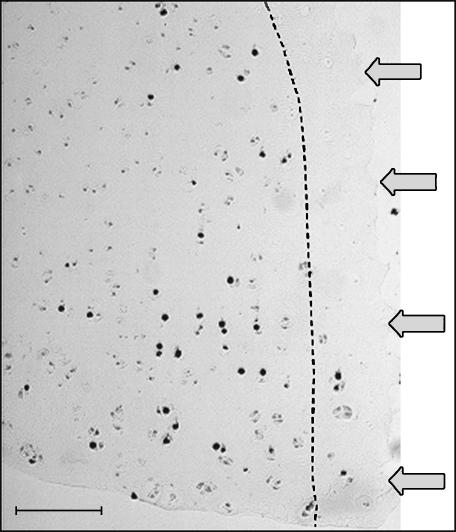
Section showing the deep zone of a trephine-wounded explant demonstrates incorporation of 3H-thymidine predominately behind the zone of cell death as represented by the dotted line. Image shows remaining viable cells within the zone of cell death either labelled with 3H-thymidine or existing as a doublet. Scale bar, 50 µm; arrows indicate wound edge, and the microautoradiograph was counterstained with haematoxylin and eosin.
In terms of matrix incorporation, both the proline and the sulphate showed similar results. In the case of trephine-made wounds, there was a band of matrix up-regulation behind the zone of cell death whereas with scalpel-made wounds, incorporation was enhanced up to the lesion edge (Fig. 6). The latter observation was confirmed by scintillation counting of incorporated label with both isotopes, which revealed that within the first 100 µm from the lesion edge, proline incorporation was enhanced by 100% and 150% by days 2 and 5, respectively, and sulphate by 150% and 300% over the same time period when compared with unwounded controls. These data are informative for two reasons. First, the up-regulation of matrix synthesis is not associated with the zone of cell death observed in trephine-wounded explants given that it is observed at the lesion edge in scalpel-wounded explants. Secondly, the latter effect can be considered beneficial in terms of integration with a reparative matrix or tissue construct as the high synthetic rate (and presumably turnover) would facilitate initial integration.
Fig. 6.
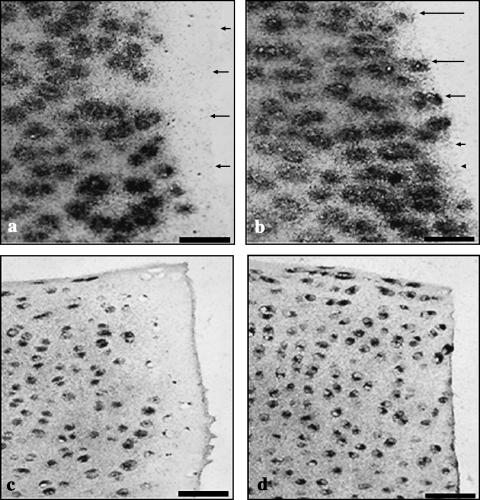
Sections showing incorporation of 35S-sulphate (a,b) and 3H-proline (c,d) by wounded explants 2 days after wounding. The trephine wound (a,c), demonstrates a lack of label incorporation adjacent to the wound edge. After wounding with a scalpel (b,d), however, label incorporation is observed adjacent to the wound edge. Scale bar, 50 µm; microautoradiographs counterstained with safranin O (a,b) or haematoxylin and eosin (c,d). Arrows indicate the lesion edge.
Can chondrocytes colonize a foreign matrix?
Clearly, in order to obtain functional integration, the extracellular matrix (ECM) must coalesce into a continuum. However, we have also posed whether a chondrocyte can invade and colonize an existing matrix. We carried out a number of combination experiments that involved placing freshly isolated chondrocytes and chondrocyte pellets in apposition. By devitalizing a 7-day-old chondrocyte pellet. By subjecting it to repeated freeze–thaw cycles, empty lacunae are visible and thus any nascent ECM is clearly detected by filling in of these spaces (Fig. 7b). We observed that chondrocytes were able to ‘fill in’ the lacunae of devitalized cartilage pellets resulting in a broad band of hypocelluar tissue reminiscent of the zone of acellularity that is present at wound edges and in osteoarthritic (OA) cartilage (Fig. 7c).
Fig. 7.
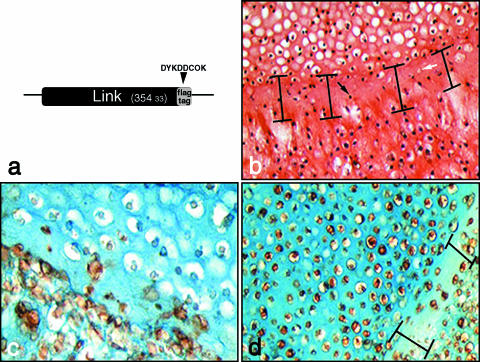
Tracking extracellular matrix deposition in fused cartilage using recombinant FLAG-tagged link protein marker. Link protein was cloned with an eight amino acid FLAG-tag linker sequence (a) in order to detect recombinant expression in transfected chondrocytes using anti-FLAG antibodies. Fusion of devitalized cartilage pellet (upper) and overlaid chondrocytes (lower) following 7 days in culture (b). A zone of hypocellularity (indicated) within which are dead cells from the devitalized pellet (black arrow) and filled lacunae (white arrow) are formed at the junction between pellet and overlaid chondrocytes. The same experiment conducted using chondrocytes transfected with recombinant FLAG-tagged link protein shows nascent matrix formation in empty lacunae but limited colonization of the devitalized matrix (c). A combination of live chondrocyte pellet overlaid with transfected chondrocytes expressing recombinant link protein (d) also produces a zone of hypocellularity (indicated).
To confirm that nascent matrix was secreted by the overlaid chondrocytes, we constructed a plasmid-expressing link protein tagged at the C-terminal end with an eight amino acid FLAG epitope for immunohistochemical disclosure (Fig. 7a, c, d). Anti-FLAG antibodies were able to identify tagged cells and also track the deposition of link protein in the matrix (Fig. 7c). Isolates of primary chondrocytes were grown in high-density pellet cultures (5 × 105 cells per pellet) for 7 days, subjected to repeated freeze–thaw cycles and then overlaid with a fresh isolate of primary chondrocytes that had been electroporated with pCMVlink-FLAG. Pellet composites were grown for a further 7 days then processed for histology and immunohistochemical analysis.
We observed that devitalized cartilage pellets were occasionally infiltrated by overlaid chondrocytes, and that recombinant link protein was deposited in nascent ECM (Fig. 7c), specifically in the empty lacunae. Interestingly, when the same experiment was conducted using ‘live’ pellets overlaid with transfected chondrocytes, a zone of hypocellularity was also present at the boundary between both pellets (Fig. 7d). In the ‘live’ pellet combination, the hypocellular boundary region was depleted of sulphated proteoglycan as it stained less readily with alcian blue. Another interesting feature of the ‘live’ pellet combination was that link protein was deposited in the matrix, particularly the pericellular matrix, of chondrocytes residing in the untransfected pellet. As the pellets are rapidily growing, the latter observation may be explained by freely circulating recombinant link protein integrating into nascent ECM.
These results indicate that formation of the zone of hypocellularity may not be related to cell death as the zone was observed with devitalized and live combinations of chondrocyte pellet and overlaid chondrocytes. This is an important finding because the zone of hypocellularity is the region where initial microfractures occur that eventually lead to the failure of integration between host and repair tissue. Although it appears that cellular infiltration of cartilage does occur, the zone of hypocellularity, which is paradoxically relatively depleted of proteoglycans, appears to be inhibitory to cellular colonization. Therefore, it will be vital to characterize the matrix in the hypocellular zone in order to understand why integration, which in one respect could be seen as a failure of cellular colonization, does not occur.
Osteoarthritis – can we reverse or repair the condition?
Most of what has been discussed above relates to ACI and the treatment of small localized defects. As indicated above, this is because of the rather small number of chondrocytes that can be generated from the available cartilage at the joint periphery and the limitations of expansion due to loss of phenotype. More recently, we have isolated a progenitor cell from the surface of immature and mature bovine cartilage (Dowthwaite et al. 2004) that unlike mature chondrocytes can be expanded in monolayer extensively (40+ population doublings) and maintains the ability to express the chondrogenic phenotype through maintenance of the cartilage transcription factor Sox-9. Thus, by utilizing this cell, it becomes possible to consider treating more extensive defects including OA defects. This possibility of course assumes that we can also address the cause of OA. Again, as with blunt trauma, a main characteristic of OA cartilage is extensive cell death occurring in early and late disease states. Hence, any reagents or factors that can reduce or limit the progressive cell death would have a major impact in the potential biological treatment of OA. Using another in vitro wound model, we investigated whether the proximity of differing chondrocyte subpopulations had any effects on cell death that resulted from trauma?
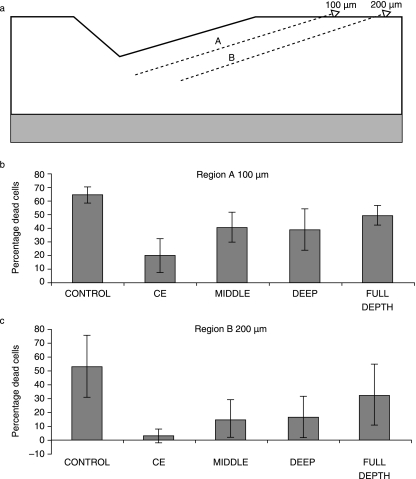
Using the immature bovine cartilage described previously, we used a specially manufactured gouge that could make reproducible chondral defects that were 800 µm wide, 400 µm long and 200 µm deep. We then isolated four chondrocyte subpopulations that were chondroprogenitor-enriched isolated by differential adhesion onto fibronectin (Dowthwaite et al. 2004), and chondrocytes isolated from the mid zone and deep zone of the cartilage together with chondrocytes isolated from the full tissue depth. Pellet cultures of each subpopulation were made in Eppendorf tubes at 250 000 cells in 500 µL medium comprising Dulbecco's modified Eagle's medium/F12 (1 : 1) supplemented with 10% fetal calf serum, ascorbate (50 µg mL−1) and gentamycin. Pellets were grown for 3 days prior to implantation into freshly made defects. After 7 days, explants were processed for routine histology and cell death analyses using ethidium homodimer. Cell death was analysed between the lesion edge and 100 µm depth and between 100 and 200 µm depth.
Compared with the control lesions that did not contain added cells, individual subpopulations all had an effect in reducing cell death to a significant degree (Figs 8 and 9), whereas full-depth populations had no significant effect (Fig. 8). When chondroprogenitor, mid- and deep-zone subpopulations were compared, we found that the chondroprogenitor-enriched subpopulations had the greatest effect in reducing cell death (to approximately 10%), with the mid-zone cells next and the deep zone cells having the least significant effect (Fig. 9). We do not know what factors are responsible for these effects, but placing control explants in medium conditioned by these subpopulations had no effect on cell death. However, given that there can be little or no cell contact between the cells in the matrix and the pelleted subpopulations suggests that the effect is via diffusible molecules even if they are intermediary in nature.
Fig. 8.
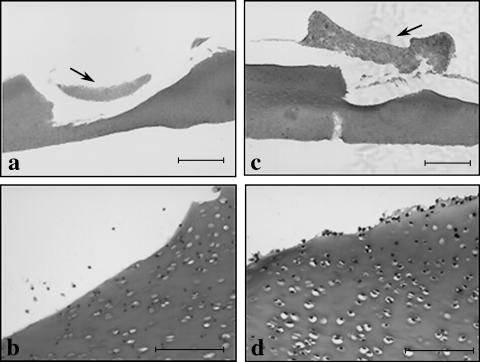
A representative wounded full-depth explant transplanted with full-depth (FD) derived pellet (arrowed) and stained with safranin O (a). (b) Apoptotic bodies were present around the wound margin and deep in the wound. The FD pellet contained a mixture of both small rounded cells and large vacuolated cells and stained strongly for glycosaminoglycans (GAGs). (c) Wounded representative full-depth explant transplanted with chondroprogenitor-enriched (CE) derived pellet (arrowed) and stained with safranin O. (d) Apoptotic bodies were present around the wound margin and deep into the wound. Note: during histological processing, the pellets became detached from the defect although clusters of chondroprogenitor-enriched cells are observed adhering to the host matrix. Scale bars (a,c) 500 µm; (b,d) 100 µm.
Fig. 9.
Histograms representing cell death between the surface of the lesion and 100 µm of tissue depth and 100–200 µm tissue depth in response to transplanted full-depth chondrocytes and enriched chondroprogenitor cells.
There is growing evidence implicating oxidative stress in articular cartilage degeneration. Activated macrophages and neutrophils produce high levels of reactive oxygen species during inflammatory episodes in the synovial joint; however, chondrocytes are themselves capable of generating a significant oxidative respiratory burst. Studies have shown that cytokine-stimulated chondrocytes release greater amounts of hydrogen peroxide than pulmonary alveolar macrophages, a well-characterized macrophage cell type (Tiku et al. 1990). Chondrocytes synthesize all the components of the NADPH oxidase complex, and stimulation with calcium ionophore ionomycin results in the release of superoxide ions in a concentration-dependent manner (Hiran et al. 1997). The toxic superoxide radical typically has a half-life of milliseconds and is rapidly dismutated by superoxide dismutase (SOD) to hydrogen peroxide. Hydrogen peroxide has a longer half-life (seconds) and can diffuse rapidly out of cells. Catalase is the second defence against the toxic intermediates of oxygen acting by dismuting hydrogen peroxide to molecular oxygen and water. Superoxide ions and hydrogen peroxide both appear to be required for cartilage homeostasis (Henrotin et al. 2005) and are co-factors in many signalling pathways; for example, they differentially mediate the actions of the catabolic cytokine IL-1b (Mendes et al. 2003). If the production rate of the superoxide radical under stress exceeds SOD activity, then oxidative damage results. This damage may take the form of direct molecular degradation of ECM components, disruption of redox-sensitive intracellular signalling networks, or through inducing necrosis or apoptosis of chondrocytes (Henrotin et al. 2005). There is indirect evidence to suggest that mechanical stress of articular cartilage could potentiate an oxidative burst from activated or dying chondrocytes (Kurz et al. 2004). Therefore, oxidative stress at the point of injurious compression could significantly affect cell viability and the integrity of the remaining ECM.
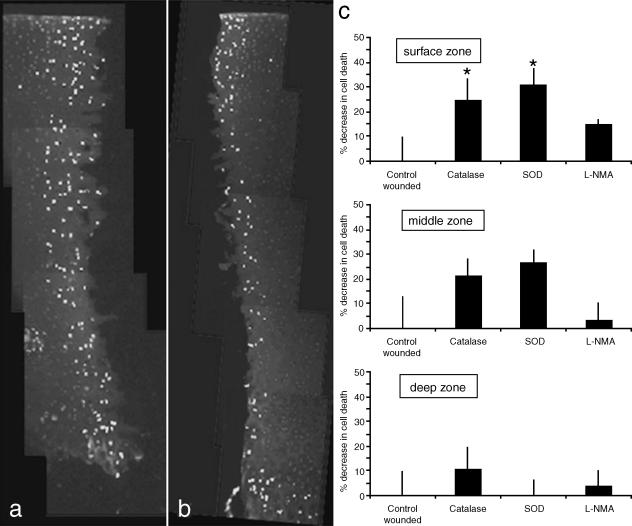
In order to test this hypothesis we experimentally wounded immature bovine articular cartilage using a trephine (Tew et al. 2000) in the presence or absence of SOD, catalase and NG-monomethyl-l-arginine (L-NMA), a non-specific inhibitor of nitric oxide synthase (Fig. 10). Cell death was quantified by counting ethidium homodimer-positive cells and total cell number in a 200-µm2 area immediately adjacent to the wound margin in the surface, middle and deep zones following 7 days of incubation in serum-containing medium. We observed that cell death was significantly decreased in the surface zone of explants that were pre-incubated and maintained in SOD- (31.2 ± 6.6%, n = 8, P < 0.05, decrease compared with control) or catalase- (24.6 ± 8.6%, P < 0.05, decrease) containing medium. Although a similar magnitude of reduction of cell death was observed in the middle zone of injured articular cartilage pre-incubated with either SOD or catalase, the greater variation in cell death in control cartilage was probably due to the combination of the nature of blunt trauma (as opposed to using a sharp scalpel) and human operation (in order to simulate clinical situations), meant the results were not significant. No significant difference in cell death was detected in the deeper zones following treatment, or in any zone with explants that had been pre-incubated with L-NMA. Antioxidant enzymes SOD and catalase clearly reduce the impact of injurious compression, and this involves rescuing chondrocytes from cell death at the wound edge.
Fig. 10.
Antioxidant enzymes reduce cell death in the surface zone of experimentally wounded immature articular cartilage. Explants were untreated or pre-incubated for 30 min with either SOD 100 U mL−1, catalase 400 U mL−1 or L-NMA 1 mm. Explants were wounded using a 3-mm trephine and then cultured for a further week in serum-containing medium. (a,b) Composite images showing tissue sections labelled with ethidium homodimer from untreated and SOD-treated explants, respectively. (c) Graphs of percentage reductions in cell death in the various zones of articular cartilage of explants treated with SOD, catalase and L-NMA compared with untreated controls. *P < 0.05. (Optimum enzyme concentrations were determined empirically.)
Cell death at the wound edge is progressive, and it is thought that proteolytic degradation of type II collagen fibrils at the wound margin as a result of the original insult and/or as a consequence of reparative remodelling of the tissue may initiate apoptosis (Tew et al. 2000). Lo & Kim (2004) have shown that chondrocyte apoptosis is induced by collagenase treatment of monolayer cultured primary chondrocytes in both a time- and dose-dependent manner Furthermore, incubation with a non-specific caspase inhibitor, Z-VAD, or IGF-1 inhibited chondrocyte apoptosis induced by collagenase treatment. Collagenases preferentially cleave type II collagen between Gly794 and Leu795, generating two fragments that are one-quarter and three-quarters the size of the original. Following cleavage, the collagen triple helix unwinds, providing a denatured substrate that can be subjected to further proteolytic degradation. The monoclonal antibody Col2-3/4m detects an epitope in the three-quarters fragment in the unwound denatured collagen helix and is a good marker of collagen degradation (Hollander et al. 1994).
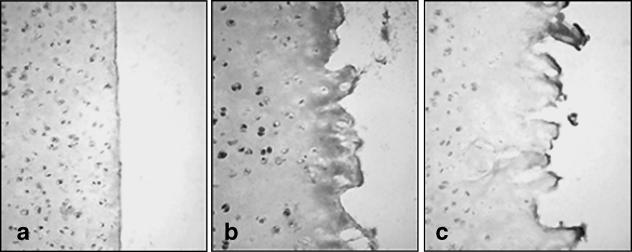
Immature cartilage sliced with a scalpel displays little perturbation of collagen structure beyond the immediate wound edge when screened with Col2-3/4m (Fig. 11). Some cellular labelling is present probably due to antibody binding nascent collagen prior to forming mature fibrils. In complete contrast, in trephine-wounded cartilage there is widespread labelling throughout the ECM from the wound edge and further into the tissue. We observed intense cellular staining in chondrocytes behind the zone of cell death, where labelling represents either increased synthetic activity to replace damaged ECM or proteolytic degradation as part of a remodelling phase of the tissue. Therefore, apoptosis resulting from reduced attachment to the surrounding ECM may be a natural consequence of tissue remodelling. Labelling with Col2-3/4m is present only at the immediate wound margin in SOD-pretreated explants. Labelling of cells and ECM was similar to that as observed in scalpel-cut cartilage. Therefore, SOD directly or indirectly interferes with corruption of the collagen fibril architecture and, thus, attenuation of proteolytic degradation may also be associated with a reduction in cell death at the wound margin.
Fig. 11.
Collagen denaturation is attenuated in antioxidant-treated explant cultures. (a) Scalpel-cut, (b) trephine-wounded and (c) trephine-wounded in the presence of SOD 100 U mL−1. Note low levels of label in both scalpel- and trephine-wounded explants with SOD. Explants were incubated with 1 mg mL−1 hyaluronidase to expose collagen epitopes for 1 h at 37 °C. Epitopes were reacted with Coll2-3/4m (0.5 µg mL−1) overnight at 4 °C and visualized using anti-mouse HRP-conjugated secondary antibodies and 3′3-diaminobenzidine peroxidase substrate.
In summary, we have addressed some of the issues surrounding the challenge of enhancing tissue integration in cartilage repair procedures. In terms of cartilage wound management, there is a need for bespoke instruments to ensure precision debridement resulting in the least possible matrix damage at the lesion edge. It is also clear that the presence of cells at the lesion edge can have marked effects on cell viability within the immediate tissue and that cartilage progenitors appear to have maximal effect in reducing cell death. When considering the potential to repair OA cartilage, we need to consider the significant role of the generation of free radicals as a major contributor to both cell death and matrix damage. Lastly, there are still many issues that need to be resolved before we will be in a position to have defined procedures to predict successful integration in repair procedures, be they cell based or construct based, that may possess an existing matrix.
Acknowledgments
We are grateful to the EPSRC and Smith & Nephew for financial support.
References
- van de Breevaart Bravenboer J, In der Maur CD, Bos PK, Feenstra L. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther. 2004;6:R469–R476. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiakulus JJ. The specificity and differential fusion of cartilage derived mesoderm and mesectoderm. J Exp Zool. 1957;136:287–300. doi: 10.1002/jez.1401360206. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Englert C, McGowan KB, Klein TJ, Giurea A, Schumacher BL, Sah RL. Inhibition of integrative cartilage repair by protoglycan 4 in synovial fluid. Arthritis Rheum. 2005;52:1091–1099. doi: 10.1002/art.20986. [DOI] [PubMed] [Google Scholar]
- Fyfe DM, Hall BK. Lack of association between avian cartilages of different embryological origins when maintained in vitro. Am J Anat. 1979;154:485–496. doi: 10.1002/aja.1001540404. [DOI] [PubMed] [Google Scholar]
- Gratz KR, Wong VW, Chen AC, Fortier LA, Nixon AJ, Sah RL. Biomechanical assessment of tissue retrieved after in vivo cartilage defect repair: tensile modlus of repair tissue and integration with host cartilage. J Biomechanics. 2006;39:138–146. doi: 10.1016/j.jbiomech.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Gross AE. Cartilage resurfacing: filling defects. J Arthroplasty. 2003;18:14–27. doi: 10.1054/arth.2003.50084. [DOI] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage. Amsterdam: Elsevier; 2005. [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hiran TS, Moulton PJ, Hancock JT. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic Biol Med. 1997;23:736–743. doi: 10.1016/s0891-5849(97)00054-3. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Heathfield TF, Webber C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- Johnson TS, Xu JW, Zaporojan VV, et al. Integrative repair of cartilage with articular and nonarticular chondrocytes. Tissue Engineering. 2004;10:9–10. doi: 10.1089/ten.2004.10.1308. [DOI] [PubMed] [Google Scholar]
- Kurz B, Lemke A, Kehn M, et al. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50:123–130. doi: 10.1002/art.11438. [DOI] [PubMed] [Google Scholar]
- Lee D, Bentley G, Archer CW. Proteoglycan depletion alone is not sufficient to stimulate proteoglycan synthesis in cultured bovine cartilage explants. Osteoarthritis Cartilage. 1994;2:175–185. doi: 10.1016/s1063-4584(05)80067-6. [DOI] [PubMed] [Google Scholar]
- Lo MY, Kim HT. Chondrocyte apoptosis induced by hydrogen peroxide requires caspase activation but not mitochondrial pore transition. J Orthop Res. 2004;22:1120–1125. doi: 10.1016/j.orthres.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Mendes AF, Caramona MM, Carvalho AP, Lopes MC. Differential roles of hydrogen peroxide and superoxide in mediating IL-1 induced NF-kappa B activation and iNOS expression in bovine articular chondrocytes. J Cell Biochem. 2003;88:783–793. doi: 10.1002/jcb.10428. [DOI] [PubMed] [Google Scholar]
- Quinn TM, Hunziker EB. Controlled enzymatic matrix degradation for integrative cartilage repair: effects on viable cell density and proteoglycan depostion. Tissue Engineering. 2002;8:799–806. doi: 10.1089/10763270260424150. [DOI] [PubMed] [Google Scholar]
- Redman SN, Dowthwaite GP, Thomson BM, Archer CW. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthritis Cartilage. 2004;12:106–116. doi: 10.1016/j.joca.2002.12.001. [DOI] [PubMed] [Google Scholar]
- Schaefer DB, Wendt D, Moretti M, et al. Lubricin reduces cartilage–cartilage integration. Biorheology. 2004;41:503–508. [PubMed] [Google Scholar]
- Tew S, Kwan APL, Hann A, Thomson B, Archer CW. The reactions of articular cartilage to experimental wounding. Arthritis Rheumatism. 2000;43:215–225. doi: 10.1002/1529-0131(200001)43:1<215::AID-ANR26>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Tew S, Redman S, Walker E, et al. Differences in repair responses between immature and mature cartilage. Clin Orthopaedics Related Res. 2001;391S:142–152. doi: 10.1097/00003086-200110001-00014. [DOI] [PubMed] [Google Scholar]
- Tiku ML, Liesch JB, Robertson FM. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J Immunol. 1990;145:690–696. [PubMed] [Google Scholar]
- Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci USA. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling E. Morphogenetic phases in development. Devel Biol. 1968;2:S184–S207. [Google Scholar]