Abstract
The osteogenic molecular signals of the transforming growth factor-β (TGF-β) superfamily, the bone morphogenetic/osteogenic proteins (BMPs/OPs) and uniquely in primates the TGF-β isoforms per se, pleiotropic members of the TGF-β supergene family, induce de novo endochondral bone formation as a recapitulation of embryonic development. Naturally derived BMPs/OPs and gamma-irradiated human recombinant osteogenic protein-1 (hOP-1) delivered by allogeneic and xenogeneic insoluble collagenous matrices initiate de novo bone induction in heterotopic and orthotopic sites of the primate Papio ursinus, culminating in complete calvarial regeneration by day 90 and maintaining the regenerated structures by day 365. The induction of bone by hOP-1 in P. ursinus develops as a mosaic structure with distinct spatial and temporal patterns of gene expression of members of the TGF-β superfamily that singly, synergistically and synchronously initiate and maintain tissue induction and morphogenesis. The temporal and spatial expressions of TGF-β1 mRNA indicate a specific temporal transcriptional window during which expression of TGF-β1 is mandatory for successful and optimal osteogenesis. Highly purified naturally derived bovine BMPs/OPs and hOP-1 delivered by human collagenous bone matrices and porous hydroxyapatite, respectively, induce bone formation in mandibular defects of human patients. By using healthy body sites as bioreactors it is possible to recapitulate embryonic developments by inducing selected biomaterials combined with recombinant proteins to transform into custom-made prefabricated bone grafts for human reconstruction. The osteogenic proteins of the TGF-β superfamily, BMPs/OPs and TGF-βs, the last endowed with the striking prerogative of inducing endochondral bone formation in primates only, are helping to engineer skeletal reconstruction in molecular terms.
Keywords: bone morphogenetic proteins, osteogenic proteins, primates, redundancy, structure/activity profile, TGF-β superfamily members
Bone: formation by autoinduction
Our understanding of the initiation, maintenance and promotion of tissue induction and morphogenesis has provided the rationale for enunciating the rules for tissue engineering, primarily for the induction of bone (Reddi, 1994, 1998, 2000; Ripamonti, 2006). Which are the molecular signals that initiate bone: formation by autoinduction (Urist, 1965)? Elucidating the nature and interactions of the signalling molecules that direct the initiation of bone formation is a major challenge of contemporary molecular, cellular, developmental and surgical biology (Ripamonti, 2006).
The distilled summary of several years of research efforts is surprisingly simple. First, tissue regeneration in postnatal life recapitulates events that occur in the normal course of embryonic development and morphogenesis. Secondly, both embryonic development and tissue regeneration are equally regulated by selected and highly conserved families of proteins, gene products of the transforming growth factor-β (TGF-β) superfamily (Wozney et al. 1988; Reddi, 1994, 1998, 2000; Ripamonti, 2003, 2006). The mechanistic understanding of bone development in embryogenesis has been instrumental to set the rules that control tissue engineering in postnatal life (Reddi, 1994, 2000). Nature relies on common yet limited molecular mechanisms that initiate the emergence of specialized tissues and organs. Common molecular initiators, the bone morphogenetic/osteogenic proteins (BMPs/OPs), are deployed for embryonic development and the induction of bone formation in postnatal life (Reddi, 1994, 2000; Ripamonti, 2003, 2006).
A striking and discriminatory prerogative of BMPs/OPs is the induction of endochondral bone formation when implanted in heterotopic extraskeletal sites (Fig. 1) (Wozney et al. 1988; Reddi, 2000; Ripamonti et al. 2001, 2005; Ripamonti, 2005, 2006). Importantly, each single recombinant human protein sets into motion the cascade of bone differentiation by induction in heterotopic extraskeletal sites (Ripamonti, 2006).
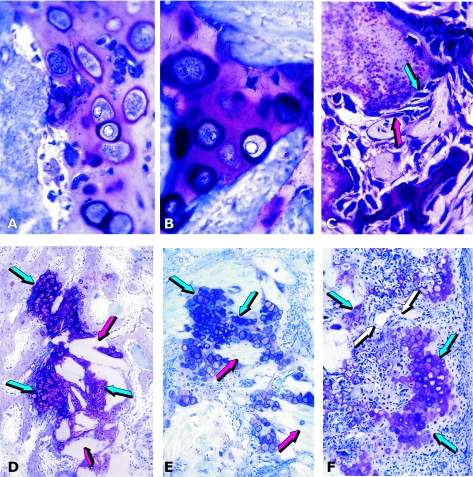
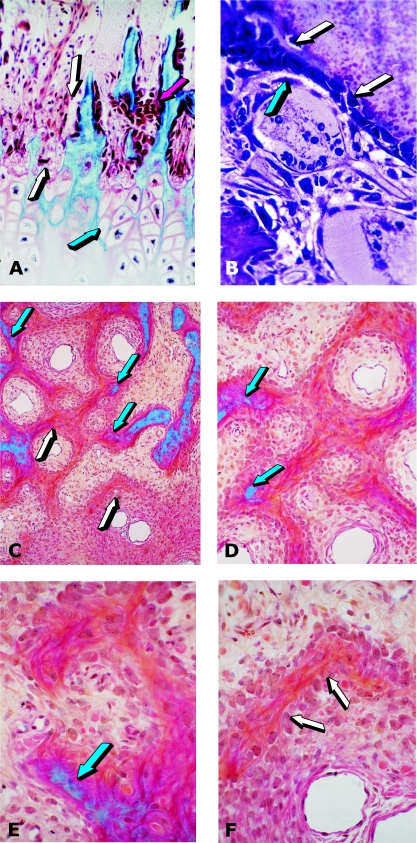
Fig. 1.
Tissue induction and morphogenesis by naturally derived and recombinant human osteogenic proteins delivered by collagenous matrices as carrier in the rodent bioassay. (A,B) Induction of chondrogenesis by 0.1–0.5 µg osteogenin purified to apparent homogeneity after electroendosmotic elution of baboon osteogenic fractions; and (C) osteogenin induces osteoblastic cells secreting bone matrix and capillary invasion with differentiating mesenchymal cells migrating from the vascular compartment (red arrow) to the bone matrix compartment (blue arrow) with secreting osteoblastic cells attached to the matrix. (D–F) Induction of endochondral bone differentiation by 2 µg hOP-1 delivered by insoluble collagenous bone matrix (red arrows) as carrier implanted in the subcutaneous space of the rodent bioassay and harvested on day 11. Islands of cartilage induction as a recapitulation of embryonic development (blue arrows), vascular invasion (white arrows in F), chondrolysis and bone differentiation.
The BMP/OP family is indeed an elegant example of nature's parsimony in programming multiple, pleiotropic, specialized functions by deploying molecular isoforms with minor variation in amino acid motifs within highly conserved carboxy terminal regions (Wozney et al. 1988; Ripamonti & Duneas, 1998; Reddi, 1998, 2000; Ripamonti et al. 2004b, 2005; Ripamonti, 2006).The BMPs/OPs are members of the TGF-β superfamily and play critical roles as soluble mediators of tissue morphogenesis (Wozney et al. 1988; Reddi, 2000; Ripamonti et al. 2005; Ripamonti, 2006). Beside postnatal osteogenesis, BMPs/OPs play multiple roles in embryonic development and are involved in inductive events unrelated to bone induction that control pattern formation during embryonic organogenesis (Fig. 2) (Ripamonti, 2004a, 2006; Ripamonti et al. 2005). BMPs/OPs act as soluble signals in epithelial/mesenchymal interactions during nephrogenesis (Vukicevic et al. 1994, 1996; Dudley et al. 1995; Luo et al. 1995; Solursh et al. 1996; Simic & Vukicevic, 2005), tooth morphogenesis (Vainio et al. 1993; Heikinheimo, 1994; Helder et al. 1995; Thesleff et al. 1995; Åberg et al. 1997; Thomadakis et al. 1999) and control pattern formation during organogenesis of such disparate tissues and organs as the kidney, eye, central and peripheral nervous systems, lung, skin, heart, teeth, cementum and the periodontal ligament (Fig. 2) (Hogan, 1996; Thomadakis et al. 1999; Kishigami & Mishina, 2005).
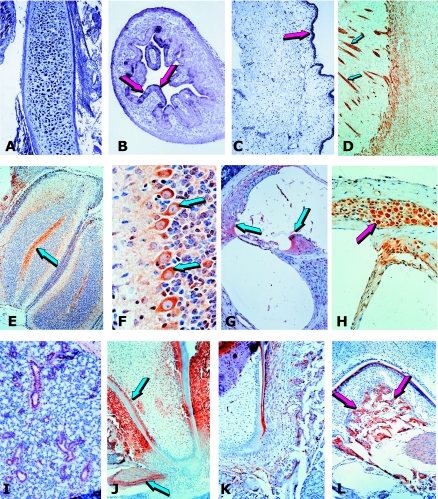
Fig. 2.
Pleiotropism of bone morphogenetic proteins: from bone to central and peripheral nervous system expression and localization. (A) In situ hybridization of OP-1 mRNA in the developing femur of a human embryo. (B) OP-1 mRNA localized in the intestinal epithelium of the human fetus and (C) epidermis (red arrows). (D) Sixteen-day-old pup: BMP-3 immunolocalization in the cerebral cortex delineating neurite axonal patterns (arrows). (E) Thirteen-day-old pup; low-magnification view of the cerebellar folia with immunolocalization of BMP-3 in the cerebellar white matter (arrow). (F) Detail of previous section: BMP-3 in the cytoplasm of Purkinjie cells (arrows). (G) Sixteen-day-old pup: OP-1 immunolocalization in inner ear: spiral limbus and interdentate cells, and the spiral ligament (blue arrows) with absence of staining in the stria vascularis of the cochlea. (H) Thirteen-day-old mouse pup: BMP-3 immunolocalization in the spiral ganglion (arrow). (I) Sixteen-day-old pup: BMP-3 immunolocalization in the ductal system of the submandibular salivary gland. (J) Thirteen-day-old pup: developing mandibular molar. BMP-3 in three components of the periodontium: alveolar bone, periodontal ligament and cementum. Note localization in predentine, odontoblasts and inferior alveolar nerve (arrows). (K) Sixteen-day-old mouse pup: immunolocalization of OP-1: developing root of mandibular molar. Strong localization of OP-1 during cementogenesis in cementoblasts and developing fibres inserting into the newly deposited cementum. (L) Sixteen-day-old mouse pup: furcation area of developing mandibular molar showing immunolocalization of BMP-2 in alveolar bone (arrows).
Expression of BMPs/OPs in different tissues and organs also induces programmed cell death, or apoptosis, in the mesenchyme of the interdigital spaces (Gañan et al. 1996; Zou & Niswander, 1996) resulting in the spatial distribution of digital rays. Morphogenesis induced by BMPs/OPs is attained by instructing cells to proliferate and differentiate into specific tissues or, alternatively, by regulating programmed cell death thereby orchestrating the emergence of patterned skeletal morphologies (Gañan et al. 1996; Zou & Niswander, 1996; Ripamonti & Duneas, 1998; Ripamonti et al. 2005; Ripamonti, 2006).
The high levels of homology between dpp and 60A genes in Drosophila melanogaster with human BMP-2 and BMP-4, and BMP-5 and BMP-7, respectively, indicate the primordial role of BMPs/OPs during the emergence and development of the vertebrates (Sampath et al. 1993; Reddi, 1998; Ripamonti, 2004a, 2006). Because of their evolutionary and functional conservation, the secreted proteins retain common developmental roles. The most compelling evidence that the genes have been conserved for at least 800 million years is that recombinant human Drosophila proteins DPP and 60A induce heterotopic endochondral bone formation in mammals (Sampath et al. 1993).
This has indicated that a phylogenetically ancient signalling process used in dorsal–ventral patterning in the fruit fly D. melanogaster also operates to produce a unique vertebrate trait, i.e. the induction of bone and skeletogenesis (Sampath et al. 1993; Ripamonti, 2006). Nature has thus found it easier to usurp phylogenetically ancient amino acid sequences deployed for dorsal–ventral patterning in D. melanogaster to induce the emergence of the skeleton, rather than developing new gene products responsible for the initiation of the unique vertebrate traits of bone formation and skeletogenesis (Ripamonti, 2003, 2004b, 2006).
Modified amino acid sequences in homologous carboxy terminal domains yielded the BMP/OP family, thus phylogenetically ancient gene products are recruited to molecularly initiate the induction of bone formation, skeletogenesis, and the emergence of the vertebrates (Ripamonti, 2006). The pleiotropic activity of BMPs/OPs is due to minor amino acid sequence variations in the carboxy-terminal domain of each isoform (Ripamonti, 2004a, 2006; Ripamonti et al. 2005, 2006), as well as signal transduction by distinct signalling pathways effected by individual Smad proteins after transmembrane serine–threonine kinase receptor activation and expression of the downstream antagonists Smad-6 and Smad-7 (Miyazono et al. 2005; Ripamonti, 2006). The molecular cloning and expression of recombinant human (h) BMPs/OPs has provided the potential for predictable bone tissue engineering in clinical contexts (Reddi, 2000; Friedlander et al. 2001; Ripamonti et al. 2001; Govender et al. 2002; Ripamonti, 2004a, 2006). Information on the morphogenetic potential of naturally derived and recombinant hBMPs/OPs in non-human primates is of critical importance to design therapeutic applications in humans (Figs 3 and 4) (Ripamonti et al. 1996, 2001, 2005; Ripamonti, 2003, 2004b).
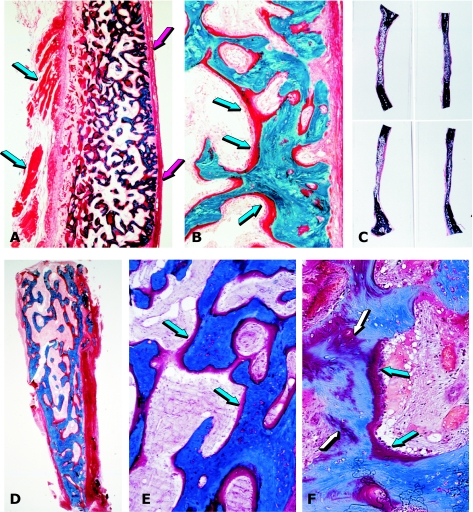
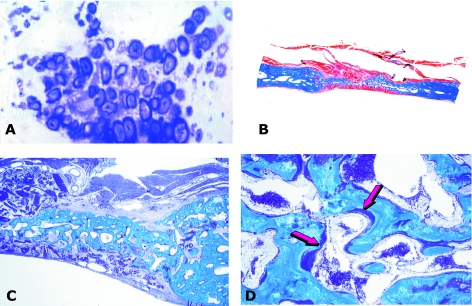
Fig. 3.
Tissue induction and morphogenesis by bone morphogenetic proteins in non-human and human primates. (A) Calvarial regeneration on day 30 by highly purified BMPs/OPs from baboon bone matrices. (B) Detail of previous section showing newly formed mineralized bone in blue surfaced by osteoid seams (arrows) populated by contiguous osteoblasts. (C) Regeneration of non-healing calvarial defects in the baboon 90 days after implantation of doses of highly purified BMPs/OPs extracted and purified from baboon bone matrices. (D) Bioptic material of newly induced bone by BMPs/OPs highly purified from bovine bone matrices implanted in large mandibular defects of human patients. (E) detail of previous section showing newly formed mineralized bone in blue surfaced by osteoid seams (arrows) in orange-red. (F) Remnants of collagenous matrix as carrier (white arrows) after induction of newly formed mineralized bone and osteoid (blue arrows) populated by contiguous osteoblasts.
Fig. 4.
Induction of bone by the human osteogenic device of gamma-irradiated hOP-1 and bovine insoluble collagenous matrix as carrier. (A,B) Induction of a mineralized corticalized ossicle by the 2.5-mg hOP-1 osteogenic device per gram of bovine matrix as carrier 30 days after implantation in the rectus abdominis muscle of an adult baboon. (B) Detail of previous section showing thick osteoid seams (arrowheads) surfacing newly formed mineralized bone in blue. (C,D) Morphology of calvarial regeneration and tissue induction 15 days after the single application of 0.1 mg hOP-1 osteogenic device per gram of bovine matrix as a carrier. Induction of bone endocranially just above the dural layer and pericranially below the temporalis muscle with numerous trabeculae of newly formed and mineralized bone (D) surfaced by osteoid seams (arrows). Substantial vascular and mesenchymal tissue invasion within the treated defect with scattered remnants of the collagenous matrix as carrier between the pericranial and endocranial osteogenetic fronts. (E,F) Complete regeneration of a calvarial defect in the baboon 90 days after the single application of the 0.5-mg hOP-1 osteogenic device. (F) Detail of previous section showing extensive osteogenesis with the induction of solid blocks of mineralized and corticalized bone with diploic spaces above the dural layer (arrow).
Different strategies for bone induction
How is bone induction initiated? The fundamental work of Sacerdotti & Frattin (1901), Huggins (1931), Levander (1938, 1945), Lacroix (1945), Bridges & Pritchard (1958), Moss (1958), Trueta (1963), Urist (1965), Urist et al. (1967) and Reddi & Huggins (1972) has indicated that the extracellular matrices of uroepithelium, bone and dentine contain morphogenetic signals endowed with the striking prerogative of initiating bone formation by induction in heterotopic extraskeletal sites of recipient animal models.
Devitalized alcohol or acetone extracts of the muscular wall of the ileum, urinary bladder and uterus, bone and bone callus of healing fractures de novo induced the formation of bone when implanted in heterotopic sites, i.e. beneath the kidney capsule, subcutaneously in the ear and intramuscularly in the rectus femori in the rabbit (Levander, 1938; Bridges & Pritchard, 1958). Levander (1938) stated that when the devitalized bone graft is transplanted into a recipient animal in heterotopic sites a ‘specific bone forming substance is liberated from the bone tissue and is carried by the tissue lymph to the surrounding areas where it is able to activate the mesenchymal tissue in such a way that this becomes differentiated into bone tissue – either directly or by means of the embryonic pre-existing stage of bone and cartilaginous tissue’. Levander subsequently stated that the ‘substance extracted by alcohol from the skeletal tissue has the power to activate the non-specific mesenchymal tissue into the formation of bone tissue’ (Levander, 1938). Attempts to extract the osteogenetic factors from alcoholic solutions had failed although so-called crude fatty-acid fractions were reported to carry the greatest ‘osteogenic activity’ in the heterotopic induction of bone formation (Levander, 1945; Levander & Willestaedt, 1946; Willestaedt et al. 1950).
Of importance were the subsequent observations of Levander (1945), who stated that: ‘There is every reason to assume that the same chemical substances are active both during the embryonal differentiation and during post-foetal growth. Regeneration of tissues, in other words, is a repetition of embryonal development.’
Levander, when publishing in Nature in 1945, stated that ‘the circumstance that a tissue is able to affect another in a specifically differentiating direction I have termed “induction” – a term borrowed from embryology, introduced, as is well known, by Spemann and his school at the turn of the century.’
The osteogenic potency of various intact and partially extracted matrices became manifest as ‘osteogenic activity’ (Urist & McLean, 1952) previously defined by Levander as ‘specific bone forming substance’ (Levander, 1938). Lacroix (1945) named this osteogenic activity he had obtained from ethanol extracts of cartilage as ‘osteogenin’ and collectively the work of Levander (1938, 1945), Bertelsen (1945), Lacroix (1945), Levander & Willestaedt (1946), Urist & McLean (1952) and Bridges & Pritchard (1958) strongly suggested that the chemical nature of the inductor(s) was proteic in nature. Intracerebral implantation of a paste of bovine bone in rats showed, in all cases, extensive osteogenic activity (Moss, 1958). Moss noted and reported the important histological observation that the ‘area of induced osteogenesis never extended beyond the area of implantation’ (Moss, 1958).
The fact that several tissues and alcohol and acid/alcohol extracts were all endowed with different osteogenic potency as determined by the heterotopic induction of bone was the first indication of the multifactorial pleiotropic nature of the then elusive osteogenic proteins. These studies prepared the way for the discovery of the critical role of selected osteogenic proteins beyond bone (Fig. 2) (Reddi, 2005). Osteogenic proteins are thus involved in epithelial/mesenchymal interactions, which encompass the design and control of many tissues and organs such as nephrogenesis and tooth morphogenesis. This has necessitated the proposal of the alternative nomenclature of body morphogenetic proteins (Reddi, 2005). The unique observations of Sacerdotti & Frattin (1901) later expounded by the classic work of Huggins (1931) on the phenomenon of uroepithelial osteogenesis confirmed that the induction of uroepithelial osteogenesis is the only de novo bone formation induced without using matrices prepared from the skeleton (Levander, 1938).
In classic experiments at the beginning of the last century, ligation of the renal artery produced ossification along different sections of the urinary tract including the kidney in rabbits (Sacerdotti & Frattin, 1901). The autogenous transplantation of the dome of the bladder into the rectus abdominis muscle, and of the fascia of the rectus abdominis into the bladder, resulted in the induction of membranous osteogenesis (Huggins, 1931). This phenomenon, first described in dogs by Huggins (1931) and defined as uroepithelial osteogenesis, has been also investigated in the adult non-human primate Papio ursinus (Ripamonti et al. 2002). Membranous ossification is induced within the bladder upon the transplantation of the rectus abdominis fascia. The early observations of Levander (1938) further suggested that the ‘bone forming substance can be excreted with the urine’, indicating that such a substance is present in the circulating blood. The research work of Levander (1945) was critical in linking tissue induction of bone with the induction and development of the embryonic organizers as defined by Spemann (1938).
Building on the previously published experimental work by Levander (1938, 1945), Lacroix (1945), Levander & Willestaedt (1946), Willestaedt et al. (1950), Bridges & Pritchard (1958) and Moss (1958), Urist [again borrowing the term ‘induction’ from Spemann (1938) and Levander (1945)] produced evidence that demineralization of the bone matrix results in bone: formation by autoinduction (Urist, 1965). In his classic paper in Science, Urist (1965) stated that evidence for osteogenesis was still entirely morphological and provided clear-cut histological evidence that osteoblastic-like cells surfacing excavation chambers of demineralized bone were derived from proliferating pluripotent mesenchymal cells of the implanted host animal. Urist further stated that in an extraskeletal or heterotopic implant of demineralized bone matrix, ‘cell-induction sequences produce an entirely new ossicle with a marrow cavity as the end-product’ (Urist, 1965). Working on both heterotopic and orthotopic sites of a variety of animal models including humans, Urist made the important statement, still poorly understood today, that ‘the orthotopic system does not offer convincing evidence of induction’, clearly indicating that the acid-test of induction is only achieved by the induction of bone in heterotopic sites (Urist, 1965). Urist's paper, however, also provides evidence of bone formation by induction upon replacement of the resected segment of the ulna in rabbits with demineralized allogeneic bone that shows radiographically new bone across the defect 12 weeks after implantation (Urist, 1965). Urist's experimental work and papers (Urist, 1965; Urist et al. 1967) question whether the demineralized bone ‘matrix produces a specific diffusible chemical agent that induces the cells of the host to differentiate into osteoblasts’ (Urist, 1965). Answering negatively, Urist indicates that ‘the system is more complex than a simple chemical stimulus and direct cell response’ (Urist, 1965).
Classically, the research works of Levander (1938, 1945), Lacroix (1945), Levander & Willestaedt (1946), Urist (1965) and Reddi & Huggins (1972) have indicated that bone contains a BMP complex endowed with the striking capacity of transforming resident mesenchymal cells into chondroblastic and osteoblastic cell lines secreting chondrogenic and osteogenic matrices, i.e. bone: formation by autoinduction (Urist, 1965). Moreover, this work has unequivocally shown that the induction of bone and the bone induction principle (Urist et al. 1967) resides within the extracellular matrix of bone (Urist et al. 1967, 1983; Sampath & Reddi, 1981). During the phenomena of bone: formation by autoinduction (Urist, 1965), the generation of cellular diversity, or differentiation, must first occur (Ripamonti & Duneas, 1998). Tissue induction, maintenance and remodelling then follow, thereby organizing differentiating osteoblastic cells into bone tissue, resulting in the generation of form and function (Reddi, 1984, 2000; Ripamonti, 2006). As a prerequisite to tissue differentiation and morphogenesis, there must exist several signalling molecules or morphogens (Turing, 1952) that are expressed and secreted by selected cells. Morphogens are thus capable of interacting with specific cell surface receptors on responding cells and initiate the pleiotropic ripple-like cascade of pattern formation and the attainment of tissue form and function, or morphogenesis (Reddi, 1984, 2000; Ripamonti & Duneas, 1998; Ripamonti, 2006).
Which are the molecular signals or morphogens as defined by Turing (1952) that initiate bone formation by induction? Although the aforementioned experiments suggested the existence of a putative BMP complex, its identification has been hindered by the fact that the extracellular matrix of bone exists in the solid state and that the small quantities of putative osteogenic proteins are tightly bound to the extracellular matrix (Sampath & Reddi, 1981; Urist et al. 1983; Ripamonti & Reddi, 1995; Reddi, 2000; Ripamonti, 2006). Intact demineralized bone and dentine matrices induce de novo endochondral bone formation in intramuscular and subcutaneous sites of rodents (Urist, 1965; Reddi & Huggins, 1972; Reddi, 1981). The matrix thus contains diffusible morphogens which interact with specific receptors on responding mesenchymal cells, which following attachment to the matrix differentiate into chondroblastic and osteoblastic cell lines synthesizing bone matrix. The matrix subsequently mineralizes to form heterotopic mineralized ossicles complete with bone marrow (Urist, 1965; Reddi & Huggins, 1972; Urist et al. 1983; Reddi, 1994, 2000; Ripamonti & Reddi, 1995; Ripamonti et al. 2005; Ripamonti, 2006).
The critical work of Urist (Urist, 1965; Urist et al. 1967, 1983) recognized the importance of demineralization of mineralized bone matrices to induce reproducible heterotopic endochondral bone and proposed the present terminology, hypothesizing the presence of a BMP complex within the bone matrix (Urist & Strates, 1971; Urist et al. 1983). Demineralization of intact bone and dentine matrices exposes the morphogenetic proteins now only bound to the organic matrix of type I and IV collagens (Sampath & Reddi, 1981; Urist et al. 1983; Ripamonti, 2006). The biochemical and molecular problem of limited morphogenetic proteins bound to both organic and inorganic components of the bone matrix in the solid state was unlocked and resolved by the solubilization of the putative osteogenic proteins from the extracellular matrix of demineralized bone (Sampath & Reddi, 1981). The classic experiments of the chaotropic extraction and reconstitution of the soluble osteogenic molecular signals with an insoluble signal or substratum restored the osteogenic activity of the intact bone matrix lost after chaotropic extraction (Sampath & Reddi, 1981). This critical experiment propelled the phenomenon of bone formation by induction into the preclinical and clinical arenas (Sampath & Reddi, 1981; Ripamonti & Reddi, 1995; Reddi, 2000; Friedlander et al. 2001; Govender et al. 2002; Ripamonti et al. 2005; Ripamonti, 2005, 2006).
The operational reconstitution of the soluble molecular signals with an insoluble substratum or signal was a key experiment that provided a bioassay for the identification of bona fide initiators of bone differentiation (Sampath & Reddi, 1981; Ripamonti et al. 1992a,b, 1993a,b, 2005; Ripamonti & Reddi, 1995; Reddi, 2000; Ripamonti, 2006). The restoration of the biological activity after recombining and reconstituting the soluble signals with an insoluble signal or substratum provided the starting point for the purification of naturally derived BMPs/OPs (Ripamonti, 2006) and was followed by the molecular cloning and expression of the recombinant human proteins (Wozney et al. 1988; Sampath et al. 1992; Reddi, 2000; Ripamonti, 2006). The availability of recombinant human proteins heralded novel molecular therapeutics for regenerative medicine of the 21st century (Reddi, 2000; Friedlander et al. 2001; Govender et al. 2002; Ripamonti et al. 2005; Ripamonti, 2005, 2006).
The foregoing discussion shows that several research experiments hypothesized that unknown substances, either called ‘specific bone forming substance’ (Levander, 1938, 1945), osteogenin (Lacroix, 1945; Sampath et al. 1987; Luyten et al. 1989; Ripamonti et al. 1992a,b,1993a,b) or BMP (Urist & Strates, 1971; Urist et al. 1983), were responsible for the initiation of bone formation by induction. Molecular cloning and expression of the recombinant human proteins (Wozney et al. 1988; Özkaynak et al. 1990) permitted the bioassay of each single recombinant human protein (Kang et al. 2004). Molecular cloning and expression have shown that the BMPs/OPs have sequence homologies with several other gene products involved in axial patterning and differentiation (Wozney et al. 1988; Reddi, 1994, 2000; Ripamonti, 2006) and because of the characteristic seven-cystein residue within the carboxy terminal domain of the proteins, the BMPs/OPs are members of the TGF-β supergene family (Wozney et al. 1988).
Last century's research has shown unequivocally that the induction of bone, i.e. the de novo endochondral bone formation in heterotopic sites of animal models, can be induced by intact demineralized extracellular matrices including demineralized bone and dentine matrices (Urist, 1965, 1994; Reddi & Huggins, 1972; Reddi, 1981; Urist et al. 1983). A quantum leap towards the mechanistic understanding of the phenomenon of bone: formation by autoinduction (Urist, 1965) has been achieved by the dissociative extraction and reconstitution of the bone matrix components (Sampath & Reddi, 1981). These experiments led eventually to the initiation of clinical trials, which have culminated in the use of recombinant hOP-1 and hBMP-2 in clinical contexts (Friedlander et al. 2001; Govender et al. 2002; Ripamonti, 2006). Purification to homogeneity of naturally derived BMPs/OPs from bovine and baboon bone matrices (Wang et al. 1988; Luyten et al. 1989; Ripamonti et al. 1992a,b) has finally resolved the ‘reality of a nebulous enigmatic myth’ (Urist, 1968). Molecular, preclinical and clinical research has dispelled the myth and as Lacroix postulated in the middle of the last century, research has shown that ‘the possibility of promoting osteogenesis at will is really within easy reach’ in the current century (Lacroix, 1945).
A different approach to bone: formation by induction (Urist, 1965) has been the emerging strategy of directing the differentiation of stem cells into the osteogenic lineage (Heng et al. 2004). Stem cell differentiation is now a major area of interest in regenerative medicine together with the potential role of adult-derived precursor stem cells as building blocks for regenerative medicine, tissue engineering and as vehicles for molecular medicine (Young et al. 2005). A different approach for the in vivo engineering of large predictable volumes of autogenous bone has also been proposed (Stevens et al. 2005). The crux of the approach rests in the deliberate creation and manipulation of an artificial space, defined as the bone bioreactor, between the tibia and the periosteum, a mesenchymal layer rich in pluripotent cells endowed with the potential to differentiate rapidly into osteoblastic-like cells secreting bone matrix (Stevens et al. 2005). The creation and manipulation of the bone bioreactor resulted in the induction of bone formation as the bioreactor space was filled by functional living bone (Stevens et al. 2005). In experiments in rabbits, transplantation of the engineered tissue constructs into contralateral tibial defects resulted in complete integration of the transplanted autogenous bone 45 days after transplantation with no apparent morbidity at the donor site (Stevens et al. 2005).
Perhaps, however, the most exciting way to initiate bone: formation by autoinduction (Urist, 1965) is to construct biomaterial matrices that per se are endowed with the striking prerogative of initiating the induction of bone formation without the exogenous application of BMPs/OPs, i.e. smart biomimetic matrices capable of differentiating osteoblast-like cells to secrete osteogenic gene products of the TGF-β superfamily, later embedding the secreted molecular signals in specific geometric configurations to self-induce bone tissue constructs in angiogenesis (Ripamonti et al. 1999, 2004, 2005, 2006; Ripamonti, 2004a, 2006). This novel strategy of tissue engineering of bone for the 21st century is highlighted below as a challenge for the future.
Osteogenesis in angiogenesis
Angiogenesis is a prerequisite for osteogenesis (Fig. 5A) (Trueta, 1963). The use of both naturally derived and recombinantly produced BMPs/OPs has indicated that capillary sprouting and invasion within the implanted matrix scaffold of the delivery system is critical for osteoblast cell differentiation and synthesis (Fig. 5B). The precursor experimental work of Levander (1938) and Trueta (1963) stressed that the specific bone-forming substance(s) (although uncertain of its nature and even its existence) (Trueta, 1963) ‘operate directly on the vascular system causing an angioblastic specific stimulation on the bone vessels of the recipient’ (Trueta, 1963). Trueta (1963) named ‘this unknown substance the vascular stimulating factor (V.S.F.)’, a first definition for the existence of the vascular endothelial growth factor (VEGF), a critical morphogen in bone formation by induction and maintenance of the induced bone constructs (Leung et al. 1989; Byrne et al. 2005) together with the endothelial cell-derived basic fibroblast growth factor (Wlodavsky et al. 1987).
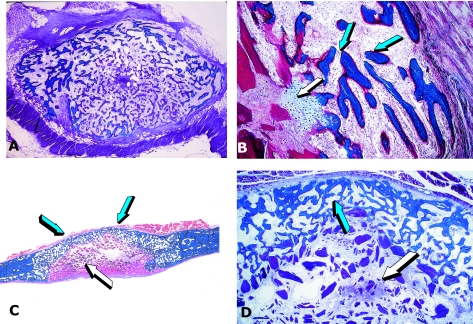
Fig. 5.
Osteogenesis in angiogenesis in rodents and the non-human primate Papio ursinus. (A) Capillary invasion and elongation (white arrows) within the chondrogenic matrix with hyperthrophic chondrocytes (blue arrow) of an embryonic growth plate. Chondrolysis and differentiation of osteoblastic-like cells (red arrows) secreting bone matrix. (B) Osteogenin (0.1–0.5 µg) purified to apparent homogeneity from baboon bone matrix induces osteoblastic cell differentiation (white arrows) with capillary invasion in very close proximity to the differentiated and secreting osteogenic cells and with vascular cells migrating from the vascular compartment (blue arrow) to the bone-forming compartment. (C,D) Capillary invasion as a scaffold for the induction of bone formation; each central vessel is surrounded by cellular condensation (white arrows) forming the Haversian canal system of the osteonic and remodelling bone of primates with newly formed trabeculae of mineralized bone (blue arrow). (E,F) Cellular condensation with foci of mineralization (blue arrows) surfaced by osteoblast-like cells (white arrows) facing the central blood vessels.
The critical role of the vessels in osteogenesis had been appropriately postulated by Levander (1938) who described in detail the tissue induced by alcoholic extracts of bone matrices as characterized by prominent capillary invasion surrounded by condensations of perivascular cells. Levander (1938) further suggested that the fully formed mesenchymal cells differentiating into osteoblastic-like cells ultimately emanate from the endothelial cells of the invading capillaries. This has supported the suggestion of Sir Arthur Keith that the cells that assume the bone-forming role are derived from the endothelium of the invading capillaries (Keith, 1927). The critical importance of angiogenesis as a prerequisite for osteogenesis has been fully described by Trueta (1963) who introduced and defined the ‘osteogenetic vessels’ and its contribution to the induction of bone.
The morphological studies of Trueta (1963) highlighted that endothelial cells of the vessels of the Haversian canals are in immediate contact with the lining osteoblasts, suggesting that all these cells, from endothelium to osteocyte, remain attached by intercellular cytoplasmic connections. The development of long interconnecting cell processes similar to the canalicular network observed in bone has been observed during in vitro experiments of osteoblast-like cells cultured on basement membrane matrix components when regulated by specific molecular domains of laminin (Vukicevic et al. 1990). The results obtained in vitro prompted the authors to indicate that in vivo the ‘memory’ of the contact of both endothelial cells and osteoblasts with laminin of the basement membrane sets into motion a ripple-like cascade of cell differentiation within the osteogenetic vessels, leading to continuous osteoblastic-cell differentiation and bone matrix synthesis (Vukicevic et al. 1990).
Trueta (1963) described the genesis of newly formed bone as dictated by the radiating vessels with differentiation and deposition of bone as provisional trabeculae of mesenchymal condensations differentiating around each newly formed capillary. Trueta (1963) reported the genesis of osteogenetic vessels exactly moulding the newly forming bone on the vascular pattern. Unique morphological images of bone initiating in angiogenesis by moulding on the vascular pattern are shown in Fig. 5(C–F). Cellular condensations around each capillary or ‘osteogenetic vessel’ delineate the three-dimensional morphological structure of the primate Haversian bone with foci of mineralization within condensations eventually leading to the complex sculpting of the Haversian canal system (Fig. 5C–F). Remarkably, invading capillaries sprouting within interconnected porosities of implanted scaffolds in P. ursinus show cellular layers of highly stained alkaline phosphatase (Ripamonti et al. 1993a,b).
Biological significance of redundancy and synergistic interaction
Studies in the primate P. ursinus have provided evidence that the hOP-1 osteogenic device directly influences the expression levels of OP-1, type IV collagen, BMP-3 and TGF-β1 mRNAs during the induction of bone formation both in heterotopic and in orthotopic calvarial sites with both increases and decreases of mRNA expression (Ripamonti, 2005, 2006). In marked contrast to results obtained in other animal species, i.e. rodents, we have shown that the TGF-β isoforms so far tested in our laboratories are endowed with the striking prerogative of initiating bone formation by induction when implanted in heterotopic sites of P. ursinus (Ripamonti et al. 1997, 2000a; Duneas et al. 1998; Ripamonti, 2003, 2004a,b, 2006). When using the hOP-1 osteogenic device, the temporal and spatial expressions of TGF-β1 mRNAs are relatively high on day 30 as compared with expression patterns on days 15 and 90. This suggested a specific temporal window during which TGF-β1 mRNA expression is mandatory for optimal osteogenesis (Ripamonti, 2005, 2006).
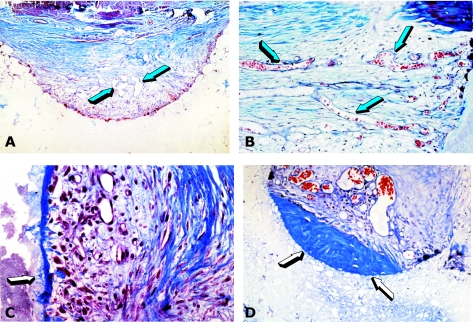
The pleiotropy of the signalling molecules of the TGF-β superfamily and the apparent redundancy of molecular signals initiating endochondral bone induction exclusively in the primate (Ripamonti et al. 1997, 2000a,b; Ripamonti, 2003, 2004a,b, 2006) are further emphasized by the finding that ebaf/Lefty-A, a new member of the TGF-β superfamily, induces chondrogenesis and bone regeneration in calvarial defects of the baboon on days 30 and 90, respectively (Fig. 6) (Ripamonti, 2004b, 2006; Ripamonti et al. 2005).
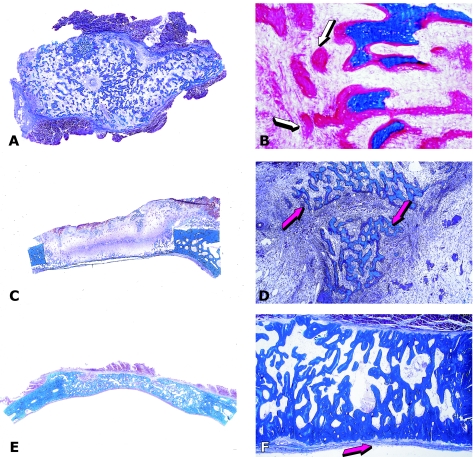
Fig. 6.
Induction of chondro-osteogenesis by 100 µg ebaf/Lefty-A protein in calvarial defects of the baboon. (A) Chondrogenesis by ebaf/Lefty-A 30 days after implantation in a calvarial defect delivered by 1 g of allogeneic insoluble collagenous matrix as carrier. (B) Osteogenesis across the defect 90 days after implantation of 100 µg ebaf/Lefty-A. (C,D) Details of previous section showing newly formed mineralized bone in blue surfaced by osteoid seams facing newly formed diploic marrow spaces (red arrows).
BMPs/OPs and TGF-β proteins act in concert to initiate singly, synchronously and synergistically the attainment of tissue form and function during the cascade of osteogenic differentiation initiated by the hOP-1 osteogenic device (Ripamonti, 2005, 2006). The temporal pattern of gene expression in both heterotopic and orthotopic sites indicates a sequence of steady-state RNA expression during osteogenic differentiation and the induction of bone formation. The high expression levels of auto-induced OP-1 mRNA together with high expression of type IV collagen mRNA mechanistically explains the continuous sustained osteogenesis in angiogenesis as evaluated by morphology and histomorphometry (Ripamonti, 2005).
In the bona fide bioassay for bone induction in heterotopic sites of rodents, the TGF-β isoforms, either purified from natural sources or expressed by recombinant techniques, do not initiate endochondral bone formation (Roberts et al. 1986). Strikingly, however, the TGF-β isoforms so far tested in non-human primates have shown a remarkable osteoinductive activity when implanted in heterotopic sites of the rectus abdominis muscle of adult P. ursinus (Fig. 7) (Ripamonti et al. 1997; Duneas et al. 1998; Ripamonti, 2000, 2003, 2004a,b, 2006).
Fig. 7.
Redundancy of soluble molecular signals initiating endochondral bone formation in the non-human primate Papio ursinus. (A,B) Low-magnification views of large ossicles induced after heterotopic implantation of 5 µg hTGF-β1 (A) and 25 µg hTGF-β2 delivered by 100 mg of allogeneic insoluble collagenous matrix as carrier; (B) island of chondrogenesis (white arrows) surrounded by trabeculae of newly formed and mineralized bone surfaced by osteoid seams (blue arrows). (C,D) Osteogenesis, albeit thinner than the original calvarium, across the defect 90 days after implantation of 100 µg hTGF-β2 delivered by 1 g of allogeneic collagenous bone matrix as carrier. Pericranial osteogenesis (blue arrows) but lack of endocranial osteogenesis above the dural layer with scattered remnants of the collagenous matrix (white arrow). (D) Detail of the pericranial central region as shown in C showing newly formed mineralized bone (blue arrow) just beneath the pericranium with scattered remnants of the collagenous matrix as carrier (white arrow) below the newly formed mineralized bone in blue.
Orthotopic calvarial implantation of the tested TGF-β isoforms does not induce bone formation in calvarial defects on day 30; there is limited pericranial bone formation across the defect on day 90 post-implantation only (Fig. 7C,D). The presence of molecularly related but different soluble signals with osteogenic activity in the primate only (Ripamonti, 2003, 2004b, 2006) raises important questions about the biological significance of this apparent redundancy. Moreover, these multiple interactions during embryonic development may be of great importance when designing regenerative therapies in clinical contexts (Ripamonti, 2003, 2004a,b, 2006).
It is likely that the endogenous mechanisms of bone repair and regeneration necessitate the deployment and concerted action of several BMPs/OPs resident within the natural milieu of the extracellular matrix (Ripamonti et al. 2000a,b; Ripamonti, 2003, 2004b). The presence of molecularly different but homologous molecular signals with osteogenic activity only in the primate also points to synergistic interactions both in embryonic development and in postnatal tissue regeneration and homeostasis (Ripamonti, 2003, 2004a, 2006). We have shown in non-human primates that relatively low doses of naturally derived or DNA-recombinantly produced TGF-β1 synergize with hOP-1 to induce massive heterotopic ossicles in the rectus abdominis muscle as early as 15 days after heterotopic implantation (Fig. 8) as well as rapid and synergistic regeneration of non-healing calvarial defects in P. ursinus (Ripamonti et al. 1997, 2000a,b; Duneas et al. 1998; Ripamonti, 2003, 2004a,b, 2006).
Fig. 8.
Synergistic interaction and rapid induction of bone by binary applications of recombinant hOP-1 and hTGF-β1 in the rectus abdominis of adult baboons. (A) Large heterotopic ossicle induced after binary application of 25 µg hOP-1 and 1.5 µg hTGF-β1 15 days after implantation. (B) Large corticalized heterotopic ossicle generated 30 days after the binary application of 25 µg hOP-1 with 0.5 µg hTGF-β1. (C,D) Large heterotopic constructs after binary applications of hOP-1 (25 µg) and doses of hTGF-β1 with generation of tissue constructs with chondrogenic induction (arrowheads in D) resembling a rudimentary embryonic growth plate.
Deploying biological principles for human osteoinduction
The repair of skeletal defects in a human patient most often requires the harvesting of bone from a distant donor site. The current gold standard for the repair of skeletal defects in humans is autogenous bone, which requires the harvesting from a distant site, creating donor site morbidity (Habal, 1994). A further limitation is the finite volume of bone available from any one donor site. Adapting the donor bone to fit the shape of the recipient defect is a final challenge to autogenous bone grafting. Although embryonic osteogenesis is the result of very complex spatial and temporal interactions of several members of the TGF-β superfamily, deploying bone formation by induction to repair skeletal defects in humans may not require perfect recapitulation of embryonic or postnatal events. With this in mind, and in view of the demonstrated apparent redundancy among osteoinductive morphogens, a strategy for human osteoinduction can be developed that is based on the application of a single morphogen with a viable substratum or delivery system and may provide a suitable alternative to the harvesting of bone for regenerative medicine in clinical contexts. Successful application of this biotechnology requires consideration of several key aspects.
The choice of a suitable morphogen remains pragmatic as no morphogen has shown performance superiority at equivalent doses over another (Kang et al. 2004). It is therefore likely that any of the recombinant hBMPs/OPs are suitable for human application. Naturally derived BMPs/OPs intuitively provide a synergistic combination of proteins that may reflect the natural milieu of the extracellular bone matrix as evolved from embryonic bone development and thus may enjoy biological advantages over recombinant human proteins (Ripamonti et al. 2000a; Ripamonti, 2003). Highly purified naturally derived BMPs/OPs delivered by human demineralized bone matrix as carrier have been used to treat human mandibular defects (Fig. 3D–F) (Ferretti & Ripamonti, 2002; Ripamonti & Ferretti, 2002). To date, the only two recombinant proteins tested are hOP-1 and hBMP-2, particularly in spinal fusion and tibial non-union models (Groeneveld & Burger, 2000; Friedlander et al. 2001; Govender et al. 2002).
The complex consideration of dose requires further elucidation. In vitro effects become evident at concentrations at the femto- and nanomolar ranges. However, clinically significant induction of bone is only achieved in the milligram range of BMPs/OPs, both naturally derived and recombinant human proteins. It has been well established also in primates (Ripamonti et al. 1996, 2000a,b; Ripamonti, 2005) that the induction of bone by BMPs/OPs is dose-dependent with none occurring below a threshold dose and increasing in a dose-dependent manner to a peak. Achievement of successful osteoinduction therefore requires above-threshold doses, but to remain economically viable selected doses must not exceed maximal effect. The extrapolation of data from animal models is difficult, including from non-human primate species, as it appears that dosage is species-specific. The result is that clinical trials have used doses that are several hundred-fold greater than the doses suggested by results in animal models and by the concentration of BMPs/OPs in mammalian bone (Friedlander et al. 2001; Govender et al. 2002, Boyne et al. 2005).
It is unclear whether lower doses in humans would still be effective; however, these supraphysiological doses suggest both ineffective and/or limited biological activity after DNA recombination processes and a flaw in the single morphogen approach to human osteoinduction for regenerative medicine. First, the glycosilation of the recombinant protein after DNA processing and secretion may not be optimal, reducing the osteogenic activity of the recombinant protein. Secondly, the substantially increased efficacy of binary applications of hOP-1 with relatively low doses of human recombinant and naturally derived TGF-β proteins has been well documented in P. ursinus and may provide an alternative that more closely resembles the mammalian osteoinductive cascade when applied in clinical contexts (Ripamonti et al. 1997, 2000a,b, 2001; Duneas et al. 1998; Ripamonti, 2003, 2004, 2006).
Additionally, synergistic binary applications provide a highly inductive therapeutic option, resulting in osteogenic devices that initiate rapid bone formation of critical importance in elderly patients subjected to delayed repair phenomena (Ripamonti et al. 1997; Ripamonti, 2003, 2006). It is therefore possible that more accurate replication of the developmental osteogenic cascade (which would require multiple growth factors) will result in superior clinical performance at considerably reduced doses but at the cost of greatly increased complexity. Finally, a dose strategy for an osteogenic device must account for the proposed recipient site. Highly vascular sites such as skeletal muscle and healthy skeletal sites require lower doses to produce viable bone, whereas subcutaneous sites, and maxillary sinus require greater doses (Groeneveld &, Burger, 2000, Boyne et al. 2005).
The therapeutic deployment of morphogens for osteoinduction requires both spatial and temporal control and this is provided by the substratum or delivery vehicle. An ideal delivery vehicle should be adaptable to the various shapes of bone defects, provide immediate structural support to the reconstructed bone and elicit a minimal immunological response (Ripamonti et al. 2001; Ripamonti, 2006). Once implanted it must temporally control diffusion of the selected morphogen. Although great strides have been made in elucidating the time frame of gene expression during bone formation by induction (Chen et al. 1997; Honda et al. 1997; Yeh et al. 2000; Roman-Roman et al. 2003; Spector et al. 2001; Ripamonti, 2005) it must be conceded that at this stage the application of this knowledge in clinical settings is not possible and close control of morphogen diffusion rates are out of our reach. Nevertheless, it is unclear whether more accurate replication of the temporal sequence of gene expression during bone formation will translate into improved clinical performances. Importantly, the delivery system should undergo resorption closely coupled to the advancing osteogenetic front. With this in mind several delivery systems have been used, including insoluble collagenous bone matrices, other collagen derivatives, resorbable synthetic biomaterials and hydroxyapatite, all of which have certain advantages but all of which fail to provide the requirements for an ideal delivery system. The development of smart biomaterials that induce bone without exogenously applied osteogenic proteins of the TGF-β supergene family is an alternative that is currently in its infancy but is a promising avenue for future research (Ripamonti, 2006; Ripamonti et al. 2006).
A final and critical consideration for human application of bone: formation by autoinduction (Urist, 1965) is the suitability of the donor site. Bone induction requires not only appropriate morphogens and substrata but also responding cells. It therefore follows that clinically successful induction can occur only in surgical sites that are highly vascular and enjoy high cellularity. The majority of skeletal defects in human patients are plagued by hypovascularity, hypocellularity and fibrosis due to repeated surgeries, severe trauma, infection and radiation. Therefore, a recipient bed needs to be carefully assessed before the decision to proceed with the deployment of BMPs/OPs in clinical contexts.
The currently accepted treatment modality to bypass the above problem is the incorporation of vascularized composite myo-osseous and osseo-myocutaneous flaps from distant sites. The disadvantages of such flaps are their donor site morbidity, sometimes limited availability and difficulty in shaping the bone flap to fit the anatomical recipient defect. Recently, the bone induction principle has been exploited further to address the above-mentioned problems in reconstructive surgery. By implanting biomaterials with recombinant morphogens in distant healthy, vascular and tissue-rich sites, we can use the body as a bioreactor to induce heterotopic bone in custom-made, prefabricated shapes that fit the requirements of the recipient defect. The concept of using BMPs and prefabricating specific bone shapes was first demonstrated by Khouri et al. (1991) in rodents.
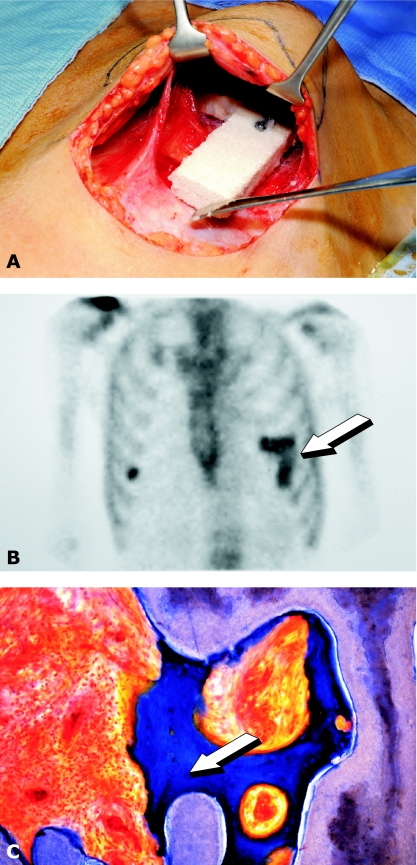
Two previously published cases of custom-made bone grafts grown in extraskeletal sites in humans deployed a cocktail of BMPs and cancellous bone implanted in the dorsal fascia, caudal to the scapula (Orringer et al. 1999) and hOP-1 and bone marrow aspirate from the iliac crest implanted into the latissimus dorsi muscle (Warnke et al. 2004). We went further by clearly demonstrating that with a single recombinant morphogen (hOP-1) and a hydroxyapatite carrier, we could induce osteogenesis in a human outside the skeleton, without the addition of cortical bone, bone marrow aspirates or any other bone precursors to engineer a custom-made prefabricated bone flap for human reconstruction (Fig. 9) (Heliotis et al. 2006). By using healthy body sites as bioreactors, we could recapitulate embryonic events by inducing the biomaterial matrix combined with morphogens to transform into custom-made, prefabricated bone grafts for human reconstruction (Heliotis et al. 2006). Hence, by combining osteoinduction with established reconstructive techniques, we have extended the reconstructive algorithm to include bioengineered flaps (Heliotis et al. 2006).
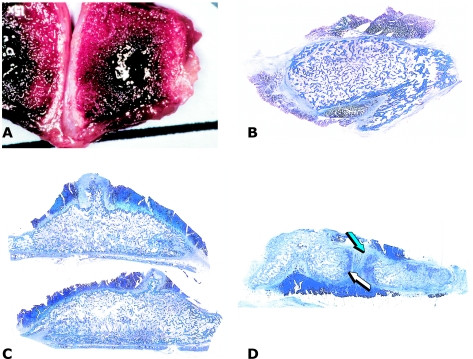
Fig. 9.
Heterotopic bone induction in humans and transplantation of the newly formed bone in a mandibular defect after ablative surgery. (A) Insertion of a porous hydroxyapatite scaffold combined with hOP-1 into the pectoralis major muscle. (B) Scintigraphic image demonstrating osteogenesis in the prefabricated heterotopic implant (arrow). (C) Undecalcified section of bioptic material showing newly formed bone by induction (arrow) attached to the hydroxyapatite scaffold.
Challenges for the future
Tissue engineering starts by erecting scaffolds of smart biomimetic matrices controlling the expression of soluble molecular signals of the TGF-β and VEGF supergene families (Ripamonti, 2006). Newly developed biomimetic matrices for bone tissue engineering and regenerative medicine should be designed to obtain specific biological responses (Ripamonti, 2006; Ripamonti et al. 2006). The use of biomaterials capable of initiating bone formation via osteoinduction is fast altering the horizons of therapeutic regenerative medicine (Reddi, 1994, 2000; Ripamonti et al. 1993a,b, 1999, 2002, 2004; Ripamonti, 2000, 2002, 2004,b, 2006). Our studies on bone tissue engineering in the past several years have focused on the critical role of the geometry of biomimetic matrices used as scaffold for bone regeneration (Ripamonti et al. 1993a, 1999, 2006; Ripamonti, 2000, 2002, 2004a,b, 2006), providing evidence that tissue induction and morphogenesis can be greatly altered by the geometry of the carrier as shown in rodents (Reddi & Huggins, 1973; Ripamonti et al. 1992a,b).
Two recent reviews have focused on the role of the geometric configuration of a variety of carriers controlling the phenotypic expression of bone formation with and without the exogenous applications of BMPs/OPs from rodents to non-human primates (Kuboki et al. 2001; Ripamonti et al. 2001). The seminal work of the geometry of the carrier substratum has been shown in heterotopic sites of rodents by Reddi & Huggins (1973). Several studies in vitro (Gray et al. 1996) and in vivo (Kuboki et al. 2001; Ripamonti et al. 2001, 2006; Ripamonti, 2006) followed in an attempt to design and fabricate biomimetic matrices endowed with the striking prerogative of initiating bone formation by induction even without the exogenous application of the soluble molecular signals of the TGF-β superfamily (Ripamonti, 2006; Ripamonti et al. 2006).
We have developed biomimetic biomaterial matrices endowed with intrinsic osteoinductivity, capable of initiating de novo bone formation in heterotopic sites of primates even in the absence of exogenously applied osteogenic proteins of the TGF-β superfamily, i.e. biomaterial matrices that per se initiate bone differentiation in extraskeletal sites of P. ursinus (Fig. 10) (Ripamonti et al. 1999, 2004, 2006; Ripamonti, 2000, 2004a,b, 2006). The intrinsic osteoinductivity is determined by the specific geometric configuration of the substratum; the presence of a series of repetitive concavities across the porous spaces of the biomimetic matrices initiates the ripple-like cascade of bone differentiation by induction (Ripamonti et al. 1999; Ripamonti, 2006). To trigger the cascade of tissue induction and morphogenesis, the osteogenic molecular signals of the TGF-β supergene family require the reconstitution with an insoluble signal or substratum (Ripamonti, 2006). The three critical requirements for successful tissue engineering of bone are a suitable extracellular matrix substratum, responding cells and soluble osteogenic molecular signals interacting with cell-surface receptors on responding cells (Reddi, 2000).
Fig. 10.
Self-inducing geometric cues and the induction of bone formation in heterotopic intramuscular sites of the baboon. (A) Angiogenesis and capillary invasion (arrows) within the soft tissues invading the concavity of a hydroxyapatite biomimetic matrix 30 days after implantation in the rectus abdominis muscle. (B) Prominent capillary invasion with elongation of the newly formed vessels (arrows) almost touching the biomimetic matrix 30 days after implantation of the biomimetic scaffold. (C) Induction of bone that had formed within the concavity of the biomimetic matrix (arrow) in close relationship with invading capillaries on day 30 after implantation. (D) Newly formed bone by induction and invading sprouting capillaries attached to the concavity of the biomimetic matrix (arrows) and harvested on day 90 after implantation in the rectus abdominis muscle.
Our research work was set to answer the following questions: can we engineer biomimetic matrices that direct cell differentiation and expression of selected mRNA species of the TGF-β superfamily embedded within specific geometric cues of the biomimetic matrix, i.e. a smart bioactive scaffold that in its own right expresses the molecular signals endowed with the striking prerogative of initiating angiogenesis culminating in self-induced osteogenesis (Ripamonti et al. 1999, 2006; Ripamonti, 2004a,b, 2006)? We have thus designed and tested a solid-state porous sintered hydroxyapatite regulatory matrix in which self-assembled repetitive sequences of concavities induce angiogenesis, culminating in the differentiation of osteoblastic-like cells expressing gene products of the TGF-β supergene family (Ripamonti, 2006; Ripamonti et al. 2006). Expression of mRNA species, i.e. OP-1, BMP-3, TGF-β1 and collagen type IV by Northern blot analyses, is followed by the embedding of the secreted gene products into the smart concavities, resulting in the induction of osteogenesis in angiogenesis as a secondary response. The induction of bone formation is preceded by induced capillary extensions towards the matrix almost touching differentiating osteoblastic-like cells resting upon the smart concavities of the substratum (Fig. 10) (Ripamonti et al. 1999, 2006; Ripamonti, 2006).
To conclude, to induce the cascade of bone differentiation by induction, the osteogenic soluble molecular signals of the TGF-β superfamily must be reconstituted with insoluble signals or substrata to trigger the bone differentiation cascade (Reddi, 2000; Ripamonti, 2006). We now propose a biomimetic biomaterial matrix that per se and intrinsically expresses the mRNA of selected osteogenic gene products of the TGF-β superfamily. Expression is followed by secretion and embedding of the molecular signals within the smart concavities that initiate bone formation by induction as a secondary response. The geometric induction of bone formation (Ripamonti et al. 1999; Ripamonti, 2000, 2006) is based on the manipulation of the geometric configuration of the biomimetic matrix that engineers regeneration by invocation of a sequence of molecular and cellular events recapitulated within the smart concavity of the substratum (Ripamonti, 2006; Ripamonti et al. 2006).
We have developed a solid-state biomimetic matrix in which concavities differentiate osteoblastic-like cells and immobilize osteogenic gene products of the TGF-β supergene family as secreted directly to the matrix within its regulatory concavities (Ripamonti, 2006). Soluble signals induce morphogenesis; physical forces imparted by the geometric topography of the insoluble signal dictate biological patterns, constructing the induction of bone by regulating the expression of selective mRNA of gene products as a function of the structure (Ripamonti, 2004a, 2006; Ripamonti et al. 2004, 2006). We propose the connubium of smart biomatrices self-inducing specific gene products resulting in tissue morphogenesis regulated by the geometry of the substratum as the true challenge for tissue engineering for the 21st century.
Acknowledgments
This work is supported by the South African Medical Research Council, the University of the Witwatersrand, Johannesburg, the National Research Foundation and by ad hoc grants of the Bone Research Unit. We thank Stryker Biotech, USA, for the preparation of the hOP-1 osteogenic devices, the Council for Scientific and Industrial Research for the preparation of the sintered biomaterial matrices, and Barbara van den Heever, June Teare and Louise Renton for unparalleled stained, free-floating undecalcified sections of bone, Laura Yeates, Karolina Kuun, Janet Patton, Thato Matsaba and N. L. Ramoshebi for the molecular and Northern blot analyses, the Central Animal Services of the University of the Witwatersrand for help with primate experimentation, Gilly Haagensen of the Photo Unit of the University of the Witwatersrand for compiling the digital images of the undecalcified sections, and Kathleen Ripamonti, Maxine Ferretti and Vivienne Griffith for critical understanding and encouragement. We are indebted to Marshall Urist and Hari A Reddi for having initiated us into the phenomena of bone: formation by autoinduction.
References
- Åberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn. 1997;210:383–396. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bertelsen A. Experimental investigations into post-foetal osteogenesis. Acta Orthop Scand. 1945;15:139–181. [Google Scholar]
- Boyne PJ, Lilly LC, Marx RE, et al. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005;63:1693–1707. doi: 10.1016/j.joms.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Bridges JB, Pritchard JJ. Bone and cartilage induction in the rabbit. J Anat. 1958;92:28–38. [PMC free article] [PubMed] [Google Scholar]
- Byrne AM, Bouchier-Hayes DJ, Harmay JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Harris MA, Rossini G, et al. Bone morphogenetic protein-2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60:283–290. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Duneas N, Crooks J, Ripamonti U. Transforming growth factor-β1: induction of bone morphogenetic protein genes expression during endochondral bone formation in the baboon, and synergistic interaction with osteogenic protein-1 (BMP-7) Growth Factors. 1998;15:259–277. doi: 10.3109/08977199809017482. [DOI] [PubMed] [Google Scholar]
- Ferretti C, Ripamonti U. Human segmental mandibular defects treated with naturally derived bone morphogenetic proteins. J Craniofacial Surg. 2002;13:434–444. doi: 10.1097/00001665-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Friedlander GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg. 2001;83A:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- Gañan Y, Macias D, Duterque-Coquillaud M, Ros MA, Hurle JM. Role of TGF-βs and BMPs as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development. 1996;122:2349–2357. doi: 10.1242/dev.122.8.2349. [DOI] [PubMed] [Google Scholar]
- Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg. 2002;84A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Gray C, Boyde A, Jones SJ. Topographically induced bone formation in vitro: implications for bone implants and bone grafts. Bone. 1996;18:115–123. doi: 10.1016/8756-3282(95)00456-4. [DOI] [PubMed] [Google Scholar]
- Groeneveld EH, Burger EH. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000;142:9–21. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- Habal MB. Bone grafting in craniofacial surgery. Clin Plast Surg. 1994;21:349–363. [PubMed] [Google Scholar]
- Heikinheimo K. Stage-specific expression of decapentaplegic-Vg-related genes 2, 4 and 6 (bone morphogenetic protein 2, 4 and 6) during human tooth morphogenesis. J Dent Res. 1994;73:590–597. doi: 10.1177/00220345940730030401. [DOI] [PubMed] [Google Scholar]
- Helder MN, Özkaynak E, Sampath KT, et al. Expression of osteogenic protein-1 (bone morhogenetic protein-7) in human mouse development. J Histochem Cytochem. 1995;43:1035–1044. doi: 10.1177/43.10.7560881. [DOI] [PubMed] [Google Scholar]
- Heliotis M, Lavery KM, Ripamonti U, Tsiridis E, Di Silvio L. Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest. Int J Oral Maxillofac Surg. 2006;35:265–269. doi: 10.1016/j.ijom.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Heng BC, Cao T, Stanton LW, Robson P, Olsen B. Strategies for directing the differentiation of stem cells into the osteogenic lineage in vitro. J Bone Min Res. 2004;19:1379–1394. doi: 10.1359/JBMR.040714. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Honda Y, Knutsen R, Strong DD, Sampath TK, Baykink DJ, Mohan S. Osteogenic protein-1 stimulates mRNA levels of BMP-6 and decreases mRNA levels of BMP-2 and -4 in human osteosarcoma cells. Calcif Tissue Int. 1997;60:297–301. doi: 10.1007/s002239900232. [DOI] [PubMed] [Google Scholar]
- Huggins CB. The formation of bone under the influence of epithelium of the urinary tract. Arch Surg. 1931;22:377–408. [Google Scholar]
- Kang Q, Sun MA, Cheng H, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- Keith A. Concerning the origin and nature of osteoblasts. Proc Royal Soc Med. 1927;21:301. [PMC free article] [PubMed] [Google Scholar]
- Khouri RK, Koudsi B, Reddi AH. Tissue transformation into bone in vivo. A potential practical application. J Am Med Assoc. 1991;226:1953–1955. [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signalling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg. 2001;83-A S1:105–115. [PubMed] [Google Scholar]
- Lacroix P. Recent investigations on the growth of bone. Nature. 1945;156:576. [Google Scholar]
- Leung DW, Cachines G, Kuang WJ, Goeddel DW, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Levander G. A study of bone regeneration. Surg Obster. 1938;67:705–714. [Google Scholar]
- Levander G. Tissue induction. Nature. 1945;155:148–149. [Google Scholar]
- Levander G, Willestaedt H. Alcohol-soluble osteogenetic substance from bone marrow. Nature. 1946;3992:587. doi: 10.1038/157587b0. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Broncers ALJJ, Sohocki M, Bradley A, Karsenty G. BNP-7 is an inducer of nephrogenesis and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Cunningham NS, Ma S, et al. Purification and partial amino-acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem. 1989;264:13377–13380. [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signalling: transcriptional targets, regulation of signals, and signalling cross-talks. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Moss ML. Extraction of an osteogenic inductor factor from bone. Science. 1958;127:755–756. doi: 10.1126/science.127.3301.755. [DOI] [PubMed] [Google Scholar]
- Orringer JS, Shaw WW, Borud LJ, Freymiller EG, Wang SA, Markowitz BL. Total mandibular and lower lip reconstruction with a prefabricated osteocutaneous free flap. Plast Reconstr Surg. 1999;104:793–797. doi: 10.1097/00006534-199909030-00028. [DOI] [PubMed] [Google Scholar]
- Özkaynak E, Rueger DC, Drier EA, et al. OP-1 cDNA encodes an osteogenic protein in the TGF-β family. EMBO J Org. 1990;9:2085–2093. doi: 10.1002/j.1460-2075.1990.tb07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi AH, Huggins CB. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci USA. 1972;69:1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi AH, Huggins CB. Influence of geometry of transplanted tooth and bone on transformation of fibroblasts. Proc Soc Exp Biol Med. 1973;143:634–637. doi: 10.3181/00379727-143-37381. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Cell biology and biochemistry of endochondral bone development. Coll Relat Res. 1981;1:209–226. doi: 10.1016/s0174-173x(81)80021-0. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Extracellular matrix and development. In: Piez KA, Reddi AH, editors. Extracellular Matrix Biochemistry. New York: Elsevier; 1984. pp. 375–412. [Google Scholar]
- Reddi AH. Symbiosis of biotechnology and biomaterials: applications in tissue engineering of bone and cartilage. J Cell Biochem. 1994;56:192–195. doi: 10.1002/jcb.240560213. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nature Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351–359. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- Reddi AH. BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 2005;16:249–250. doi: 10.1016/j.cytogfr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Ma S, Cunningham N, Yates L, Reddi AH. Initiation of bone regeneration in adult baboons by osteogenin, a bone morphogenetic protein. Matrix. 1992a;12:369–380. doi: 10.1016/s0934-8832(11)80033-8. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Ma S, Reddi AH. The critical role of geometry of porous hydroxyapatite delivery system in induction of bone by osteogenin, a bone morphogenetic protein. Matrix. 1992b;12:202–212. doi: 10.1016/s0934-8832(11)80063-6. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, van den Heever B, van Wyk J. Expression of the osteogenic phenotype in porous hydroxyapatite implanted extraskeletally in baboons. Matrix. 1993a;13:491–502. doi: 10.1016/s0934-8832(11)80115-0. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Ma S, Cunningham N, Yeates L, Reddi AH. Reconstruction of bone-bone marrow organ by osteogenin, a bone morphogenetic protein, and demineralized bone matrix in calvarial defects of adult primates. Plast Reconstr Surg. 1993b;91:263–267. doi: 10.1097/00006534-199301000-00005. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Reddi H. Bone morphogenetic proteins: applications in plastic and reconstructive surgery. Adv Plast Reconstr Surg. 1995;11:47–73. [Google Scholar]
- Ripamonti U, van den Heever B, Sampath TK, Tucker MM, Rueger DC, Reddi AH. Complete regeneration of bone in the baboon by recombinant human osteogenic protein-1 (hOP-1, bone morphogenetic protein-7) Growth Factors. 1996;13:273–289. doi: 10.3109/08977199609003228. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Duneas N, van den Heever B, Bosch C, Crooks J. Recombinant transforming growth factor-β1 induces endochondral bone in the baboon and synergizes with recombinant osteogenic protein-1 (bone morphogenetic protein-7) to initiate rapid bone formation. J Bone Miner Res. 1997;2:1584–1595. doi: 10.1359/jbmr.1997.12.10.1584. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Duneas N. Tissue morphogenesis and regeneration by bone morphogenetic proteins. Plast Reconstr Surg. 1998;101:227–239. doi: 10.1097/00006534-199801000-00040. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Crooks J, Kirkbride AN. Sintered porous hydroxyapatites with intrinsic osteoinductive activity: geometric induction of bone formation. S Afr J Sci. 1999;95:335–343. [Google Scholar]
- Ripamonti U. Smart biomaterials with intrinsic osteoinductivity: geometric control of bone differentiation. In: Davis JE, editor. Bone Engineering. Toronto: EM2 Corporation; 2000. pp. 215–222. [Google Scholar]
- Ripamonti U, Crooks J, Matsaba T, Tasker J. Induction of endochondral bone formation by recombinant human transforming growth factor-β2 in the baboon (Papio ursinus) Growth Factors. 2000a;17:269–285. doi: 10.3109/08977190009028971. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, van den Heever B, Crooks J, Rueger DC, Reddi AH. Long-term evaluation of bone formation by osteogenic protein-1 in the baboon and relative efficacy of bone-derived bone morphogenetic proteins delivered by irradiated xenogeneic collagenous matrices. J Bone Miner Res. 2000b;15:1798–1809. doi: 10.1359/jbmr.2000.15.9.1798. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Ramoshebi LN, Matsaba T, Tasker J, Crooks J, Teare J. Bone induction by BMPs/OPs and related family members in primates. The critical role of delivery systems. J Bone Joint Surg. 2001;83-A S1:16–27. [PubMed] [Google Scholar]
- Ripamonti U. Tissue engineering of bone by novel substrata instructing gene expression during de novo bone formation. Science in Africa. 2002. http://www.scienceinafrica.co.za/2002/march/bone2.htm.
- Ripamonti U, Ferretti C. Mandibular reconstruction using naturally-derived bone morphogenetic proteins: A clinical trial report. In: Lindholm TS, editor. Advances in Skeletal Reconstruction Using Bone Morphogenetic Proteins. Singapore: World Scientific Publishers Co; 2002. pp. 277–289. [Google Scholar]
- Ripamonti U, van den Heever B, Heliotis M, Dal Mas I, Hahnle U, Biscardi A. Local delivery of bone morphogenetic proteins using a reconstituted basement membane gel: tissue engineering with Matrigel. S Afri J Sci. 2002;98:429–433. [Google Scholar]
- Ripamonti U. Osteogenic proteins of the TGF-β superfamily. In: Henry HL, Norman AW, editors. Encyclopedia of Hormones. TX: Austin Academic Press; 2003. pp. 80–86. [Google Scholar]
- Ripamonti U. Soluble, insoluble and geometric signals sculpt the architecture of mineralized bone. J Cell Mol Med. 2004a;8:169–180. doi: 10.1111/j.1582-4934.2004.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripamonti U. Molecular signals in geometrical cues sculpt bone morphology. S Afr J Sci. 2004b;100:355–367. [Google Scholar]
- Ripamonti U, Ramoshebi LN, Patton J, Matsaba T, Teare J, Renton L. Soluble signals and insoluble substrata: Novel molecular cues instructing the induction of bone. In: Massaro EJ, Rogers JM, editors. The Skeleton. Totowa, NJ: Humana Press; 2004. pp. 217–227. [Google Scholar]
- Ripamonti U. Bone induction by recombinant human osteogenic protein-1 (hOP-1, BMP-7) in the primate Papio ursinus with expression of mRNA of gene products of the TGF-β superfamily. J Cell Mol Med. 2005;9:911–928. doi: 10.1111/j.1582-4934.2005.tb00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripamonti U, Herbst N-N, Ramoshebi LN. Bone morphogenetic proteins in craniofacial and periodontal tissue engineering: experimental studies in the non-human primate Papio ursinus. Cytokine Growth Factors Rev. 2005;16:357–368. doi: 10.1016/j.cytogfr.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Ripamonti U. Soluble osteogenic molecular signals and the induction of bone formation. Biomaterials. 2006;27:807–822. doi: 10.1016/j.biomaterials.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Richter PW, Thomas ME. Self-inducing shape memory geometric cues embedded within smart hydroxyapatite-based biomimetic matrices. Plast Reconstr Surg. 2006 doi: 10.1097/01.prs.0000287133.43718.89. in press. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Roman S, Garcia T, Jackson A, et al. Identification of genes regulated during osteoblastic differentiation by genome-wide expression analysis of mouse calvaria primary osteoblasts in vitro. Bone. 2003;32:474–482. doi: 10.1016/s8756-3282(03)00052-8. [DOI] [PubMed] [Google Scholar]
- Sacerdotti C, Frattin G. Sulla produzione eteroplastica dell'osso. R Accad Med Torino. 1901;27:825–836. [Google Scholar]
- Sampath TK, Reddi AH. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci USA. 1981;78:7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath TK, Muthukumaran N, Reddi AH. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci USA. 1987;84:7109–7113. doi: 10.1073/pnas.84.20.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath TK, Maliakal JC, Hauschka PV, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352–20362. [PubMed] [Google Scholar]
- Sampath TK, Rashka KE, Doctor JS, Tucker RF, Hoffmann FM. Drosophila TGF-β superfamily proteins induce endochondral bone formation in mammals. Proc Natl Acad Sci USA. 1993;90:6004–6008. doi: 10.1073/pnas.90.13.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic P, Vukicevic S. Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev. 2005;16:438–443. doi: 10.1016/j.cytogfr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Solursh M, Langille RM, Wood J, Sampath TK. Osteogenic protein-1 is required for mammalian eye development. Biochem Biophys Res Commun. 1996;218:438–443. doi: 10.1006/bbrc.1996.0078. [DOI] [PubMed] [Google Scholar]
- Spector JA, Luchs JS, Mehrara BJ, Greenwald JA, Smith LP, Longaker MT. Expression of bone morphogenetic proteins during membranous bone healing. Plast Reconstr Surg. 2001;107:24–34. doi: 10.1097/00006534-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Spemann H. Embryonic Development and Induction. New Haven: Yale University Press; 1938. [Google Scholar]
- Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. In vivo engineering of organs: The bone bioreactor. Proc Natl Acad Sci USA. 2005;102:11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I, Vaahkari A, Kettunen P, Åberg T. Epithelial-mesenchymal signaling during tooth development. Connect Tissue Res. 1995;32:9–15. doi: 10.3109/03008209509013700. [DOI] [PubMed] [Google Scholar]
- Thomadakis G, Crooks J, Rueger DC, Ripamonti U. Immunolocalization of bone morphogenetic protein-2 and -3 and osteogenic protein-1 during murine tooth root morphogenesis and in other craniofacial structures. Eur J Oral Sci. 1999;107:368–377. doi: 10.1046/j.0909-8836.1999.eos107508.x. [DOI] [PubMed] [Google Scholar]
- Trueta J. The role of the vessels in osteogenesis. J Bone Joint Surg. 1963;45B:402–418. [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. Philos Trans Royal Soc Lond. 1952;237:37. [Google Scholar]
- Urist MR, McLean FC. Osteogenetic potency and new bone formation by induction in transplants to the anterior chamber of the eye. J Bone Joint Surg. 1952;34A:443–476. [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Urist MR, Silverman BF, Büring K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop. 1967;53:243–283. [PubMed] [Google Scholar]
- Urist MR. The reality of a nebulous, enigmatic myth. Clin Orthop. 1968;59:3–5. [Google Scholar]
- Urist MR, DeLange RJ, Finnerman GAM. Bone cell differentiation and growth factors. Science. 1983;220:680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Urist MR. The search for and discovery of bone morphogenetic protein (BMP) In: Urist MR, O'Conner BT, Burwell RG, editors. Bone Grafts, Derivatives and Substitutes. London: Butterworth Heinemann; 1994. pp. 316–362. [Google Scholar]
- Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971;50:1392–1406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- Vainio S, Karanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- Vukicevic S, Luyten FP, Kleinman HK, Reddi AH. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990;63:437–445. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- Vukicevic S, Helder MN, Luyten FP. The developing human lung and kidney are major sites of synthesis of bone morphogenetic protein-3 (osteogenin) J Histochem Cytochem. 1994;42:869–875. doi: 10.1177/42.7.8014470. [DOI] [PubMed] [Google Scholar]
- Vukivevic S, Kopp JB, Luyten FP, Sampath TK. Induction of nephrogenic mesenchyme by osteogenic protein-1 (bone morphogenetic protein-7) Proc Natl Acad Sci USA. 1996;93:9021–9026. doi: 10.1073/pnas.93.17.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EA, Rosen V, Cordes P, et al. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci USA. 1988;85:9484–9488. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke PH, Springer ING, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- Willestaedt H, Levander G, Hult L. Studies in osteogenesis. Acta Orthop Scand. 1950;21:419–432. doi: 10.3109/17453675008991101. [DOI] [PubMed] [Google Scholar]
- Wlodavsky I, Folkman J, Sullivan R, Fridman R, Ishai-Michaeli R, Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci USA. 1987;84:2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yeh LC, Unda R, Lee JC. Osteogenic protein-1 differentially regulates the mRNA expression of bone morphogenetic proteins and their receptors in primary cultures of osteoblasts. J Cell Physiol. 2000;185:87–97. doi: 10.1002/1097-4652(200010)185:1<87::AID-JCP8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Young HE, Duplaa C, Katz R, et al. Adult-derived stem cells and their potential for use in tissue repair and molecular medicine. J Cell Mol Med. 2005;9:753–769. doi: 10.1111/j.1582-4934.2005.tb00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]