Abstract
We have previously shown that MRL/MpJ mice have a capacity for regeneration instead of scar formation following an ear punch wound. Understanding the differences that occur between scar-free regeneration or repair with scarring will have great impact upon advances in skin tissue engineering. A key question that remains unanswered in the MRL/MpJ mouse model is whether regeneration was restricted to the ear or whether it extended to the skin. A histological analysis was conducted up to 4 months post-wounding, not only with 2-mm punch wounds to the ear but also to the skin on the backs of the same animals. MRL/MpJ mouse ear wounds regenerate faster than control strains, with enhanced blastema formation, a markedly thickened tip epithelium and reduced scarring. Interestingly, in the excisional back wounds, none of these regenerative features was observed and both the C57BL/6 control and MRL/MpJ mice healed with scarring. This review gives an insight into how this regenerative capacity may be due to evolutionary processes as well as ear anatomy. The ear is thin and surrounded on both sides by epithelia, and the dorsal skin is devoid of cartilage and under greater tensile strain. Analysis of apoptosis during ear regeneration is also discussed, assessing the role and expression of various members of the Bcl-2 family of proteins. Ongoing studies are focusing on de novo cartilage development in the regenerating ear, as well as understanding the role of downstream signalling cascades in the process. Identification of such signals could lead to their manipulation and use in a novel tissue-engineered skin substitute with scar-free integration.
Keywords: MRL/MpJ, regeneration, repair, skin, wound healing
Engineering new skin – can lessons be learned from regenerative mechanisms?
Tissues and organs in short supply through harvesting from living donors or cadavers can be potentially engineered by a variety of methods. One of the new challenges facing scientific researchers is discovering the means to build, repair and regenerate human tissues with living biological materials by integrating engineering technologies with those developed in the life sciences. Most involve integrating chemical matrices and biological cells or growth factors, or controlling the differentiation of progenitor or stem cells. Such technologies should enable the manufacture of structural and functional analogues to normal tissues, or allow the controlled delivery of cells and proteins to restore tissue function through in vivo regeneration.
Autologous, full-thickness and split-thickness skin grafts are still the most common ways to treat extensive skin defects. Skin substitutes, including cadaveric and porcine grafts, have been used as an alternative to autologous skin grafts, but essentially only as a temporary biological dressing (Chiu & Burd, 2005). Additionally, allograft dermis and bilayer collagen matrices have been developed that allow invasion from the host fibroblast, remodelling and final replacement of the initial structure with native skin. Such substitutes have proved very difficult to sustain in the tissue engineering market (Metcalfe & Ferguson, 2005). Mesenchymal stem cells (MSCs) have also been used in the treatment of skin defects. The transplantation of MSCs onto the surface of deep thermal burns has resulted in the enhancement of wound regeneration and neoangiogenesis (Rasulov et al. 2005). Similarly, a recent study using MSCs together with basic fibroblast growth factor-soaked skin substitutes showed accelerated cutaneous wound healing in an animal full-thickness skin defect model (Nakagawa et al. 2005). These technologies are, however, in their infancy and require further development.
In humans and domestic animals, cutaneous wound healing following trauma or surgery is a complex process that leads to the formation of a permanent scar in adult skin. It often results in loss of function, restriction of tissue movement and or growth and adverse psychological effects (reviewed in Ferguson & O'Kane, 2004). At present, there are no preventative prescription drugs for the treatment of dermal scarring. Within our group we are investigating new methodologies for tissue engineering skin, specifically to assist with the healing of wounds or replacement of tissue following burns injury. In this review, we will consider what has been employed to overcome the clinical problems of skin wounds. The present solutions will be discussed along with how regenerative mechanisms could theoretically be applied to improve the situation. As a group, we are also identifying novel therapeutic manipulations to improve the degree of integration between a tissue-engineered dermal construct and the host. This approach not only involves utilizing our prior expertise in the prevention of scarring but developing a better understanding of regeneration. To this end, the differences between repair and regeneration will also be considered, citing the MRL/MpJ mouse as a model system to elucidate some of the mechanisms of mammalian regeneration.
Mammalian regeneration – evolutionary interference
Throughout evolution mammals have increasingly lost their ability to regenerate structures. There are limited examples of where regeneration occurs in mammals which will be discussed later in this review, but the real question that this raises is why has this amazing reparative capacity been selectively lost or removed? There are many reasons as to why this might have happened, but as Goss (1980) stated, the absence of regeneration in mammals is as important as its presence and loss of this potential may have arisen as a consequence of evolution. Some of the key transitions in vertebrate evolution have included movement from aquatic to terrestrial habitats and the modification of poikilothermic to homeothermic metabolism (Goss, 1992). This poses another question: is warm bloodedness and regeneration incompatible? In simple terms, the ability to regenerate may simply be a metabolic problem, as an elevated rate of metabolism in warm-blooded vertebrates requires a higher nutrient intake (Goss, 1980). This incompatibility, however, is just one theory and by no means the absolute explanation. Take for example the axolotl, which is known to be able to regenerate limbs and tail but fails to regenerate the retina or lens. A close relative, the newt, however, can regenerate these structures. From this example alone it can be seen that very similar species have very different regenerative capacities. The process of regeneration, in evolutionary terms, is complex and as yet not well understood.
The evolutionary change brought about by a habitat transition from water to land also rendered the regeneration of weight-bearing limbs impractical; life on terra firma favours rapid wound healing and scar formation, which, it is believed, effectively precludes blastema formation (Goss, 1992). For example, a mammal with an amputated leg could not regenerate such a structure fast enough to evade starvation or capture brought about by such immobility. There would be no selective advantage in maintaining a regenerative capacity for this in nature – potentially a reason for selecting against it (Goss, 1980). Rapid healing would obviously not save an amputee from capture by a predator whereas for more minor injuries, quick repair mechanisms are probably advantageous.
Mammals are very adept at repairing wounds, given that it is vital for an injured animal to heal a wound rapidly to minimize infection. Such repair mechanisms may also have been one of the many contributing factors that have resulted in the demise of wound regeneration in mammals. Scarring in this situation is of secondary evolutionary importance, although it does seem to be a process incompatible with regeneration (Goss, 1980).
Normal repair mechanisms vs. regeneration
In higher vertebrates injury to the skin initiates a series of events including inflammation, tissue formation and remodelling that lead to a partial reconstruction of the damaged area (Chettibi & Ferguson, 1999). Rapid repair is essential following injury as a process to stop bleeding and lower the risk of infection. As the healing process continues granulation tissue is formed, composed of fibroblasts and inflammatory cells, along with new capillaries embedded in a loose extracellular matrix (ECM) of collagen, fibronectin, hyaluronic acid and glycosaminoglycans (GAGs). As the wound matures, collagen is reorganized into regularly aligned bundles orientated along the stress lines of the healing wound. Remodelling then occurs and as it progresses, there is a gradual reduction in cellularity and vascularity of the repairing tissue that results in the formation of a collagen scar (reviewed in Chettibi & Ferguson, 1999; Ferguson & O'Kane, 2004).
Regeneration, unlike repair, leads to the complete replacement of the injured structures, culminating in a complete restoration of tissue function. Interestingly, regeneration often occurs at body sites that could be considered to be non-essential and has for some time been thought to be exclusive to invertebrates, lower vertebrates and fetuses.
Lessons from embryonic development: the scar-free approach
Normally, the wound repair process in the skin begins with an acute inflammatory response. However, one of the most important aspects of scar-less fetal wound repair appears to be a markedly attenuated inflammatory response, suggesting that inflammation promotes scar formation in the skin. The inflammatory response in embryonic wounds consists of lower numbers of less differentiated inflammatory cells (Cowin et al. 1998). This, together with high levels of morphogenetic molecules involved in skin growth and morphogenesis, means that the growth factor profile in a healing embryonic wound is very different from that in an adult wound (Whitby & Ferguson, 1991a,b; O'Kane & Ferguson, 1997; Shah et al. 2000; Cowin et al. 2001). Thus, embryonic wounds that heal without a scar have low levels of transforming growth factor beta 1 (TGFβ1) and beta 2 (TGFβ2), low levels of platelet-derived growth factor (PDGF) and high levels of TGFβ3 (reviewed in Ferguson & O'Kane, 2004). We have experimentally manipulated healing adult wounds in mice, rats and pigs to mimic the scar-free embryonic profile, e.g. neutralizing PDGF, neutralizing TGFβ1 and TGFβ2 or adding exogenous TGFβ3 (Shah et al. 1992, 1994, 1995; for reviews see also McCallion & Ferguson, 1996; O'Kane & Ferguson, 1997; Shah et al. 2000; Ferguson & O'Kane, 2004). These experiments resulted in scar-free wound healing in the adult.
Lessons from adult vertebrates: ear regeneration in mammals
Ear regeneration has been a focus of scientific research for many years. Rabbits were one of the first mammals discovered to have the capacity to regenerate new tissue for repairing through-and-through holes to their ears (observation originally described by Markelova, 1953, translated by Vorontsova & Liosner, 1960). A growing number of investigations followed that early work, as ear tissue is one of the few good examples of epimorphic regeneration in mammals (Goss & Grimes, 1972, 1975; Williams-Boyce & Daniel, 1980, 1986). It is, however, important to note that this phenomenon extends to other species apart from lagomorphs. Goss & Grimes (1975) claimed that the lost ear tissue in dogs and sheep will not regenerate, due to a lack of the development of epidermal downgrowths, which they believed form as a result of chondroepithelial interactions. Epidermal downgrowths are also thought to be an important anti-scarring feature of the regeneration process (Goss, 1980). It was also postulated that in the absence of these downgrowths, scar tissue forms and therefore interferes with blastema cell accumulation. Goss (1980) also proposed that this is the main reason why regeneration does not occur with holes created in the ears of deer, armadillo, opossum, chinchilla, guinea-pig, hamster, gerbil, cavy, rat or mouse. Ear regeneration also occurs in cats, hares and the pika (Goss, 1980). In the ears of fruit bats, through-and-through holes did not close but there are certain species of echolocating bats in which the ear holes filled but the regenerated areas had no cartilage. These studies were expanded by Williams-Boyce & Daniel (1986), who demonstrated limited ear regeneration in chinchilla, mouse and rat, in contrast to the work of Goss (1980).
More recently, Clark et al. (1998) described similar ear punch regeneration in the inbred MRL/MpJ mouse strain. In this model, 2-mm excisional wounds in the ears of the MRL/MpJ mouse were found to regenerate completely after 4–5 weeks, whereas in the control strain, C57BL/6, the wounds remained and did not regenerate. The genetics surrounding this phenomenon have been studied, and genomic-level quantitative trait loci mapping has been performed (McBrearty et al. 1998). This methodology demonstrated that candidate genes for ear regeneration were present on most of the mouse chromosomes but identification of any true possible genes for regeneration has proved difficult (Masinde et al. 2001, 2005; Heber Katz et al. 2004; Yu et al. 2005). Other recent studies have suggested that components of the inflammatory system may play a significant role in the regeneration/repair process (X. Li et al. 2000, 2001; F. Li et al. 2001; Gawronska-Kozak, 2004; Ueno et al. 2005).
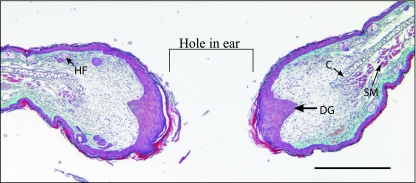
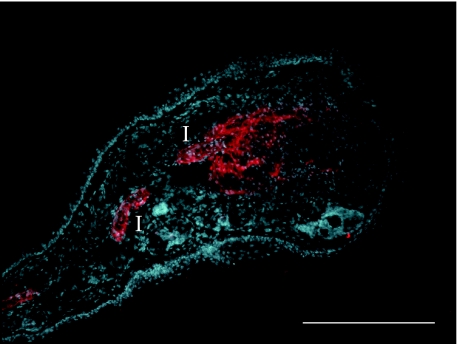
We have demonstrated considerable variability in the MRL/MpJ regenerating mouse ear model (Rajnoch et al. 2003; Metcalfe & Ferguson, 2005). The extent of complete regeneration was dependent on the degree of trauma imposed, with wounds created by an animal husbandry ear punch much less likely to regenerate than those created by a sharp surgical biopsy punch (Rajnoch et al. 2003). Histological analysis suggests that the regenerative capacity of the MRL/MpJ mouse may only occur under specific conditions, and in situations where substantial amounts of necrosis and inflammation occur, indicating that a default repair mechanism overrides the regenerative mechanism. It was also determined that C57BL/6 mice did possess a limited regenerative capacity, with biopsy punch wounds closing to just over half their original size by 4 months post wounding (Rajnoch et al. 2003). This was in contrast to the original phenotypic description of these mice, where no significant decrease in wound size was observed in these animals. MRL/MpJ mice regenerated through-and-through holes in their ears by developing a blastema-like structure, similar histologically to the structures seen in regenerating amphibian limbs (Fig. 1; Echeverri et al. 2001; Rageh et al. 2002; see reviews by Endo et al. 2004; Stocum, 2004; Brockes & Kumar, 2005). Interestingly, the regeneration observed in the MRL/MpJ mice was characterized in part by a thickened apical epithelium and also by development of de novo cartilage ‘islands’ within the mesenchyme (Fig. 2). The cartilage ‘islands’ also appear to be responsible for laying down GAGs into the mesenchymal space. De novo cartilage regeneration was further investigated using the cartilage progenitor cell marker aggrecan (Fig. 2). Staining for this chondro-progenitor cell marker was observed in the blastemal mesenchyme during both early and late stages of ear regeneration. As with the findings of Goss (1980) this infers an important interaction between the cut cartilage and the blastemal-like epithelium.
Fig. 1.
MRL/MpJ ear 14 days post wounding. Blastema-like structures either side of the punch biopsy hole. Thickened tip epithelia can be seen along with epidermal downgrowths (DG), cartilage (C), skeletal muscle (SM) and hair follicles (HF). Bar, 1 mm.
Fig. 2.
MRL/MpJ ear wound showing a blastema-like structure 21 days post biopsy punch wounding, stained with an anti aggrecan antibody (TRITC), counterstained with the nuclear dye DAPI. Cartilage ‘islands’ (I), stained with the anti-aggrecan antibody, are clearly visible in the regenerating MRL/MpJ ear. Aggregan is also diffusely expressed in the mesenchyme, extending from the leading cartilage ‘island’. Bar, 500 µm.
Regeneration and repair mechanisms coexist in the same animal
We have recently demonstrated further variability in the MRL/MpJ regenerative response. Using the same animals in which the ear punches were created, equivalent 4-mm dorsal skin punch biopsy excisional wounds were also made. On analysis of those back wounds of the MRL/MpJ mice, no apparent regeneration occurred (Metcalfe & Ferguson, 2005). Instead, in the back wounds, scars of similar size and quality to those of the control animals (C57BL/6) developed. Analysis showed that early macrophage recruitment was equivalent between the two strains as was the deposition of α-smooth muscle actin. This suggests that the mechanism responsible for regeneration in the ear was not functioning in the skin.
This result was surprising as it contrasts quite markedly with earlier results observed in MRL/MpJ mice obtained after 2-mm through-and-through biopsy punching of the ear (Clark et al. 1998; Rajnoch et al. 2003). The regenerating blastema-like structures were not observed in MRL/MpJ dorsal skin wounds but were seen in ear wounds created in the same animals (Rajnoch et al. 2003). Significantly, rather than regenerating like the ears, the dorsal wounds repaired with scarring (Metcalfe & Ferguson, 2005). This demonstrates that repair in the back and regeneration in the ear of the same animal can occur. This fundamentally important phenomenon suggests that the mechanisms governing these two healing processes are likely to be controlled by similar molecules that diverge in subtly different ways dependent upon the location of injury (Ferguson & O'Kane, 2004).
Regeneration and apoptosis
Another process known to be involved in the response to injury and thought to have a role in regeneration is apoptosis. Although little is known about apoptosis in the blastema, recently a study in planarians has suggested that apoptosis may be involved in controlling cell numbers, eliminating unnecessary tissues or cells and remodelling the old tissues of regenerating body parts (Hwang et al. 2004). Bcl-2 family proteins are the main regulators of the apoptotic process, acting either to inhibit or to promote it (Metcalfe et al. 1999). Pro-and anti-apoptotic Bcl-2 family members should function in harmony to regulate the apoptotic machinery, and their relative levels are critical for cell fate throughout development (Cory et al. 2003; Metcalfe et al. 2004; Sorenson, 2004). Within the MRL/MpJ mouse ear wound model we have observed a reduction of expression of some proapoptotic members of the Bcl-2 family during the formation of the blastema-like structure. Immunohistochemical analysis of MRL/MpJ ear sections at day 14 post wounding demonstrate a reduction in the expression of Bax, Bim and Bid as compared with a day 5 post wounding ear section. This chronologically altered expression of apoptosis-related proteins may be a causative factor in the accelerated regeneration observed in the blastema-like structure. Further work is underway to understand the role of apoptosis in the processes of repair and regeneration.
Mechanical and architectural influences on regeneration
The lack of regeneration in the dorsal skin is as yet uncharacterized, but may be a reflection of differences in the architecture of the two sites. The ear is a very thin structure with epidermis on both sides, as well as containing a supporting cartilage framework. The blastema-like structures that develop in the wounded ear are manifested adjacent to the cartilage at the wound edge. Regeneration of the ear is likely to be controlled by a multitude of factors. These include not only cell signalling cascades but also de novo synthesis of cartilage, the ingrowth of regenerating nerves, as well as deposition of GAGs and ECM within the developing primitive mesenchyme of the blastema-like structure. By contrast, the skin on the body is loose, cartilage-free, much thicker structurally than the ear and contains a substantial subcutaneous fat layer. Unlike the ear, bound by epithelia on both sides, there is only one layer of epithelia in the back.
Contraction of the back wounds is also likely to play a significant role in the repair process. Mechanotransductive forces may therefore be a significant contributing factor towards whether a wound repairs or regenerates. Mechanical forces cause direct stretching of protein–cell surface integrin binding sites that occur on all eukaryotic cells. Stress-induced conformational changes in the ECM may alter integrin structure and lead to activation of several secondary messenger pathways within the cell, as well as having effects on growth factor shedding and cell proliferation (Silver & Siperko, 2003; Tschumperlin et al. 2004; Yano et al. 2004). It is thought that cells are able to sense mechanical stress through autocrine loops localized to compliant extracellular spaces (Tschumperlin et al. 2004). If this is the case, then dorsal skin injuries will result in cellular stresses that are probably much different from the ones that impact on cells in the injured ear. This phenomenon may play a role in the subsequent mechanisms governing whether a wound closes by simple repair or by scar-less regeneration.
In summary, unlike the regeneration observed in the ears of MRL/MpJ mice and the partial regeneration seen in C57BL/6 mice, dorsal skin wounds heal with scarring similar to that seen in other strains of mice. We believe that along with the physical dimensions and mechanics of ear wounds, the chondro-epithelial interactions in ear regeneration are also an important consideration. The most exciting and significant finding of our present study is that regeneration and repair can occur simultaneously within the same animal, as has also been noted to occur in humans (Ferguson & O'Kane, 2004). This suggests that similar molecules and signals are likely to be required for both mechanisms but in some way are capable of being reprogrammed, dependent upon the location of injury within the body.
The future: harnessing regeneration and other technologies for tissue engineering
Apart from understanding mammalian regeneration from model systems such as the MRL/MpJ mouse, there are other technological areas of research that the tissue engineer might use to replace tissues. Mesenchymal stem cells have been used in the treatment of skin defects (Badiavas & Falanga, 2003; Nakagawa et al. 2005; Rasulov et al. 2005). Embryonic stem cells (ESCs) have also proven capable of adopting various cells fates and could in theory facilitate the development and regeneration of tissues. Although stem cells may have the potential to regenerate a variety of tissues, as indicated by a number of preliminary reports, ethical issues and safety considerations seem to preclude the use of human ESCs in clinical use (Bongso & Richards, 2004; Romano, 2004). If these initial problems can be overcome and with a greater conceptual understanding of their use, these cells still represent an important facet for the future of regenerative medicine. The expanding clinical applications of bone marrow progenitors, the anticipated discovery of novel growth factors, and the better understanding of the biological behaviour of important cellular elements and of pathways of the healing process of tissues will also offer novel treatment strategies in the enhancement of tissue regeneration. Additionally, the introduction of nanotechnologies may provide scientists and clinicians with new techniques of delivering growth factors and cytokines to the affected tissues such as skin, with non-invasive procedures (reviewed in Cross & Roberts, 2004).
Creating and delivering a fully functional ‘off-the-shelf’, living tissue-engineered skin substitute is proving to be a long process. A primary concern is finding a good source of cells to engineer the tissue, and equally important is having a greater understanding of the immune system's response to those cells and their persistence. Improving vascularization of the graft is also a key factor, as is developing better preservation techniques for the newly engineered tissues. A revolution in biology and the field of tissue regeneration has begun. Model systems of mammalian regeneration coupled with some of these new technological advances will pave the way for therapeutic regeneration in humans. In the years to come the hope is that many patients will benefit in terms of restoration of tissue loss, function and an improved health state in general.
Acknowledgments
This study was carried out within the UK Centre for Tissue Engineering, and we are grateful to the BBSRC, MRC and EPSRC for their support.
References
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003. pp. 510–516. [DOI] [PubMed]
- Bongso A, Richards M. History and perspective of stem cell research. Best Pract Res Clin Obstet Gynaecol. 2004;18:827–842. doi: 10.1016/j.bpobgyn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Chettibi S, Ferguson MWJ. Wound repair: an overview. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 865–881. 3. [Google Scholar]
- Chiu T, Burd A. ‘Xenograft’ dressing in the treatment of burns. Clin Dermatol. 2005;23:419–423. doi: 10.1016/j.clindermatol.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Brosnan MP, Holmes TM, Ferguson MWJ. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dynamics. 1998;212:385–393. doi: 10.1002/(SICI)1097-0177(199807)212:3<385::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Holmes TM, Brosnan P, Ferguson MWJ. Expression of TGF-3 and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol. 2001;11:424–431. [PubMed] [Google Scholar]
- Cross SE, Roberts MS. Physical enhancement of transdermal drug application: is delivery technology keeping up with pharmaceutical development? Curr Drug Deliv. 2004;1:81–92. doi: 10.2174/1567201043480045. [DOI] [PubMed] [Google Scholar]
- Echeverri K, Clarke JD, Tanaka EM. In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev Biol. 2001;236:151–164. doi: 10.1006/dbio.2001.0312. [DOI] [PubMed] [Google Scholar]
- Endo T, Bryant SV, Gardiner DM. A stepwise model system for limb regeneration. Dev Biol. 2004;270:135–145. doi: 10.1016/j.ydbio.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Ferguson MWJ, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawronska-Kozak B. Regeneration in the ears of immunodeficient mice: identification and lineage analysis of mesenchymal stem cells. Tissue Eng. 2004;10:1251–1265. doi: 10.1089/ten.2004.10.1251. [DOI] [PubMed] [Google Scholar]
- Goss RJ, Grimes LN. Tissue interactions in the regeneration of rabbit ear holes. Am Zool. 1972;12:151–157. [Google Scholar]
- Goss RJ, Grimes LN. Epidermal downgrowths in regenerating rabbit ear holes. J Morph. 1975;146:533–542. doi: 10.1002/jmor.1051460408. [DOI] [PubMed] [Google Scholar]
- Goss RJ. Why mammals don't regenerate – or do they? News Phys Sci. 1980;2:112–115. [Google Scholar]
- Goss RJ. The evolution of regeneration: adaptive or inherent. J Theor Biol. 1992;159(2):241–260. doi: 10.1016/s0022-5193(05)80704-0. [DOI] [PubMed] [Google Scholar]
- Heber Katz E, Chen P, Clark L, Zhang XM, Troutman S, Blankenhorn EP. Regeneration in MRL mice: further genetic loci controlling the ear hole closure trait using MRL and M.m. Castaneus mice. Wound Repair Regen. 2004;12:384–392. doi: 10.1111/j.1067-1927.2004.012308.x. [DOI] [PubMed] [Google Scholar]
- Hwang JS, Kobayashi C, Agata K, et al. Detection of apoptosis during planarian regeneration by the expression of apoptosis-related genes and TUNEL assay. Gene. 2004;333:15–25. doi: 10.1016/j.gene.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Li F, Jin F, Freitas A, Szabo P, Weksler ME. Impaired regeneration of the peripheral B cell repertoire from bone marrow following lymphopenia in old mice. Eur J Immunol. 2001;31:500–505. doi: 10.1002/1521-4141(200102)31:2<500::aid-immu500>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Miyakoshi N, Baylink DJ. Differential protein profile in the ear-punched tissue of regeneration and non-regeneration strains of mice: a novel approach to explore the candidate genes for soft-tissue regeneration. Biochim Biophys Acta. 2000;1524:102–109. doi: 10.1016/s0304-4165(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Baylink DJ. Analysis of gene expression in the wound repair/regeneration process. Mamm Genome. 2001;12:52–59. doi: 10.1007/s003350010230. [DOI] [PubMed] [Google Scholar]
- Masinde GL, Li X, Gu W, Davidson H, Mohan S, Baylink DJ. Identification of wound healing/regeneration quantitative trait loci (QTL) at multiple time points that explain seventy percent of variance in (MRL/MpJ and SJL/J) mice F2 population. Genome Res. 2001;11:2027–2033. doi: 10.1101/gr.203701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masinde GL, Li R, Nguyen B, et al. New quantitative trait loci that regulate wound healing in an intercross progeny from DBA/1J and 129x1/SvJ inbred strains of mice. Funct Integr Genomics. 2006;6(2):157–163. doi: 10.1007/s10142-005-0004-1. [DOI] [PubMed] [Google Scholar]
- McBrearty BA, Clark LD, Zhang XM, Blankenhorn EP, Heber-Katz E. Genetic analysis of a mammalian wound-healing trait. Proc Natl Acad Sci USA. 1998;95:11792–11797. doi: 10.1073/pnas.95.20.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallion RL, Ferguson MWJ. Fetal wound healing and the development of anti-scarring therapies for adult wound healing. In: Clarke RAF, editor. The Molecular and Cellular Biology of Wound Repair. 2. New York: Plenum; 1996. pp. 561–600. [Google Scholar]
- Metcalfe AD, Gilmore A, Klinowska T, et al. Developmental regulation of Bcl-2 family protein expression in the involuting mammary gland. J Cell Sci. 1999;112:1771–1783. doi: 10.1242/jcs.112.11.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe AD, Hunter HR, Bloor DJ, et al. Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol Reprod Dev. 2004;68:35–50. doi: 10.1002/mrd.20055. [DOI] [PubMed] [Google Scholar]
- Metcalfe AD, Ferguson MWJ. Harnessing wound healing and regeneration for tissue engineering. Biochem Soc Trans. 2005;33:413–417. doi: 10.1042/BST0330413. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153:29–36. doi: 10.1111/j.1365-2133.2005.06554.x. [DOI] [PubMed] [Google Scholar]
- O'Kane S, Ferguson MWJ. Transforming growth factor betas and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- Rageh MA, Mendenhall L, Moussad EE, Abbey SE, Mescher AL, Tassava RA. Vasculature in pre-blastema and nerve-dependent blastema stages of regenerating forelimbs of the adult newt, Notophthalmus viridescens. J Exp Zool. 2002;292:255–266. doi: 10.1002/jez.10015. [DOI] [PubMed] [Google Scholar]
- Rajnoch C, Ferguson S, Metcalfe AD, Herrick SE, Willis HS, Ferguson MWJ. Regeneration of the ear after wounding in different mouse strains is dependent on the severity of wound trauma. Dev Dyn. 2003;226:388–397. doi: 10.1002/dvdy.10242. [DOI] [PubMed] [Google Scholar]
- Rasulov MF, Vasilchenkov AV, Onishchenko NA, et al. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med. 2005;1:141–144. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- Romano G. Stem cell transplantation therapy: controversy over ethical issues and clinical relevance. Drug News Perspect. 2004;17:637–645. doi: 10.1358/dnp.2004.17.10.873915. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MWJ. Control of scarring in adult wounds by neutralising antibodies to transforming growth factor beta (TGF3) Lancet. 1992;339:213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MWJ. Neutralising antibody to TGFβ1,2, reduces scarring in adult rodents. J Cell Sci. 1994;107:1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MWJ. Neutralisation of TGFβ1 and TGFβ2 or exogenous addition of TGFβ3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Shah M, Rorison P, Ferguson MWJ. The role of transforming growth factors beta in cutaneous scarring. In: Garg HG, Longaker MT, editors. Scarless Wound Healing. New York: Marcel Dekker; 2000. pp. 213–226. [Google Scholar]
- Silver FH, Siperko LM. Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix? Crit Rev Biomed Eng. 2003;31:255–331. doi: 10.1615/critrevbiomedeng.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- Sorenson CM. Bcl-2 family members and disease. Biochim Biophys Acta. 2004;1644:169–177. doi: 10.1016/j.bbamcr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Stocum DL. Amphibian regeneration and stem cells. Curr Top Microbiol Immunol. 2004;280:1–70. doi: 10.1007/978-3-642-18846-6_1. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Dai G, Maly IV, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83–86. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Lyons BL, Burzenski LM, et al. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest Ophthalmol Vis Sci. 2005;46:4097–4106. doi: 10.1167/iovs.05-0548. [DOI] [PubMed] [Google Scholar]
- Vorontsova MA, Liosner LD. Asexual Propagation and Regeneration. New York: Pergamon Press; 1960. [Google Scholar]
- Whitby DJ, Ferguson MW. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991a;147:207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Whitby DJ, Ferguson MW. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development. 1991b;112:651–668. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]
- Williams-Boyce PK, Daniel JC. Regeneration of rabbit ear tissue. J Exp Zool. 1980;212:243–253. doi: 10.1002/jez.1402120211. [DOI] [PubMed] [Google Scholar]
- Williams-Boyce PK, Daniel JC. Comparison of ear tissue regeneration in mammals. J Anat. 1986;149:55–63. [PMC free article] [PubMed] [Google Scholar]
- Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K. Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. J Invest Dermatol. 2004;122:783–790. doi: 10.1111/j.0022-202X.2004.22328.x. [DOI] [PubMed] [Google Scholar]
- Yu H, Mohan S, Masinde GL, Baylink DJ. Mapping the dominant wound healing and soft tissue regeneration QTL in MRL x CAST. Mamm Genome. 2005;16:918–924. doi: 10.1007/s00335-005-0077-0. [DOI] [PubMed] [Google Scholar]