Abstract
Teeth develop from a series of reciprocal interactions that take place between epithelium and mesenchyme during development of the mouth that begin early in mammalian embryogenesis. The molecular control of key processes in tooth development such as initiation, morphogenesis and cytodifferentiation are being increasingly better understood, to the point where this information can be used as the basis for approaches to produce biological replacement teeth (BioTeeth). This review outlines the current approaches, ideas and progress towards the production of BioTeeth that could form an alternative method for replacing lost or damaged teeth.
Keywords: BioTeeth, teeth, tooth replacement
Why teeth?
Despite major advances in surgical techniques and more effective immune suppressive drugs, the number of organ transplants (heart, lung and kidney) per year has remained static (or in the case of heart, decreased) in the UK for the last 10 years (NHS statistics). Shortage of suitable organ donors is the major reason that more transplants are not being carried out. The use of xenografts continues to be explored, but issues of safety mean that it is doubtful that xenografts will be a practical and viable alternative to human organs. The possibility that human organs might be made de novo in the laboratory and then used for transplantation is obviously a very attractive idea. Moreover, if such organs could be made from a patient's own cells (autologous), this would revolutionize organ transplantation as lifetime immune-suppression would not be necessary and organ replacement would thus become a routine surgical procedure. Regardless of whether autologous or allogeneic cells are used, the concept of laboratory-constructed (engineered) organs has immense appeal.
There are two obvious major challenges that need to be overcome. Methods have to be developed to reproduce the highly complex specialized arrangements of differentiated cells that constitute an organ. This is a huge challenge in itself but even if it were possible today to construct such organs the second challenge is perhaps even more daunting, namely testing these laboratory-constructed organs in patients. The very reason most research is concentrated on major internal organs is that they are essential for life and thus testing engineered organs on patients is going to be a difficult process. This is confounded by the fact that the patients themselves are often seriously ill and by their very nature, major organs require major surgery. The future benefits of laboratory-engineered organs for transplantation are exceptional, but getting there is going to be a long and torturous process. An alternative pathway to follow is to work on organs that are not essential for life. The laboratory engineering processes are likely to involve similar approaches for most organs. By developing these techniques for organs where there is a clinical need for transplantation, yet where the organs themselves are non-essential, the concept of using laboratory-engineered organs for transplants can be established.
Teeth are an ectodermal organ and as such, in common with other ectodermal organs such as hair, skin, sweat glands and salivary glands, they are located close to the extremity of the body. These organs develop in the embryo via interactions between the ectoderm and the underlying mesenchyme. Teeth are thus easily accessible organs that can be visualized in the mouth. Their removal and replacement does not involve major surgery and most significantly teeth are not essential for life. This means that not only is surgery carried out on healthy patients, but should anything go wrong with the transplant, the patient is unlikely to die and moreover, because of ease of accessibility, the transplant could straightforwardly be replaced. Despite not needing them to survive, we place an incredible amount of importance upon our teeth. In the western world, an estimated 85% of adults have had some dental treatment. Seven per cent have lost one or more teeth by the age of 17. After the age of 50, an average of 12 teeth stand to have been lost (Sharpe & Young, 2005). This means that there is a huge patient base that is a significant drain on healthcare resources. Teeth thus have important advantages for providing a test case for proof of concept of organ engineering. By carrying out research on laboratory engineering of an organ such as teeth, in which the final stage of clinical trials will be simple, the whole field of organ engineering can benefit in the long term.
Do we need a biological method of teeth replacement?
Replacement of missing or damaged teeth currently involves fixed or removable prostheses or dental implants. The use of dental implants is the most rapidly growing area of dentistry, currently increasing by 15–20% per year. Dental implants involve drilling a hole into the jawbone into which a titanium rod is screwed that is capped by a plastic or ceramic ‘tooth’ crown. In general, implants work well and are most successful when there is sufficient bone into which the titanium rod can integrate. Implants are more difficult and less successful when tooth loss is accompanied by bone loss. Despite being the current ‘state-of-the-art’ in tooth replacement, the technology on which implants are based has been around for thousands of years. Ancient Egyptians, the Mayans and the Romans all practised forms of dental implantation using a variety of materials including shell and iron (Crubez et al. 1998; Westbroek & Martin, 1998) (Fig. 1).
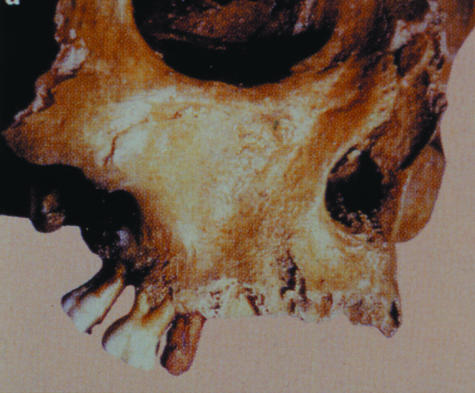
Fig. 1.
The skull of an ancient Roman excavated in London that has an iron dental implant hammered into the jawbone. The ability to replace a missing tooth with a biological replacement (BioTooth) produced by organ engineering is an attractive alternative to having a metal rod screwed into the jaw. In a recent survey carried out by us, 70% of Implantologists and Dental Surgeons indicated they would use a BioTooth instead of an implant if one were available (Odontis Ltd).
Teeth are not just one organ
Having extolled the advantages of teeth as an organ engineering model, there is one disadvantage that needs to be considered: teeth are different shapes. The human dentition is composed of three basic tooth shapes, incisors, canines and multicuspids (molars, premolars). However, each tooth in the upper and lower jaw quadrants is different, making a possible eight different shapes per jaw (ignoring the left–right differences in shape). This means that teeth cannot be considered to be a single organ, but somewhere between three and 16 organs and thus, in order even to begin to think about engineering a BioTooth, the regulation of tooth shape must be understood.
Tooth shape is determined very early in development by expression of different genes in different regions of the mesenchyme of the jaw primordia. These genes, principally homeobox genes, act to provide cells with positional information that directs the cells that participate in tooth development down specific pathways of morphogenesis (Tucker & Sharpe, 2004). The best example of this involves the Barx1 homeobox gene, which is expressed in mesenchymal cells of the jaw primordial, from where molar teeth will develop. Misexpression of Barx1 in the mesenchyme from where incisors will develop results in molar-shaped teeth developing from incisor primordia (Miletich et al. 2005). The ectopic expression of Barx1 in the incisor mesenchyme has redirected the cells to follow a molar pathway of morphogenesis. These events occur before overt tooth development has begun and thus early tooth primordia (from the bud stage in Fig. 2) already know what shape they are to become.
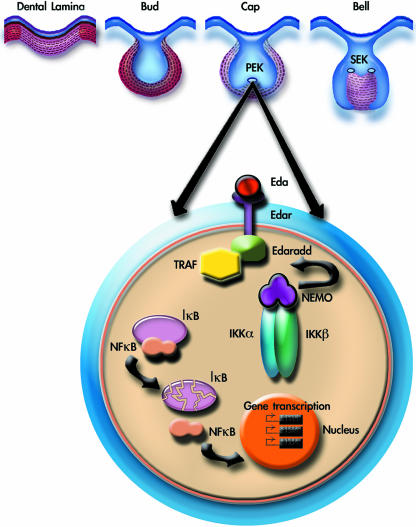
Fig. 2.
Diagrammatic representation of the NF-κB pathway stimulated by Eda in primary enamel knot (PEK) during tooth development. PEK, primary enamel knot; SEK, secondary enamel knot; EDA, ectodysplasin; EDAR, ectodysplasin receptor; EDARADD, ectodysplasin-associated death domain; TRAFs, tumour necrosis factor receptor-associated (Frs); NFαB, nuclear factor kappa (B); IαB, inhibitor of kappa B; NEMO, nuclear factor kappa B esential modifier; IKKα, inhibitor of kappa kinase alpha; IKKβ, inhibitor of kappa kinase beta.
Tooth development
Teeth are ectodermal organs that develop from reciprocal interactions between oral ectoderm (epithelium) and neural crest-derived mesenchyme. Signals from the oral epithelium act to initiate tooth development and control gene expression in the mesenchyme (Thesleff et al. 1995; Thesleff & Sharpe, 1997; Tucker et al. 1998; Thesleff, 2003). Spatial distributions of mesenchymal gene expression provide ‘positional information’ to direct the pathways of tooth morphogenesis. Homeobox genes such as Barx1, for example, the spatially restricted expression of which in presumptive molar mesenchyme is regulated by antagonistic interactions between FGF8 and BMP4, is necessary and sufficient for molar tooth morphogenesis (Tucker et al. 2004). Homeobox genes such as Barx1 act upon mesenchymal cells prior to tooth initiation and this positional information must thus be interpreted by the cells and used to direct epithelial folding to produce tooth shape. The enamel knot is a transient epithelial signalling centre observed at the cap stage of tooth development that is involved in regulating crown morphogenesis.
A specific challenge of producing a BioTooth based on understanding development is to be able to control tooth crown shape, and an understanding of the role of genes such as Barx1 and the nuclear factor κB (NF-κB) pathway is thus particularly important.
The mechanisms that the cells use to interpret this information generated by homeobox proteins such as Barx1 are not fully understood, but evidence has emerged that the NF-κB pathway plays an important role. Patients with forms of hypohydrotic ectodermal dysplasia have absent or abnormal ectodermal organs, including misshapen and missing teeth. Mutations in several different genes in these patients have been identified and shown in mouse models to participate in the activation of the NF-κB pathway. EDA (ectodysplasin) is a member of the tumour necrosis factor (TNF)-family of signalling proteins that binds to receptors (EDAR) and transmits the signal into the cytoplasm via an adaptor protein (EDARADD) and various TRAFs (tumour necrosis factor receptor-associated factors), including TRAF6 (Naito et al. 2002; reviewed by Ohazama & Sharpe, 2004; Courtney et al. 2005). The outcome is the activation of the IKK complex that phosphorylates IκB, which dissociates itself from NF-κB allowing NF-κB to enter the nucleus and regulate gene transcription. Loss-of-function mutations in EDA, EDAR and EDARADD have been identified in patients with ectodermal dysplasia and in spontaneous mouse mutants (Tabby, Downless and Crinkled) that show features of ectodermal dysplasia (Ferguson et al. 1997; Headon & Overbeek, 1999; Monreal et al. 1999; Headon et al. 2001). Mice with loss of function mutations in Ikkα, Traf6 and NF-κB all have molar teeth with abnormal cusps similar to Tabby. Further evidence that this pathway has a key role in tooth morphogenesis comes from transgenic mice that express a ligand-independent activated form of Edar that results in overactivation of the pathway and leads to molar teeth with extra cusps. The level of activation of the NF-κB pathway thus appears to regulate cusp patterns in molariform teeth and this pathway is likely therefore to be downstream of Barx1. In terms of understanding the control of tooth shape for organ engineering purposes, the presence or absence of Barx1 expression is diagnostic of the distinction between multicuspid and monocuspid tooth shape. The cusp patterns themselves found on different teeth, premolar, molar, etc., can potentially be modified by regulating the level of NF-κB activation during development. Several small-molecule modulators of NF-κB signalling are available (Brennan & O'Neil, 1996).
How to make a BioTooth
There are basically four ways to make a Biotooth: try and reconstruct the mature tooth as it appears in the mouth; try and reproduce the embryonic development of a tooth in the mouth; try and ‘induce’ a third dentition; and create a matrix (scaffold) in the shape of a tooth, throw in some cells and hope for the best. All four of these approaches have been suggested and two are currently being tried (Fig. 3).
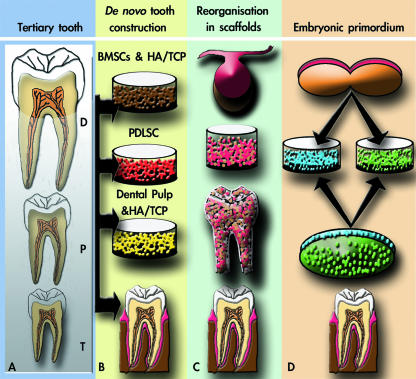
Fig. 3.
Schematic summary representation of four different possible approaches to tissue engineering teeth. (A) Stimulation of third dentition (tertiary tooth) (Otto et al. 1997). (B) Construction of an adult tooth de novo (Robey, 2005). (C) Seeding of dissociated third molar tooth bud cells into tooth-shaped scaffolds (Young et al. 2002; Duailibi et al. 2004). (D) Generation of a tooth primordium from cultured stem cells (Ohazama et al. 2004). T: tertiary; P: primary; D, deciduous.
The idea that a third dentition might be somehow locally induced to replace missing teeth has been around for some time, and is obviously an attractive concept (Fig. 3A). This approach is generally presented in terms of adding molecules to induce de novo tooth initiation in the adult mouth following permanent tooth loss. Such molecules might be those that are involved in embryonic tooth induction or in successional tooth formation. The identification of mutations in RUNX2 causing cleidocranial dysplasia, in which patients have a third set of teeth, has attracted attention as a possible route towards creating BioTeeth (Otto et al. 1997). The idea that de novo activation of genes such as RUNX2 might be used to induce new tooth formation in the adult mouth does, however, pose obvious dangers as RUNX2 plays a key role in other cellular processes, including bone formation. Moreover, one additional problem with this type of approach is that the cells from which teeth develop are not present in the adult jaw, and thus there is ‘nothing’ for any inductive molecules to act upon.
The idea that a complete adult tooth might be constructed has recently been proposed by Pamela Robey and colleagues (e.g. Robey, 2005) (Fig. 3B). In this approach the constituent parts of a tooth are proposed to be made individually, a process comparable with constructing a building. Because teeth require anchorage, bone marrow stromal cells (BMSCs) and hydroxyapaptite/tricalcium phosphate (HA/TCP) could be used to engineer the alveolar bone. The dental pulp and enamel could be constructed using dental pulp cells and HA/TCP in an enamel-like crown mould, whilst the periodontal ligament attaching the tooth to bone could be obtained from periodontal ligament stem cells (PDLSCs). Despite a very high level of technical difficulty, this approach has the attraction that here should be a high level of control in the construction and the process might lend itself to automation and scale-up.
One of the most successful techniques for tissue engineering of simple tissues is the use of biodegradable scaffolds into which cells are seeded and adopt the shape of the scaffold. The well-documented ‘ear on the back of a mouse’ experiment carried out in 1997 by Vacanti and co-workers (Cao et al. 1997) is a vivid (if impractical) demonstration of the use of scaffolds. Today scaffolds are used in a variety of contexts and the idea that they might be used to construct complex organs, including teeth, has been investigated. The pioneering work of Shirley Glasstone-Hughes (1952) demonstrated that early-stage embryonic tooth primordia can be split in two and each half can generate a complete normal size tooth. This established that early-stage tooth primordia have an inherent level of plasticity and regenerative capacity. This regenerative capacity was utilized in experiments by Young et al. (2002) who, in collaboration with Vacanti, made scaffolds in the shape of different teeth and seeded these with cells dissociated from early-stage third molar tooth germs from pigs and rats (Fig. 3C). The seeded scaffolds were grown in the omentum of immune compromised rats, and histological analysis revealed the formation of tiny (1–2 mm) tooth crowns 20–30 weeks after in vivo implantation using porcine tooth buds (Young et al. 2002). The rat molar tooth germ, however, developed just after 12 weeks of in vivo implantation (Duailibi et al. 2004). The interpretation placed on these results at the time was that they demonstrated the existence of stem cells in tooth primordia that are able to regenerate teeth. A simpler and more likely explanation, however, is that small numbers of dissociated dental epithelial and mesenchymal cells recombined in the scaffold and initiated tooth formation in a process suggested by the Glasstone-Hughes experiments. This explanation is further supported by the fact that the teeth produced were tiny and thus probably formed from small groups of epithelial and mesenchymal cells reaggregating from the dissociated population. Regardless of the mechanism, the real question is: does this approach have any potential as a method of producing human BioTeeth? Its main attraction is that the control of tooth shape is in theory very simple, by making a scaffold in the shape of the required tooth. The problem, however, is that the mini-teeth that developed did not adopt the shape of the scaffold. An additional but important aspect of this approach is that bone was not formed in the process. A BioTooth process must involve the formation of new bone into which the tooth can attach and develop its roots. These experiments have, however, confirmed the remarkable ability of early dental cells to reorganize themselves and this itself may have potential uses in BioTooth production.
The final approach to consider is the one pursued in our laboratory that aims to reproduce, in the adult mouth, tooth development as it occurs in the embryo. This approach is based on a simple premise, that because complex organs are produced in the embryo, in order to produce them in vitro, you must understand their embryonic development (Fig. 3D).
Production of BioTeeth using stem cells
The starting point for tooth formation is the first interactions between oral epithelium and neural crest-derived mesenchyme cells in the jaw primordia. At this time (embryonic day 9.5 in mice and 6 weeks in humans) the mesenchyme cells are undifferentiated and can respond in different ways to the epithelial signals. We have reasoned that this initial, simple stage is the one to try and reproduce in vitro from cultured cells. The dental epithelial cells form the ameloblasts of the tooth whereas the mesenchyme cells form all the other functional cell types, including odontoblasts, cementoblasts, pulp cells and periodontal ligament. The first challenge therefore is to identify cell populations that can replace neural crest-derived embryonic mesenchyme and interact with oral epithelium to form these mesenchymal cell types of the tooth. A feature of neural crest cells is their stem cell-like multipotentiality and thus at the time when they receive the first signals from the oral epithelium they are uncommitted and able to follow different pathways of differentiation. We therefore investigated the capacity of other stem cell populations to replace these cells in tooth formation.
Mouse embryonic stem (ES) cells, fetal neural stem cells and adult bone marrow stromal cells were cultured and aggregated to form a semisolid mass. The aggregates were combined with embryonic oral epithelium and cultured for 3 days. The initiation of tooth development was assayed histologically and with molecular markers, and in all three cases, genes were expressed in the stem cell-derived mesenchyme that were indicative of early odontogenesis (Ohazama et al. 2004). Having established that these stem cell-derived mesenchyme cells could respond to epithelial signals and begin tooth formation, the explants were allowed to develop further by transferring them to renal capsules of adult mice for 10 days. Under these conditions the explants from bone marrow stromal cells formed complete tooth crowns of the same approximate size as mouse molars and contained all the same cell types as normal teeth. Bone marrow stromal cells thus have the ability to respond to the appropriate signals from embryonic oral epithelium and participate in tooth development. Ongoing research is directed towards finding a cell source to replace embryonic oral epithelium in order to produce a BioTooth entirely from cultured cells.
Acknowledgments
We would like to thank Atsushi Ohazama for critically reading the manuscript. Work in the authors’ laboratory is funded by the MRC and Wellcome Trust.
References
- Brennan P, O'Neil LAJ. 2-Mercaptoethanol restores the ability of nuclear factor κB (NFκB) to bind DNA in nuclear extracts from interleukin 1-treated cells incubated with pyrollidine dithiocarbomate (PDTC) Biochem J. 1996;320:975–981. doi: 10.1042/bj3200975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Vacanti JP, Paige KT, et al. Transplantation of chondrocytes utilising a polymer–cell construct to produce tissue engineered cartilage in the shape of a human ear. Plast Recontr Surg. 1997;100:297–302. doi: 10.1097/00006534-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Courtney J-M, Blackburn J, Sharpe PT. The ectodysplasin and NF-κB signalling pathways in odontogenesis. Arch Oral Biol. 2005;50:159–163. doi: 10.1016/j.archoralbio.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Crubez E, Murail P, Girard L, et al. False teeth of the roman world. Nature. 1998;391:29. doi: 10.1038/34067. [DOI] [PubMed] [Google Scholar]
- Duailibi MT, Duailibi SE, Young CS, et al. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- Ferguson BM, Brockdorff N, Formstone E, et al. Cloning of Tabby, the murine homolog of the human EDA gene: evidence for a membrane-associated protein with a short collagenous domain. Human Mol Genet. 1997;6:1589–1594. doi: 10.1093/hmg/6.9.1589. [DOI] [PubMed] [Google Scholar]
- Glasstone-Hughes S. The development of halved tooth germs; a study in experimental morphology. J Anat. 1952;86:12–25. [PMC free article] [PubMed] [Google Scholar]
- Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet. 1999;22:370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- Headon DJ, Emmal SA, Ferguson BM. Gene defect in ectodermal dysplasia implicates a death domain. Nature. 2001;414:913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- Miletich I, Buchner G, Sharpe PT. Barx1 and evolutionary changes in feeding. J Anat. 2005;207:619–622. doi: 10.1111/j.1469-7580.2005.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monreal AW, Ferguson BM, Headon DL, et al. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohydrotic ectodermal dysplasia. Nat Genet. 1999;22:366–369. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- Naito A, Yoshida H, Nishioka E, et al. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci USA. 2002;99:8766–8771. doi: 10.1073/pnas.132636999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohazama A, Modino SAC, Miletich I, et al. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Sharpe PT. TNF signalling in tooth development. Current Opinion Genet Dev. 2004;14:513–519. doi: 10.1016/j.gde.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Robey PG. Post-natal stem cells for dental and craniofacial repair. Oral Biosci Med. 2005;2:83–90. [Google Scholar]
- Sharpe PT, Young CS. Test-tube teeth. Sci Am. 2005;293:34–41. doi: 10.1038/scientificamerican0805-34. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol. 1995;39:35–50. [PubMed] [Google Scholar]
- Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- Thesleff I. Epithelial mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Matthews KL, Sharpe PT. Transformation of tooth type induced by inhibition of BMP signalling. Science. 1998;282:1136–1138. doi: 10.1126/science.282.5391.1136. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Headon DJ, Courtney JM, Overbeek P, Sharpe PT. The activation level of the TNF family receptor, Edar, determines cusps number and tooth number during tooth development. Dev Biol. 2004;268:185–194. doi: 10.1016/j.ydbio.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Tucker A, Sharpe PT. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- Westbroek P, Martin FA. A marriage of bone and nacre. Nature. 1998;392:861–862. doi: 10.1038/31798. [DOI] [PubMed] [Google Scholar]
- Young CS, Terada S, Vacanti JP, et al. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;10:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]