Abstract
The involvement of the immune system in the response to tissue injury has raised the possibility that it might influence tissue, organ or appendage regeneration following injury. One hypothesis that has been discussed is that inflammatory aspects may preclude the occurrence of regeneration, but there is also evidence for more positive roles of immune components. The vertebrate eye is an immunoprivileged site where inflammatory aspects are inhibited by several immunomodulatory mechanisms. In various newt species the ocular tissues such as the lens are regenerative and it has recently been shown that the response to local injury of the lens involves activation of antigen-presenting cells which traffic to the spleen and return to displace and engulf the lens, thereby inducing regeneration from the dorsal iris. The activation of thrombin from prothrombin in the dorsal iris is one aspect of the injury response that is important in the initiation of regeneration. The possible relationships between the immune response and the regenerative response are considered with respect to phylogenetic variation of regeneration in general, and lens regeneration in particular.
Keywords: inflammation, lens, lymphocyte, salamander
Introduction
The involvement of the immune system in vertebrate regeneration is an interesting crossover topic which is increasing in importance. This review considers the different connections that have been proposed, along with some examples. We then consider in more detail the case of regeneration within the eye, where recent work provides a new perspective on the relationships between immune mechanisms and regeneration. Any satisfying account of such relationships must eventually encompass questions of evolution and phylogenetic variation, and we conclude with some remarks on these issues. The paper is written from the primary orientation of regenerative biology and in order to be accessible to immunologists and others with an interest in these problems, we have tried to summarize relevant aspects of regeneration research, for example the events of lens regeneration.
Many adult animals are able to regenerate complex structures from the body plan following tissue injury or removal (Thouveny & Tassava, 1988; Sánchez Alvarado, 2000; Brockes et al. 2001). The salamanders, or urodele amphibians, are the vertebrates with the most extensive ability to respond in this way. An adult newt, a species of acquatic salamander, can regenerate its limbs and tail (Tassava & Huang, 2005; Tsonis, 1996), jaws (Ghosh et al. 1994), ocular tissues such as the lens (Reyer, 1954) and retina (Mitashov, 1996; Mitsuda et al. 2005), the intestine (O'Steen & Walker, 1962), and sections of the heart (Oberpriller et al. 1995); by comparison, the regenerative ability of a mammal appears distinctly curtailed. This contrast extends not only to the difference between a salamander and a mouse, but also to differences between species of salamander, some of which have greatly reduced ability (Scadding, 1977). Furthermore there can be differences between species that are considered to have extensive ability. The axolotl, a neotenic species of salamander, can regenerate all of the tissues outlined above for the newt except for the lens (Stone, 1967). It is a long-standing and difficult problem to understand why some animals are able to regenerate particular structures and others not, and differences in immune mechanisms and immunomodulation have been suggested as an important factor (Fahmy & Sicard, 2002; Sicard, 2002; Harty et al. 2003; Mescher & Neff, 2005).
Injury response, immunomodulation and regeneration
The acute response to tissue injury or removal generally includes the events of wound healing, hemostasis and tissue repair. One critical role played by tissue injury is likely to be the initiation of regeneration. A regenerative system has to sense the removal or damage of tissue, and it is almost a logical requirement that some aspect of the injury response should signal for regeneration to occur. There are many potential signals and a variety of candidates have been suggested, but only recently has this question been answered in two contexts. In salamander regeneration, and particularly in the case of the lens to be discussed later, it appears that the local activation of thrombin from its zymogen prothrombin is a critical signal for the activation of cell cycle re-entry and regeneration (Tanaka & Brockes, 1998; Tanaka et al. 1999; Simon & Brockes, 2002; Imokawa & Brockes, 2003; Imokawa et al. 2004). Recent evidence for mammalian liver regeneration suggests that the release of serotonin by activated platelets following hepatectomy is a critical signal for the initiation of proliferation by residual hepatocytes (Lesurtel et al. 2006). These insights are valuable in part because they place the regenerative response in a wider context. In the salamander, for example, regeneration is linked to coagulation and other thrombin-dependent aspects of the response to injury, such as platelet activation.
By contrast, it is often suggested that the ongoing events of wound healing may be incompatible with regeneration, and immune modulation is widely recognized as an important factor (Ferguson et al. 1996; Sicard, 2002). The role of the immune system in inflammation, fibrosis and scar formation in mammals is highlighted by the occurrence of scar-free wound healing in embryos at stages which precede the development of some immune cell types (Nodder & Martin, 1997). Furthermore, mice deficient in macrophages and certain antigen-presenting cells (APCs) retain the capacity for scar-free healing (Martin et al. 2003). This view of immune ‘interference’ in regeneration has been reviewed recently and will not be considered in detail here (Mescher & Neff, 2005). It has been pointed out that there is a correlation in phylogeny between the development of adaptive immunity and progressive loss of regenerative ability (Flajnik et al. 2003). Mammals have highly developed adaptive immunity and relatively poor capacity to regenerate. Among amphibians the urodeles are better regenerators but relatively immunodeficient compared with anurans such as Xenopus, as they have an IgM-based humoral immune response with slow allograft rejection (de Both 1970; Sicard, 1983a,b, 2002; Plytycz et al. 1988; Ruben et al. 1988; Washabaugh & Tsonis, 1994; Fahmy & Sicard, 2002). In Xenopus the development of adaptive immunity occurs in the late larval stages approaching metamorphosis, and this is the same period when regenerative ability in the hindlimb is progressively lost. In a comparison of genes expressed in blastemas at a regeneration competent and an older regeneration incompetent stage, many were noted to be regulators of adaptive immunity in mammals (Mescher & Neff, 2005).
At present this is no more than a correlation, and although changes in the mode of wound healing may well be incompatible with cellular and molecular events characteristic of regeneration in the salamander – for example the formation of a wound epidermis and its proximity to the underlying regeneration blastema – it is difficult to demonstrate this. Although there is evidence that modification of the profile of wound healing in mice might assist tissue repair (Ashcroft et al. 1999; Martin et al. 2003; Heber-Katz et al. 2004), it is unlikely that it would lead to regeneration of a characteristically non-regenerative structure such as the limb. One reason for saying this is that there are a number of other differences between salamander and mouse cells that prevent or limit regeneration, for example the failure of the differentiated cells to respond to the thrombin-derived signal, or lack of expression of certain Hox genes in the limb after development (Brockes & Kumar, 2005). It is likely that there are significantly more of such differences than are currently recognized, as we are far from an exhaustive analysis of the underlying processes.
Differences in immunomodulation during wound healing and inflammation may be one possibility, but it is hard to know if they are, or were at some point, sufficient in any context as an explanation for the failure to regenerate. This has been suggested as a possibility in the case of Xenopus development that was discussed above (Mescher & Neff, 2005), and it will be interesting to see if this is supported by experiments in future.
Another possibility is that immunomodulation of the injury response may play a positive or neutral role with respect to regeneration. Although wound healing and scarring may be roadblocks for regeneration in most organisms, the lizard provides us with an example that these processes can be coordinated, or at least not interfere. Some lizards can lose substantial portions of their tail by autotomy, and this results in initiation of wound healing with scarring, wound epithelium formation, blastema formation and subsequent regeneration of the tail (Daniels et al. 2003; Alibardi & Toni, 2005). After autotomy the spleen is enlarged about 14 times in weight during early stages, and splenectomy can delay wound healing without any obvious effect on the later stages of regeneration (Shah et al. 1978). It is unclear how this switch occurs from wound healing and fibrosis to the initiation of blastema formation. In summary, immunomodulation of the injury response may play various roles in relation to regeneration depending on the tissue and species contexts.
A somewhat different principle that falls under the heading of a positive role is that a component of the immune system may have an activity in regeneration that is distinct from its function in the immune response. There is currently considerable interest in the role of certain members of the complement family in regeneration (Mastellos & Lambris, 2002). There is strong evidence that C5 plays an important role in mammalian liver regeneration, probably by modulating release of the critical cytokine IL-6 from Kupffer cells (Mastellos et al. 2001). Furthermore, C3 and C5 are expressed in the newt limb blastema, and C5 in the regenerating lens (Kimura et al. 2003). An investigation of proximodistal identity in the newt limb blastema has identified the newt orthologue of CD59 as a critical determinant expressed at the cell surface (Morais da Silva et al. 2002). It is expressed at higher levels in proximal vs. distal blastemas, and elevation of its expression in distal cells of the larval axolotl converts them to proximal identity (Echeverri & Tanaka, 2005). CD59 was originally studied as an inhibitor of extracellular complement activity (Davies et al. 1989; Huang et al. 2005; Kim & Song, 2006), and it is possible that its role in pattern formation during regeneration could be related to its ability to bind complement complexes. On the other hand, it has been shown to complex selectively with the activated EGF receptor in mammalian cells, and it could be this activity that is more relevant for regeneration (Blagoev et al. 2003). The relationship between the more familiar immune role and the role in regeneration should be clarified for each of these examples by further experiments.
Immune mechanisms and regeneration in the eye
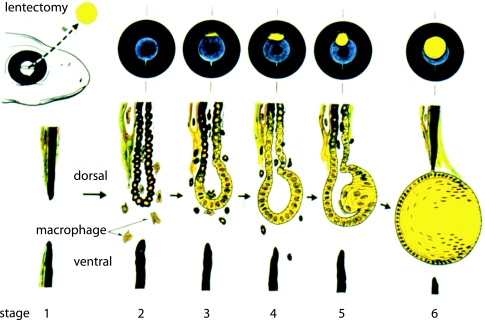
Although it is possible to point to potential circumstances of interference and synergy, it has been difficult to study regeneration, or the potential for regeneration, in the shadow of inflammation and scarring. The signals that direct healing in either the direction of regeneration or scar formation are closely linked to the early signals of injury and the immune response (Lotan & Schwartz, 1994; Ferguson et al. 1996; Sicard, 2002). In limb regeneration, for example, the restoration of cartilage, muscle, connective tissue and skin presents a complex array of events occurring at the same time. Some of these may even be competing events and the various ongoing processes make it difficult to evaluate the regenerative context. We will focus on the special environment of the eye where the issues of wound healing and inflammation are absent or at least diminished, thus allowing an examination of the interrelationships without these complications. The salamander eye is immunopriviliged and newts can regenerate the retina, iris and lens. After removal of the lens, termed lentectomy, the pigmented epithelial cells (PECs) on the pupillary margin of the iris re-enter the cell cycle (Eguchi & Shingai, 1971), lose their pigmentation, and transdifferentiate into the new lens which grows as a vesicle from this location (Fig. 1) (Tsonis et al. 2004). Although both dorsal and ventral PECs can be induced to form lens in dissociated culture (Eguchi, 1988), the response after lentectomy is strictly localized, and occurs in a tissue, the iris, that is not contiguous with the site of tissue removal. These circumstances provide a unique context in which to appreciate how tissue injury and immune mechanisms can regulate regeneration.
Fig. 1.
The events of lens regeneration in a newt. Removal of the lens (yellow circle) leads to regeneration from the dorsal pupillary margin of the iris as illustrated by the set of images at the top. The bottom part shows the microscopic events at the iris margin following lentectomy; the recruitment of macrophages, the loss of pigmentation, the induction of cell division and the formation of the lens vesicle. The figure was kindly provided by Professor Goro Eguchi.
Two complementary forms of immunity, the innate and adaptive systems, both act to thwart invading pathogens and usually employ a common pathway of inflammation in achieving this end (Kapsenberg, 2003; Reis & Sousa, 2004; Akira et al. 2006; Cooper & Alder, 2006; Karin et al. 2006). Inflammation in the eye is a grave threat to vision, and a special kind of immune protection, devoid of inflammation, has evolved (Streilein, 1999). This compromise protects the eye from most infections while avoiding the perils of inflammation, but also leaves the eye vulnerable to certain infections that require inflammation for their destruction (Streilein et al. 2002). The immune privilege of the eye is due to the special architectural features of the anterior chamber as well as the immunomodulatory molecules present in ocular fluids and expressed on ocular tissues. First, a blood–tissue barrier acts to limit access of blood-borne immune cells with reactivity for local antigens. Second, the aqueous humour of the eye has been shown to suppress inflammation by producing soluble factors that inhibit T-cell activation, shut down T-cell proliferation and inflammatory cytokine production, and convert T cells to regulatory T lymphocytes that act to suppress other T cells (Taylor, 1999). This suppression is selective, sparing cytotoxic T cells that lyse their targets but eliminating any T cell likely to cause inflammation. The aqueous humour also prevents antibodies from triggering complement activation that would lead to inflammation, but does not interfere with the effectiveness of antibodies to neutralize viruses (Ferguson & Griffith, 1997).
The modulated anti-inflammatory environment produces a restricted systemic immune response that is different from other cellular compartments, and has been termed anterior chamber-associated immune deviation (ACAID). This process generates antigen-specific regulatory T cells (CD4+ and CD8+) that inhibit the induction and expression of pro-inflammatory effector modalities (Stein-Streilein & Streilein, 2002). APCs in the stroma of the iris and the ciliary body express CD1, and their activity is modulated by soluble factors in ocular fluid (Sonoda et al. 1999). After capture of local antigens they can process and present peptides on class I and II MHC molecules, and express certain co-stimulatory molecules (such as B7-1), but they fail to secrete IL-12, and cannot up-regulate the co-stimulatory molecule CD40 that is central to induction of T cells that mediate delayed hypersensitivity (Streilein et al. 2002; Sugita et al. 2006). These APCs secrete TGFβ2 and leave the anterior chamber by migrating across the meshwork directly into the venous circulation (rather than the lymphatics), and home to the marginal zone of the spleen (Hong & Van Kaer, 1999; Sugita et al. 2006). Here they secrete a chemokine that attracts NKT cells, which recognize the CD1 on the APCs and produce the cytokine that recruits antigen-specific T cells (Sonoda et al. 1999). This aggregate induces the regulatory T cells of ACAID. These events pre-empt the normal systemic immune response to eye-derived antigens, and prevent inflammation from occurring in the eye. In animals devoid of the spleen, ACAID fails to develop (Streilein, 1999).
A new model for lens regeneration in the newt has recently been reported that clarifies how these mechanisms can be linked to the induction of regeneration (Kanao & Miyachi, 2006). If the lens is pricked once with a needle through the cornea, it undergoes vacuolation and subsequent histolysis via autophagic cell death, followed by destruction and engulfment (Fig. 2). These latter steps were mediated by dendritic cells (DCs) that migrated from the periphery to the central region of the cornea. The elimination of the lens after pricking was followed by an accelerated regeneration of a new lens from the dorsal margin of the iris (Fig. 3). In a striking demonstration, the authors isolated DCs from the eye of such animals after engulfment and transplanted them into the eyes of normal (unpricked) animals. In 70% of the cases the lens was displaced and engulfed and a new lens grew from the dorsal margin (Fig. 4). After transfer of naïve DCs, no animals showed these responses. The involvement of the ACAID pathway was implicated by removal of the spleen from the recipient before transfer of educated DCs; this abrogated the response in all animals. The receptor for vascular endothelial growth factor (VEGFR) is an important marker of angiogenesis, and its expression was observed in the central cornea and in the dorsal iris, but not the ventral iris, after pricking the lens. It was also detected after transplantation of educated DCs, but not naïve DCs. The vascularization of the cornea may enhance the absorption of the lens debris through the new vessels, while the enhanced vasculature of the dorsal iris may accelerate the local events of lens regeneration (Kanao & Miyachi, 2006).
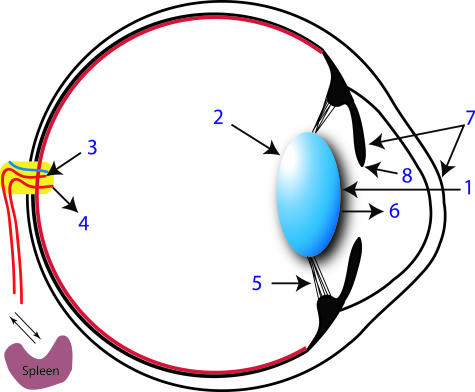
Fig. 2.
Sequence of events following pricking of the newt lens that are discussed in the text. 1, lens puncture; 2, autophagy of the lens; 3, DCs leave the eye and arrive at the spleen via the blood; 4, immune effector cells return to the eye; 5, zonular ligaments are ruptured by lens displacement; 6, lens is removed; 7, new blood vessel formation occurs in central cornea and dorsal iris; 8, regeneration is initiated from the dorsal iris.
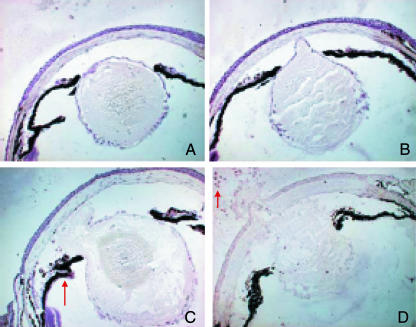
Fig. 3.
Lens destruction and removal after pricking. A–D show successive stages in the process, and examples of DCs (arrows). Note that the lens is dislodged towards the anterior chamber and ruptures at the anterior cortex. The liquified contents of the lens are extruded from the chamber (D).
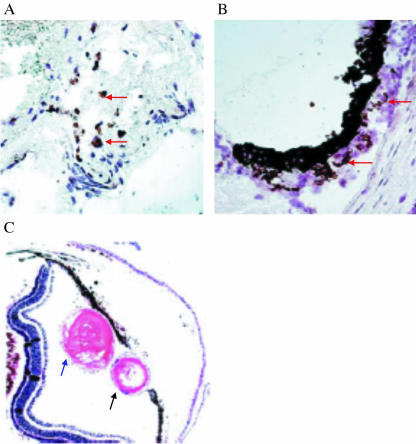
Fig. 4.
Transplantation of DCs induces lens regeneration in the recipient animal. (A) Congregation of DCs (arrows) around a lens corpse; (B) DCs around the dorsal iris after the original lens was destroyed; and (C) lens regeneration has occurred at the dorsal iris after transplantation of DC. The larger lens (blue arrow) is the original lens which is displaced, the smaller lens (black arrow) is the regenerated lens. Figures 3 and 4 are reproduced from Kanao & Miyachi (2006) with permission.
The transplantation of DCs into a normal eye provides a purely immune-mediated model of regeneration. In line with the model of ACAID, it appears that it is critical for DCs to travel to the spleen for immune amplification and subsequent homing to the eye of immune effector cells, the precise identity of which remains unclear. This is required in order to initiate lens regeneration and to induce VEGFR expression in the cornea and dorsal iris. The involvement of the DCs in ACAID and subsequent regeneration is significant in that it is apparently the immune system which initiates the concerted processes leading to lens regeneration after puncture. How might the events of lens puncture and immune-mediated lens destruction converge with the events following lentectomy to localize regeneration in both cases to the PECs on the dorsal margin? Our laboratory has provided evidence that a critical early event following lentectomy is the activation of thrombin on the dorsal margin (Imokawa & Brockes, 2003; Imokawa et al. 2004). Thrombin was found to mediate re-entry to the cell cycle by cultured newt myotubes. This effect was indirect in that thrombin was able to cleave a protein in plasma and serum leading to a product that induced re-entry in newt myotubes and iris PECs in culture, but was inactive on mouse myotubes (Tanaka et al. 1999). After lentectomy, thrombin activity appears selectively on the dorsal margin, as detected by overlaying sections of the iris with a membrane impregnated with a fluorogenic substrate (Fig. 5). If thrombin activity is blocked by introducing a protein or small molecule inhibitor into the anterior chamber, this inhibits S phase re-entry at the dorsal margin by 90%, and markedly affects the time course and extent of lens regeneration (Fig. 5) (Imokawa et al. 2004). Thrombin activation may also be critical for the recruitment of macrophages to the margin, possibly by generation of chemotactic factors; this is a cellular response to lentectomy which has long been recognized (Reyer, 1990a,b; T. Yamada 1972).
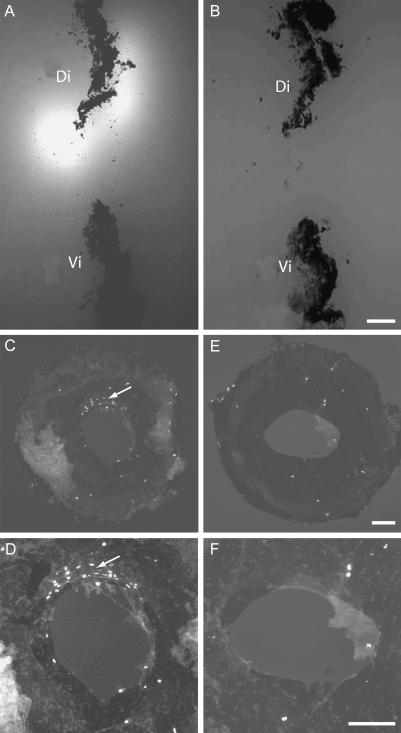
Fig. 5.
Thrombin is a signal for lens regeneration. (A) Enzyme overlay assay on a section of the anterior orbit after lentectomy showing the fluorescent reaction product associated with the dorsal but not the ventral iris. Note the strong reaction product at the dorsal pupillary margin (arrowed). (B) A parallel section reacted in the presence of the thrombin inhibitor PPACK. Note the inhibition of the reaction. (C) Whole mount of the iris from a newt injected with BrdU after lentectomy, and (D) a high-power view of C at the pupillary margin. Note the cluster of labelled nuclei at the dorsal margin. These are PECs re-entering the cell cycle, and they are the progenitors of the new lens. E and F are low- and high-power images, respectively, of an iris taken from an animal injected with PPACK to inhibit thrombin. Note the absence of BrdU-labelled cells at the dorsal margin. Figure adapted from Imokawa & Brockes (2003).
In the conventional scenario following injury, clotting factors in the blood complex with the membrane protein termed Tissue Factor (TF) that is expressed by subendothelial cells, and that leads to formation of prothrombinase and generation of thrombin at the cell surface (Mackman, 1995; Imokawa et al. 2004). We have previously suggested that expression of TF is localized to the dorsal iris in the newt (Imokawa & Brockes, 2003), and this has recently been verified (J. Godwin et al. unpublished data). The lens is suspended in the anterior chamber by the zonular ligaments, which are anchored in the muscle of the ciliary body. We suggest that removal of the lens, or its physical displacement and engulfment, can lead to rupture of vessels at the surface of the ciliary body and release of clotting factors and prothrombin into the aqueous humour. These are captured by the TF at the dorsal margin, leading to thrombin activation and the initiation of regeneration (Imokawa et al. 2004).
As mentioned above, the axolotl is excellent at regeneration but cannot regenerate its lens (Stone, 1967; Tsonis et al. 2004). In experiments where fragments of dorsal iris were transplanted between the anterior chamber of newt and axolotl, it was concluded that the axolotl iris is missing some determinant for regeneration that is present on the newt iris (Reyer, 1956). Although thrombin is activated after limb amputation in the axolotl, no activity is detectable after lentectomy (Imokawa & Brockes, 2003), and TF is not expressed by the dorsal iris. It is possible that tissue factor expression is the critical determinant, but this hypothesis will require further experiments. Interestingly, we find that the axolotl has a similar mechanism for lens destruction after pricking to that described for the newt, indicating that this aspect of the process is normal (J. Godwin et al. unpublished data).
It is interesting to compare the recent findings on ACAID-mediated lens destruction and regeneration with the role of the immune system in a model of axon regeneration in the adult rat optic nerve. Pricking the lens in this context does not result in autophagic cell death and absorption, but does result in accumulation of macrophages, astrocyte stimulation, and an increased expression of growth-associated protein GAP-43 in retinal ganglion cells that allows a degree of regeneration that does not occur without puncture (Leon et al. 2000). When macrophages were artificially stimulated, leaving the lens intact, a similar stimulation of regeneration was observed, indicating that this effect is mediated directly through macrophage induction (Yin et al. 2003).
Evolution and phylogeny
There has been little substantive progress in understanding regeneration as an evolutionary variable, and the case of lens regeneration encapsulates some of the problems and paradoxes of this area. The only vertebrates known to be able to regenerate the lens from the dorsal iris after lentectomy are the newts (Stone, 1967), and the Japanese freshwater species Misgumus, a Cobitid fish (Sato, 1961). All vertebrate PECs that have been tested, including human, are able to transdifferentiate to lens in culture (Eguchi, 1988), but this does not occur after lentectomy, even in some salamander species with extensive regenerative ability. It has been suggested that the relevant stimulus for lens regeneration in the natural habitat is not lentectomy but parasitic infection of the lens, which has been observed by Sato and by Eguchi (Okada, 2004). The events following lens puncture, including the attendant immune involvement, are therefore likely to be relevant to the mechanism for removing an infected lens and regenerating a new one. Thus, it is perhaps not surprising that the process of ACAID seems to be intact in newts in a context where regeneration occurs, and is directly involved in replacing the lens. More studies are clearly required in this context to dissect the immune mechanisms, for example to identify the effector cells that home to the eye from the spleen. It would also be interesting to know if this mechanism is shared by the Cobitid species. There may be implications from this work for our view of immune privilege in the eye. It has been suggested that the loss of cells in the eye after inflammatory episodes would lead to irreversible loss of cells or structures (Streilein, 2003), yet even in a regenerative context ACAID apparently occurs, and one might even suggest that it could have its origins in relation to ocular regeneration.
There are several points to be made about the relationship between regeneration, tissue injury and the immune response. Although the immune response plays a fundamental role in the sequence of events after puncture, it is not directly involved, in our present model, in the initiation of lens regeneration, which depends on tissue injury and rupture of vessels, a requirement that could plausibly be shared with other contexts of regeneration such as the limb (Tanaka et al. 1999). It is possible nonetheless that there may be contexts where regeneration is directly linked to an aspect of the immune response, and this would be of great interest. The prevailing view, as discussed earlier, is that the transition to an inflammatory type of rapid wound healing in adult vertebrates may have curtailed regenerative ability, at least relative to the salamanders. We express some reservation about this view, and suggest that regeneration may have been lost for other reasons and ‘overlaid’ with a different healing mechanism. In the eye, the ACAID mechanism appears to have persisted in vertebrates, although lens regeneration has been lost in salamanders such as the axolotl, and in all other species except for Cobitid fish. We hope that these issues will be clarified as our understanding of the underlying mechanisms increases.
Acknowledgments
We thank Anoop Kumar for his help, and the MRC for support by a Programme Grant and Research Professorship to J.P.B.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Toni M. Wound keratins in the regenerating epidermis of lizard suggest that the wound reaction is similar in the tail and limb. J Exp Zool A Comp Exp Biol. 2005;303:845–860. doi: 10.1002/jez.a.213. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Blagoev B, Kratchmarova I, Ong SE, et al. A proteomics strategy to elucidate functional protein–protein interactions applied to EGF signaling. Nat Biotechnol. 2003;21:315–318. doi: 10.1038/nbt790. [DOI] [PubMed] [Google Scholar]
- de Both NJ. Transplantation immunity in the axolotl (Ambystoma mexicanum) studied by blastemal grafts. J Exp Zool. 1970;173:147–158. doi: 10.1002/jez.1401730204. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A, Velloso CP. Regeneration as an evolutionary variable. J Anat. 2001;199:3–11. doi: 10.1046/j.1469-7580.2001.19910003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Daniels CB, Lewis BC, Tsopelas C, et al. Regenerating lizard tails: a new model for investigating lymphangiogenesis. FASEB J. 2003;17:479–481. doi: 10.1096/fj.02-0579fje. [DOI] [PubMed] [Google Scholar]
- Davies A, Simmons DL, Hale G, et al. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989;170:637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri K, Tanaka EM. Proximodistal patterning during limb regeneration. Dev Biol. 2005;279:391–401. doi: 10.1016/j.ydbio.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Eguchi G, Shingai R. Cellular analysis on localization of lens forming potency in the newt iris epithelium. Dev Growth Differ. 1971;13:337–349. doi: 10.1111/j.1440-169x.1971.00337.x. [DOI] [PubMed] [Google Scholar]
- Eguchi G. Cellular and molecular background of wolffian lens regeneration. Cell Differ Dev. 1988;25(Suppl.):147–158. doi: 10.1016/0922-3371(88)90111-6. [DOI] [PubMed] [Google Scholar]
- Fahmy GH, Sicard RE. A role for effectors of cellular immunity in epimorphic regeneration of amphibian limbs. In Vivo. 2002;16:179–184. [PubMed] [Google Scholar]
- Ferguson MW, Whitby DJ, Shah M, et al. Scar formation: the spectral nature of fetal and adult wound repair. Plast Reconstr Surg. 1996;97:854–860. doi: 10.1097/00006534-199604000-00029. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Griffith TS. A vision of cell death: insights into immune privilege. Immunol Rev. 1997;156:167–184. doi: 10.1111/j.1600-065x.1997.tb00967.x. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Miler K, Pasquier L. Evolution of the immune sytem. In: Pasquier L, Miller K, editors. Fundamental Immunology. Philadelphia: Lippincott Williams and Wilkins; 2003. pp. 519–570. [Google Scholar]
- Ghosh S, Thorogood P, Ferretti P. Regenerative capability of upper and lower jaws in the newt. Int J Dev Biol. 1994;38:479–490. [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, Mescher AL. Regeneration or scarring: an immunologic perspective. Dev Dyn. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E, Leferovich J, Bedelbaeva K, Gourevitch D, Clark L. The scarless heart and the MRL mouse. Philos Trans R Soc Lond B Biol Sci. 2004;359:785–793. doi: 10.1098/rstb.2004.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Van Kaer L. Immune privilege: keeping an eye on natural killer T cells. J Exp Med. 1999;190:1197–1200. doi: 10.1084/jem.190.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Smith CA, Song H, et al. Insights into the human CD59 complement binding interface toward engineering new therapeutics. J Biol Chem. 2005;280:34073–34079. doi: 10.1074/jbc.M504922200. [DOI] [PubMed] [Google Scholar]
- Imokawa Y, Brockes JP. Selective activation of thrombin is a critical determinant for vertebrate lens regeneration. Curr Biol. 2003;13:877–881. doi: 10.1016/s0960-9822(03)00294-x. [DOI] [PubMed] [Google Scholar]
- Imokawa Y, Simon A, Brockes JP. A critical role for thrombin in vertebrate lens regeneration. Philos Trans R Soc Lond B Biol Sci. 2004;359:765–776. doi: 10.1098/rstb.2004.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanao T, Miyachi Y. Lymphangiogenesis promotes lens destruction and subsequent lens regeneration in the newt eyeball, and both processes can be accelerated by transplantation of dendritic cells. Dev Biol. 2006;290:118–124. doi: 10.1016/j.ydbio.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kim DD, Song W-C. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Madhavan M, Call MK, et al. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J Immunol. 2003;170:2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesurtel M, Graf R, Aleil B, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- Lotan M, Schwartz M. Cross talk between the immune system and the nervous system in response to injury: implications for regeneration. FASEB J. 1994;8:1026–1033. doi: 10.1096/fasebj.8.13.7926367. [DOI] [PubMed] [Google Scholar]
- Mackman N. Regulation of the tissue factor gene. FASEB J. 1995;9:883–889. doi: 10.1096/fasebj.9.10.7615158. [DOI] [PubMed] [Google Scholar]
- Martin P, D'Souza D, Martin J, et al. Wound healing in the PU.1 null mouse – tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Lambris JD. Complement: more than a ‘guard’ against invading pathogens? Trends Immunol. 2002;23:485–491. doi: 10.1016/s1471-4906(02)02287-1. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Mitashov VI. Mechanisms of retina regeneration in urodeles. Int J Dev Biol. 1996;40:833–844. [PubMed] [Google Scholar]
- Mitsuda S, Yoshii C, Ikegami Y, Araki M. Tissue interaction between the retinal pigment epithelium and the choroid triggers retinal regeneration of the newt Cynops pyrrhogaster. Dev Biol. 2005;280:122–132. doi: 10.1016/j.ydbio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Morais da Silva S, Gates PB, Brockes JP. The newt ortholog of CD59 is implicated in proximodistal identity during amphibian limb regeneration. Dev Cell. 2002;3:547–555. doi: 10.1016/s1534-5807(02)00288-5. [DOI] [PubMed] [Google Scholar]
- Nodder S, Martin P. Wound healing in embryos: a review. Anat Embryol (Berl) 1997;195:215–228. doi: 10.1007/s004290050041. [DOI] [PubMed] [Google Scholar]
- O'Steen WK, Walker BE. Radioautographic studies or regeneration in the common newt. III. Regeneration and repair of the intestine. Anat Rec. 1962;142:179–187. doi: 10.1002/ar.1091420210. [DOI] [PubMed] [Google Scholar]
- Oberpriller JO, Oberpriller JC, Matz DG, Soonpaa MH. Stimulation of proliferative events in the adult amphibian cardiac myocyte. Ann NY Acad Sci. 1995;752:30–46. doi: 10.1111/j.1749-6632.1995.tb17404.x. [DOI] [PubMed] [Google Scholar]
- Okada TS. From embryonic induction to cell lineages: revisiting old problems for modern study. Int J Dev Biol. 2004;48:739–742. doi: 10.1387/ijdb.041918to. [DOI] [PubMed] [Google Scholar]
- Plytycz B, Potter SW, Cohen N, Bayne CJ. In vitro fusion of newt macrophages. J Exp Zool. 1988;246:319–323. doi: 10.1002/jez.1402460312. [DOI] [PubMed] [Google Scholar]
- Reis E, Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004;16:21. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Reyer RW. Regeneration of the lens in the amphibian eye. Q Rev Biol. 1954;29:1–46. doi: 10.1086/399936. [DOI] [PubMed] [Google Scholar]
- Reyer RW. Lens regeneration from homoplastic and heteroplastic implants of dorsal iris into the eye chamber of Triturus viridescens and Amblystoma punctatum. J Exp Zool. 1956;133:145–189. [Google Scholar]
- Reyer RW. Macrophage invasion and phagocytic activity during lens regeneration from the iris epithelium in newts. Am J Anat. 1990a;188:329–344. doi: 10.1002/aja.1001880402. [DOI] [PubMed] [Google Scholar]
- Reyer RW. Macrophage mobilization and morphology during lens regeneration from the iris epithelium in newts: studies with correlated scanning and transmission electron microscopy. Am J Anat. 1990b;188:345–365. doi: 10.1002/aja.1001880403. [DOI] [PubMed] [Google Scholar]
- Ruben LN, Beadling C, Langeberg L, Shiigi S, Selden N. The substitution of carrier priming of helper function in the common American newt, Notophthalmus viridescens by lectins and human lymphokines. Thymus. 1988;11:77–87. [PubMed] [Google Scholar]
- Sánchez Alvarado A. Regeneration in the metazoans: why does it happen? Bioessays. 2000;22:578–590. doi: 10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sato T. Uber die Linsenregeneration bei den Cobitiden Fishen I. Misgumus anguillicaudatus. Embryologia (Nagoya) 1961;6:251–290. [Google Scholar]
- Scadding S. Pylogenetic distribution of limb regeneraton capacity in adult amphibia. J Exp Zool. 1977;202:57–68. [Google Scholar]
- Shah RV, Kothari JS, Hiradhar PK. Histological changes in spleen during tail regeneration in the gekkonid lizard. J Anim Morph Physiol. 1978;25:167–171. [Google Scholar]
- Sicard RE. Blood cells and their role in regeneration. I. Changes in circulating blood cell counts during forelimb regeneration. Exp Cell Biol. 1983a;51:51–59. doi: 10.1159/000163173. [DOI] [PubMed] [Google Scholar]
- Sicard RE. Blood cells and their role in regeneration. II. Effects of putative immunological manipulations on circulating blood cell counts during regeneration. Exp Cell Biol. 1983b;51:109–114. [PubMed] [Google Scholar]
- Sicard RE. Differential inflammatory and immunological responses in tissue regeneration and repair. Ann NY Acad Sci. 2002;961:368–371. doi: 10.1111/j.1749-6632.2002.tb03126.x. [DOI] [PubMed] [Google Scholar]
- Simon A, Brockes JP. Thrombin activation of S-phase reentry by cultured pigmented epithelial cells of adult newt iris. Exp Cell Res. 2002;281:101–106. doi: 10.1006/excr.2002.5650. [DOI] [PubMed] [Google Scholar]
- Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21:123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- Stone LS. An investigation recording all salamanders which can and cannot regenerate a lens from the dorsal iris. J Exp Zool. 1967;164:87–103. doi: 10.1002/jez.1401640109. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Regional immunity and ocular immune privilege. Chem Immunol. 1999;73:11–38. doi: 10.1159/000058741. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Masli S, Takeuchi M, Kezuka T. The eye's view of antigen presentation. Hum Immunol. 2002;63:435–443. doi: 10.1016/s0198-8859(02)00393-2. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- Sugita S, Ng TF, Lucas PJ, Gress RE, Streilein JW. B7+ iris pigment epithelium induce CD8+ T regulatory cells; both suppress CTLA-4+ T cells. J Immunol. 2006;176:118–127. doi: 10.4049/jimmunol.176.1.118. [DOI] [PubMed] [Google Scholar]
- T Yamada JND. Macrophage activity in wolffian lens regeneration. J Morph. 1972;136:367–383. doi: 10.1002/jmor.1051360309. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Brockes JP. A target of thrombin activation promotes cell cycle re-entry by urodele muscle cells. Wound Repair Regen. 1998;6:371–381. doi: 10.1046/j.1524-475x.1998.60413.x. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Drechsel DN, Brockes JP. Thrombin regulates S-phase re-entry by cultured newt myotubes. Curr Biol. 1999;9:792–799. doi: 10.1016/s0960-9822(99)80362-5. [DOI] [PubMed] [Google Scholar]
- Tassava RA, Huang Y. Tail regeneration and ependymal outgrowth in the adult newt, Notophthalmus viridescens, are adversely affected by experimentally produced ischemia. J Exp Zool A Comp Exp Biol. 2005;303:1031–1039. doi: 10.1002/jez.a.242. [DOI] [PubMed] [Google Scholar]
- Taylor AW. Ocular immunosuppressive microenvironment. Chem Immunol. 1999;73:72–89. doi: 10.1159/000058738. [DOI] [PubMed] [Google Scholar]
- Thouveny Y, Tassava RA. Regeneration through phylogenesis. In: Ferretti P, Geraudie J, editors. Cellular and Molecular Basis of Regeneration: from Invertebrates to Humans. Chichester: John Wiley & Sons; 1988. pp. 9–44. [Google Scholar]
- Tsonis PA. Limb RegenerationDevelopmental and Cell Biology Series. New York: Cambridge University Press; 1996. [Google Scholar]
- Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K. A newt's eye view of lens regeneration. Int J Dev Biol. 2004;48:975–980. doi: 10.1387/ijdb.041867pt. [DOI] [PubMed] [Google Scholar]
- Washabaugh CH, Tsonis PA. Mononuclear leukocytes in the newt limb blastema: in vitro behavior. Int J Dev Biol. 1994;38:745–749. [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]