Abstract
Evolution has used many different strategies to build eyes and lenses. However, the genetic regulation involved seems to be quite conserved. Likewise, the regeneration of eye structures is remarkable, especially in salamanders. This review outlines the basic mechanisms of lens regeneration and its induction and the possibility of creating lenses by transdifferentiation of the pigment epithelial cells, by stem cells or by bioengineering.
Keywords: engineering, evolution, eye, lens, regeneration, stem cells
Nature builds eyes (and lenses)
It is believed that during evolution the eye has been invented independently as many as 40 times (Dawkins, 1996; Land & Nilsson, 2002). Interestingly, however, several types of eyes have been invented during evolution depending on how they work and on the species. These types include the compound eye, found mainly in insects and arthropoda, and the camera type found in different organisms from jellyfish to fish and to mammals. Even these two types can be subdivided depending on the photoreceptor type or optic properties (Land & Nilsson, 2002). More primitive eyes found in invertebrates consist of sensory cells or pigment cells. Perhaps the origin of vision can even be traced back to organisms such as cyonabacteria, which have specialized proteins (opsins) capable of responding to light. The variety of eye structures (especially with regard to the lens) does not end here. Several aquatic eyes have been evolved with four lenses (Bathylychnops exilis), three lenses (in the male copepod Pontella; the female has two) or two lenses (the copepod Sapphirina) (Schwab et al. 2001; Land & Nilsson, 2002). What is fascinating about eye evolution, however, is the conservation of factors that seem to control its formation. One particular gene, pax-6, has been heralded as the eye master gene. Indeed, mutations in pax-6 result in an eyeless phenotype in Drosophila, small eye phenotype in mice and aniridia in humans (Gehring, 2002). Moreover, pax-6 seems single-handedly to initiate the formation of ectopic eyes. For example, when exogenous pax-6 is expressed ectopically in Drosophila, e.g. at the limb imaginal discs, compound eyes with well-differentiated cells types are formed in the limb (Halder et al. 1995). In addition to such effects, expression of pax-6 has been clearly correlated with light-sensing structures throughout the animal kingdom. An interesting case is the cubozoan jellyfish eye. In these animals, a cluster of six different eyes are present in a sensory club, called the rhopalium. Some of the eyes are complex (with lens) whereas others are simple. In addition to these eyes, each rhopalium contains a statocyst, most likely involved in mechanosensing. All of the eyes, including the statocyst, express paxB (an ancestor of pax-6) (Piatigorsky & Kozmic, 2004; Nilsson et al. 2005).
The role of pax-6 in eye development, including the lens, has been studied in detail and a particular network has been delineated. In frogs, upstream of this network is an inhibitor of the bone morphogenetic protein (BMP) pathway and downstream regulators involve pax-6 and six-3 (Zuber et al. 2003). Six-3 is now thought to play a significant role in eye and lens development by regulating pax-6 in a feedback loop. Pax-6 is clearly involved in the differentiation of eye cells during Drosophila eye development as well (Wawersik & Maas, 2000). During vertebrate lens development, which is initiated by interactions of the surface ectoderm and the optic cup, pax-6 and six-3 are expressed in the lens, inducing competent surface ectoderm. Variations of lens development, such as by thickening of the cornea or by differentiation of transparent cells, has been observed in fly larvae as well (Dawkins, 1996; Land & Nilsson, 2002). A direct association of pax-6 and six-3 in lens differentiation has been found because they ectopically induce lens formation when injected exogenously in frog and fish embryos, respectively (Oliver et al. 1996; Altmann et al. 1997).
Re-building a lens (regeneration)
Regeneration of eye tissues is of paramount importance for the repair of damaged cells that lead to several types of blindness in humans. Remarkably, the vertebrate eye, as best reflected in some salamanders, has an amazing potential to regenerate entire organs, such as the whole retina or the lens (Del Rio-Tsonis & Tsonis, 2003). Regeneration is accomplished by transdifferentiation of the pigment epithelial cells (PECs; Fig. 1). Studies have also confirmed that such an ability of the pigment epithelium can be evoked even with human cells when cultured for a long time. In other words, the capacity of PECs for transdifferentiation is widespread; however, only in some salamanders does it occur in vivo (Del Rio-Tsonis & Eguchi, 2004; Tsonis et al. 2004a). Such repair is far superior to the one mediated by progenitor cells (mainly for retina growth).
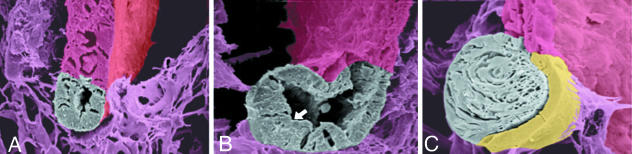
Fig. 1.
The process of lens regeneration as shown by pseudocolouring of SEM micrographs. (A) Ten days post-lentectomy, an early lens vesicle (grey) has been produced at the tip of the dorsal iris. (B) At day 15 post-lentectomy the vesicle has grown and cells at the posterior part elongate (arrow) to form the lens fibres. (C) At day 20 the posterior part forms the lens fibres, which fill the lens, and the anterior becomes the lens epithelium (yellow).
Because lens regeneration normally occurs only from the dorsal iris (and never the ventral) by transdifferentiation of the iris PECs, research in our laboratory has focused on finding key differences between the positive cells (dorsal) and the negative ones (ventral). We hoped that these differences could help induce the ventral iris (as a first step) to regenerate a lens. We decided to concentrate on genes that were important for eye development and axis formation. First we showed that pax-6, a known eye master gene, is expressed during dedifferentiation of the PECs and lens regeneration (Del Rio-Tsonis et al. 1995). These initial expression studies indicated that lens regeneration could recapitulate lens development or that in the adult newt developmental genes are not turned off. We then delineated the role of fibroblast growth factors (FGFs) and FGF receptors in lens regeneration. FGFs and FGF receptors are expressed during lens regeneration, with a preference in the dorsal iris, but they are also imperative for normal regeneration (Del Rio-Tsonis et al. 1997). When exogenous FGF was given to the eye another lens could be elicited from the dorsal iris, but not from the ventral iris (Del Rio-Tsonis et al. 1997; Hayashi et al. 2004). When FGF receptor signalling was specifically inhibited, lens regeneration was abolished (Del Rio-Tsonis et al. 1998). These data clearly showed that FGF signalling is imperative for normal regeneration. Other important factors were studied for specific expression and function during lens regeneration. These included key regulators such as Hox genes (Jung et al. 1998), Prox-1 (Del Rio-Tsonis et al. 1999), retinoic acid receptors (Tsonis et al. 2000), cyclin-dependent kinases (Tsonis et al. 2004b), complement components (Kimura et al. 2003) and the identification of a novel role for the hedgehog pathway in lens regeneration (Tsonis et al. 2004c). The goal of all these studies was to identify factor(s) that might be good candidates for the induction of lens regeneration and to apply them to the ventral iris to induce regeneration from that site. This was initially accomplished by transgenesis of the ventral iris by six-3 and treatment with retinoic acid. As mentioned above, six-3 is a major eye development regulator and collaborates with pax-6. We also found that inhibition of the BMP pathway (which lies upstream of the pax-6/six-3 loop) could do exactly the same (Fig. 2). BMPs are known ventralizers and it seems that their function controls the identity of dorsal and ventral iris (DeRobertis & Kuroda, 2004). Interestingly, six-3 was found to be expressed in both the dorsal and the ventral iris and that their association with induction is related to the level of expression. We believe that in order for the iris to become competent for regeneration, levels of expression must be elevated above established thresholds (Grogg et al. 2005).
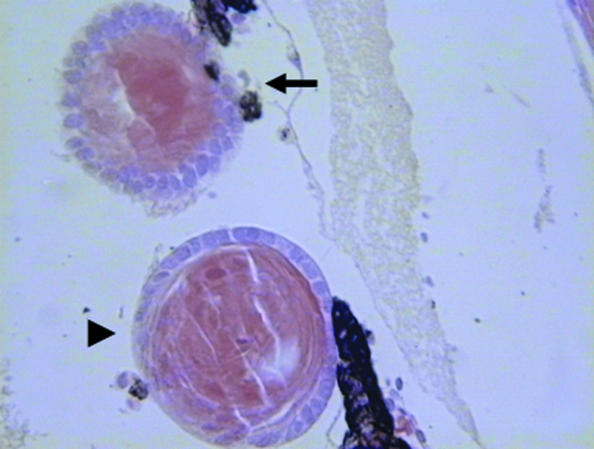
Fig. 2.
A ventral iris explant treated with bone morphogenetic protein inhibitor transdifferentiated to lens (arrowhead). The host lens from the dorsal iris is indicated by an arrow.
Is such a mechanism conserved in other animals? The answer is unclear, but we can speculate. The mode of lens regeneration, as in eye evolution, differs in other animals. For example, lens regeneration in the premetamorphic frog is achieved by transdifferentiation of the inner layer of the cornea, rather than the iris. Given the conservation of the mechanisms involved in eye induction, however, we would expect that the same players might be involved in induction of lens regeneration in other animals. However, the exact mechanistic tuning must be unique to the eye environment of the different species. This represents a major challenge in the field of lens regeneration and in the field of regeneration in general.
Mammals possess an ability to regenerate the lens, but only when the capsule is left behind. This has been studied extensively in rabbits (Gwon, 2006), and recently such studies have been extended to mice (Lois et al. 2005; Call et al. 2004). The source of the regenerated lens is the adherent lens epithelial cells that cannot be completely removed.
Lens differentiation from stem cells
The process of lens regeneration in adult newts is not mediated by stem cells. It is a clear case of transdifferentiation of the existing PECs from the dorsal iris. Such transdifferentiation can be seen even when isolated PECs are grown in single-cell cultures. In fact the capacity of PECs for in vitro transdifferentiation is not only restricted to newt dorsal iris but is widespread among the animal kingdom. Even PECs from old-aged humans are capable of lens transdifferentiation under certain conditions in vitro (Tsonis et al. 2001). This transdifferentiation leads to lentoids, rather amorphous structures containing lens fibres without a firm lens structure surrounded by lens epithelium. However, embryonic stem cells can also differentiate to lentoids (Fig. 3) (Hirano et al. 2003; Ooto et al. 2003). This has interesting implications for the biology of dedifferentiation. Is, for example, the newt producing progenitor cells by the process of transdifferentiation? Carefully designed experiments involving transplantations and examination of molecular signatures could answer this important question.
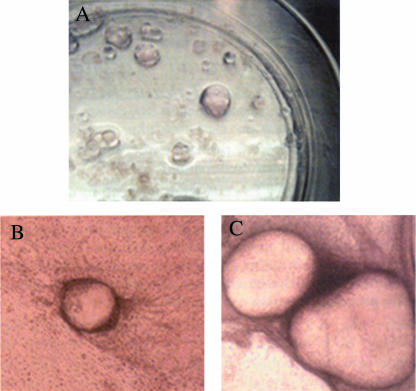
Fig. 3.
Lentoid differentiation from embryonic stem cells 23, 30 and 53 days of induction (A, B and C, respectively) (reproduced by permission of ARVO).
Lens engineering
Is it then possible that a lens can be engineered in vitro? We believe that the answer to this question is yes. Again studies with the newt lend support to this idea. When PECs are aggregated and implanted in the eye (or even the regenerating limb) they not only transdifferentiate to lens tissue, but also build a lens of the right structure with a lens epithelium and an anterior–posterior polarity (Ito et al. 1999). This means that in vitro aggregated PECs can build a perfect lens when implanted in vivo. This can be seen in Fig. 2 as well, where the ventral iris explant was induced to transdifferentiate to a perfectly structured lens. It is possible that if the PECs are grown in suitable shaped scaffolds in vitro they will be coaxed to form a normal lens. New advances in tissue engineering and lessons learned from basic systems such as the newt may allow us to build an organ in vitro. Recently, an artificial compound eye has been fabricated with optical characteristics similar to those found in nature (Jeong et al. 2006).
Acknowledgments
This work was supported by NIH grant EY10540.
References
- Altmann CR, Chow RL, Lang RA, Hemmati-Brivanlou A. Lens induction by Pax-6 in Xenopus laevis. Dev Biol. 1997;185:119–123. doi: 10.1006/dbio.1997.8573. [DOI] [PubMed] [Google Scholar]
- Call MK, Grogg MW, Del Rio-Tsonis K, Tsonis PA. Lens regeneration in mice: implications in cataracts. Exp Eye Res. 2004;78:297–299. doi: 10.1016/j.exer.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Dawkins R. Climbing Mountain Improbable. New York: W. W. Norton Co; 1996. [Google Scholar]
- Del Rio-Tsonis K, Washabaugh CH, Tsonis PA. Expression of pax-6 during urodele eye development and lens regeneration. Proc Natl Acad Sci USA. 1995;92:5092–5096. doi: 10.1073/pnas.92.11.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Jung J-C, Chiu I-M, Tsonis PA. Conservation of fibroblast growth factor function in lens regeneration. Proc Natl Acad Sci USA. 1997;94:13701–13706. doi: 10.1073/pnas.94.25.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Trombley MT, McMahon G, Tsonis PA. Regulation of lens regeneration by fibroblast growth factor receptor 1. Dev Dyn. 1998;213:140–146. doi: 10.1002/(SICI)1097-0177(199809)213:1<140::AID-AJA14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tomarev SI, Tsonis PA. Regulation of prox-1 during lens regeneration. Invest Ophthalmol Vis Sci. 1999;40:2039–2045. [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tsonis PA. Eye regeneration at the molecular age. Dev Dyn. 2003;226:211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Eguchi G. Lens regeneration. In: Lovicu F, Robinson M, editors. Development of the Ocular Lens. Cambridge: Cambridge University Press; 2004. pp. 290–311. [Google Scholar]
- DeRobertis EM, Kuroda H. Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ. The genetic control of eye development and its implications for the evolution of the various eye-types. Int J Dev Biol. 2002;46:65–73. [PubMed] [Google Scholar]
- Grogg MW, Call MK, Okamoto M, Vergara MN, Del Rio-Tsonis K, Tsonis PA. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature. 2005;438:858–862. doi: 10.1038/nature04175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwon A. Lens regeneration in mammals: a review. Surv Ophthalmol. 2006;51:51–62. doi: 10.1016/j.survophthal.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Mizuno N, Ueda Y, Okamoto M, Kondoh H. FGF2 triggers iris-derived lens regeneration in newt eye. Mech Dev. 2004;121:519–526. doi: 10.1016/j.mod.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Hirano M, Yamamoto A, Yoshimura N, et al. Generation of structures formed by lens and retinal cells differentiating from embryonic stem cells. Dev Dyn. 2003;228:664–671. doi: 10.1002/dvdy.10425. [DOI] [PubMed] [Google Scholar]
- Ito M, Hayashi T, Kuroiwa A, Okamoto M. Lens formation by pigmented epithelial cell reaggregate from dorsal iris implanted into limb blastema in the adult newt. Dev Growth Differ. 1999;41:429–440. doi: 10.1046/j.1440-169x.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- Jeong K-H, Kim J, Lee LP. Biologically inspired artificial compound eyes. Science. 2006;312:557–561. doi: 10.1126/science.1123053. [DOI] [PubMed] [Google Scholar]
- Jung JC, Del Rio-Tsonis K, Tsonis PA. Regulation of homeobox-containing genes during lens regeneration. Exp Eye Res. 1998;66:361–370. doi: 10.1006/exer.1997.0437. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Madhavan M, Call MK, et al. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J Immunol. 2003;170:2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- Land MF, Nilsson D-E. Animal Eyes. Oxford: Oxford University Press; 2002. [Google Scholar]
- Lois N, Taylor J, McKinnon AD, Forrester JV. Posterior capsule opacification in mice. Arch Ophthalmol. 2005;123:71–77. doi: 10.1001/archopht.123.1.71. [DOI] [PubMed] [Google Scholar]
- Nilsson DE, Gislen L, Coates MM, Skogh C, Garm A. Advanced optics in a jellyfish eye. Nature. 2005;435:201–205. doi: 10.1038/nature03484. [DOI] [PubMed] [Google Scholar]
- Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Ectopic lens induction in fish in response to the murine homeobox gene six-3. Mech Dev. 1996;60:233–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- Ooto S, Haruta M, Honda Y, Kawasaki H, Sasai Y, Takahashi M. Induction of the differentiation of lentoids from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2003;44:2689–2693. doi: 10.1167/iovs.02-1168. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J, Kozmik Z. Cubozoan jellyfish: an Evo/Devo model for eyes and other sensory systems. Int J Dev Biol. 2004;48:719–729. doi: 10.1387/ijdb.041851jp. [DOI] [PubMed] [Google Scholar]
- Schwab IR, Ho V, Roth A, Blankenship TN, Fitzgerald PG. Evolutionary attempts at 4 eyes in vertebrates. Trans Am Ophthalmol Soc. 2001;99:145–156. [PMC free article] [PubMed] [Google Scholar]
- Tsonis PA, Trombley MT, Rowland T, Chandraratna RAS, Del Rio-Tsonis K. Role of retinoic acid in lens regeneration. Dev Dyn. 2000;219:588–593. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1082>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Jang W, Del Rio-Tsonis K, Eguchi G. A unique aged human retinal pigmented epithelial cell line useful for studying lens differentiation in vitro. Int J Dev Biol. 2001;45:753–758. [PubMed] [Google Scholar]
- Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K. A newt's eye view of lens regeneration. Int J Dev Biol. 2004a;48:975–980. doi: 10.1387/ijdb.041867pt. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Madhavan M, Call MK, Gainer S, Rice A, Del Rio-Tsonis K. Effects of a CDK inhibitor on lens regeneration. Wound Repair Regen. 2004b;12:24–29. doi: 10.1111/j.1067-1927.2004.012107.x. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Vergara MN, Spence JR, et al. A novel role of the hedgehog pathway in lens regeneration. Dev Biol. 2004c;267:450–461. doi: 10.1016/j.ydbio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Maas RL. Vertebrate eye development as modeled in Drosophila. Hum Mol Genet. 2000;9:917–925. doi: 10.1093/hmg/9.6.917. [DOI] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]