Abstract
The visual pulvinar is part of the dorsal thalamus, and in primates it is especially well developed. Recently, our understanding of how the visual pulvinar is subdivided into nuclei has greatly improved as a number of histological procedures have revealed marked architectonic differences within the pulvinar complex. At the same time, there have been unparalleled advances in understanding of how visual cortex of primates is subdivided into areas and how these areas interconnect. In addition, considerable evidence supports the view that the hierarchy of interconnected visual areas is divided into two major processing streams, a ventral stream for object vision, and a dorsal stream for visually guided actions. In this review, we present evidence that a subset of medial nuclei in the inferior pulvinar function predominantly as a subcortical component of the dorsal stream while the most lateral nucleus of the inferior pulvinar and the adjoining ventrolateral nucleus of the lateral pulvinar are more devoted to the ventral stream of cortical processing. These nuclei provide cortico-pulvinar-cortical interactions that spread information across areas within streams, as well as information relayed from the superior colliculus via inferior pulvinar nuclei to largely dorsal stream areas.
Keywords: visual system, superior colliculus, visual cortex, thalamus
Introduction
The pulvinar complex has long been a poorly understood part of the dorsal thalamus. At a time when almost nothing was known, Cajal (1852-1934) recognized that the visual pulvinar is well developed in humans and other primates, but was rudimentary or possibly absent in rodents and many other mammals (Cajal, 1911). We now know that the superior colliculus and visual cortex inputs identify a posterior part of the thalamus as belonging to the visual pulvinar in all mammals. In non-primates, it is still common to call part or all of the pulvinar complex, the lateral posterior nucleus (see for review Lyon, et al., 2003; Lyon, 2007). The visual pulvinar is especially well-developed and differentiated into distinct nuclei in primates. The visual pulvinar of primates consists of several divisions of the inferior and lateral pulvinar, while the medial and anterior pulvinar have mainly multisensory and somatosensory functions (Stepniewska, 2003).
The visual pulvinar receives inputs from subdivisions of visual cortex and projects backto subdivisions of visual cortex. The inputs from cortex are of two types: a driving type that can provide the main source of activation and a modulating type that appears to only modulate ongoing activity (Sherman and Guillery, 2001). Other inputs to selective parts of the visual pulvinar are from the superior colliculus, and, to a limited extent, the retina (Stepniewska, 2003). The nuclei of the visual pulvinar project back to visual cortex to modulate ongoing activity and possibly drive some neurons.
Advances in understanding how visual cortex of primates is subdivided into areas of functional significance provide the groundwork for studies of how these areas interconnect with each other and subdivisions of the pulvinar. Present evidence suggests that at least some primates have on the order of 30 or more cortical areas that are largely devoted to vision (e.g., Felleman and Van Essen, 1991; Lyon, 2007). While not all of these proposed visual areas are well defined, studies of connections, the effects of lesions on behavior, and other types of evidence indicate that the connectional hierarchy of visual areas, starting with primary visual cortex (V1), is divided into two functionally distinct processing streams (Ungerleider and Mishkin, 1982; Goodale and Milner, 1992; Ungerleider and Haxby, 1994; Goodale and Westwood, 2004). A ventral stream of interconnected visual areas is largely devoted to object vision and perception, while a dorsal stream of areas is specialized for visually guiding behavior and making visuospatial judgments. Considerations of processing in the ventral and dorsal streams generally do not focus on the role that interactions with the pulvinar might play. Here we present evidence that most of the nuclei of the inferior pulvinar complex are mainly interconnected with visual areas in the dorsal stream, while the most lateral nucleus of the inferior pulvinar and the adjoining lateral pulvinar are interconnected with early processing areas that are proportionally more devoted to the ventral stream.
Cortical areas of the dorsal and ventral streams
Some of the visual areas involved in the dorsal and ventral streams of processing are shown on a dorsolateral view of an owl monkey brain (Figure 1). While much of the research on these streams has been on macaque monkeys, the areas shown are those likely to be common to all primates (Kaas, 2004a; Lyon, 2007). These visual areas are more easily illustrated on the brain of a small, New World monkey with fewer fissures than macaque monkeys. Here we focus on the areas that are early in the visual processing hierarchy, as these areas have been most clearly defined. Even for these areas, there has not been full agreement over the boundaries, extents and even the existence of some areas. All investigators recognize primary visual cortex, area 17 or V1, the second visual area, V2, and the middle temporal visual area, MT, but there is less agreement on the other areas illustrated. Most notably, the third visual area, V3, is sometimes recognized as a single field, and sometimes divided into two fields, dorsal V3 (V3d) and the ventroposterior area, VP (see Kaas and Lyon, 2001). In early studies from our laboratory, we did not recognize V3 and included the territory of V3 in other visual areas (see Kaas and Lyon, 2001). However, now we feel that the issue is largely resolved (Lyon, 2007), as a series of anatomical studies provided strong evidence for V3 (including ventral and dorsal halves) in prosimian galagos (Lyon and Kaas, 2002a) four species of New World monkeys (Lyon and Kaas, 2001; 2002b; Lyon, et al., 2002) and Old World macaque monkeys (Lyon and Kaas, 2002c). V1 and V2 both have connections that associate them with both the dorsal and ventral visual streams, but more of their outputs are directed into the ventral stream (Kaas, 2004a). Less is known about the cortical and subcortical connections of V3 due to difficulties in identifying the borders of V3. But, V3 also appears to be involved in both the dorsal and ventral streams (Kaas and Lyon, 2001).
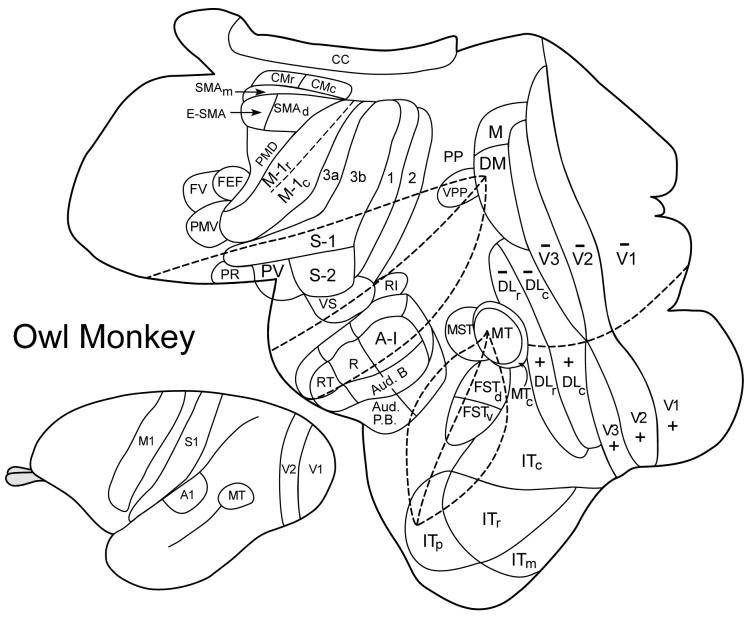
Figure 1.
Surface view of unfolded and flattened cortex of an owl monkey with cortical areas indicated. A view of the intact brain is on the bottom left. Abbreviations: auditory areas: A1, primary auditory; R, rostral area; AT, rostrotemporal area; Aud. B, auditory belt; Aud. P.B., auditory parabelt. Somatosensory areas: 3a, 3b, 1 and 2 of Brodmann (3a = primary somatosensory area, S-1), S2, second area; PV, parietal ventral area; PR, parietal rostral area; VS, parietal ventral area. Motor areas: rostral, r, and caudal, c, divisions of primary motor cortex, M1; PMD, dorsal premotor cortex; PMV, ventral premotor cortex; SMAd, dorsal supplementary motor area; SMAm, medial supplementary motor area; E-SMA, eye-supplementary motor area; CMr, rostral cingulate motor area; CMc, caudal cingulate motor area; FEF, frontal eye field; FV, frontal visual area. Visual areas: V1, V2, and V3, first, second and third visual areas; M, medial visual area; DM, dorsomedial visual area; DLr and DLc, rostral and caudal dorsolateral visual areas; ITc, ITr, ITm, and ITp, caudal, rostral, medial, and polar divisions of inferior temporal (IT) cortex; MT, the middle temporal visual area; MTc, the middle temporal crescent; MST, the middle superior temporal area; FSTv and FSTd, ventral and dorsal areas of the fundus of the superior temporal sulcus (FST); VPP, ventral posterior parietal region; PP, posterior parietal cortex. Also, cc, corpus callosum.
Another area with uncertainties is the dorsomedial visual area, DM, long ago identified by microelectrode mapping and architecture in owl monkeys (Allman and Kaas, 1975), and soon thereafter subsumed in an area termed V3a in macaque monkeys (Zeki, 1978). Early depictions of DM included part of the territory of dorsal V3, and V3 connections with V1 were interpreted as DM connections. Now we have clear evidence for separate retinotopic patterns of connections of V1 with V3 and with DM, providing strong evidence for both areas (Lyon and Kaas, 2001; 2002a; 2002b; 2002c; Lyon, 2007). DM has cortical connections that associate it with the dorsal stream (e.g., Wagor, et al., 1975; Krubitzer and Kaas, 1993; Beck and Kaas, 1998a; 1999).
The dorsolateral visual area, DL, was recognized by a pattern of retinotopic organization in early experiments on owl monkeys (Allman and Kaas, 1974). Subsequent studies led to some modification of the border of DL, and to the subdivision of DL into rostral and caudal areas, DLr and DLc, containing parallel retinotopic representations and differing in connection patterns (Cusick and Kaas, 1988; Steele and Weller, 1993). The rostral area, DLr has cortical connections that associate it with the dorsal stream while the caudal division has connections predominantly with the ventral stream. Together, DLr and DLc roughly correspond to the V4 area of macaque monkeys, especially along the proposed dorsal border, but evidence presented elsewhere suggests that the proposed ventral border of V4 extends too far ventrally (Stepniewska, et al., 2004). DL is joined ventrally by subdivisions of inferior temporal cortex (IT) belonging to the ventral stream.
The middle temporal visual area, MT (Allman and Kaas, 1971) is part of a constellation of adjoining visual areas that cortical connection patterns identify as belonging to the dorsal stream. MT receives direct inputs from V1 and from a set of band-like modules in V2 that are dominated by the M-cell pathway from the retina to cortex (Casagrande and Kaas, 1994) and is interconnected to the medial superior temporal area, MST, the fundal area of the superior temporal sulcus, FST, and the MT crescent, MTc (e.g., Krubitzer and Kaas, 1990). MTc is a narrow visual area surrounding much of MT (Kaas and Morel, 1993). It differs from the earlier proposed V4t (Ungerleider and Desimone, 1986) by being more extensive and including representations of both the upper and lower visual quadrants, instead of just the lower quadrant (Rosa and Elston, 1998). FST is divided into dorsal and ventral areas with FSTd having dense interconnections with MT and FSTv having dense interconnections with MTc (Kaas and Morel, 1993). In addition, these areas have connections with DM and DLr, and other fields of the dorsal stream.
Finally, many of the dorsal stream areas, including DM, MT, MST, FSTd, have connections with the caudal portion of posterior parietal cortex in a region we have called ventral posterior parietal cortex (e.g., Kaas and Morel, 1993). This region appears to include cortex referred to as the posterior parietal reach region, the ventral intraparietal area, and the lateral intraparietal area of macaque monkeys (see Cavada, 2001). It is not yet certain if these areas are well defined in New World monkeys. Nevertheless, it is relevant to note that these regions of posterior parietal cortex with inputs from dorsal stream visual areas interconnect with areas of motor cortex to influence motor behavior in all primates (see Rizzolatti, et al., 1997; Stepniewska, et al., 2005).
Subdivisions of the visual pulvinar
The pulvinar complex includes four major divisions: the inferior pulvinar (PI), the lateral pulvinar (PL), the medial pulvinar (PM), and the anterior or oral pulvinar (PA). The extents of these traditional subdivisions have been portrayed somewhat differently in early studies based on cytoarchitecture (e.g., Olszewski, 1952) or myeloarchitecture (Walker, 1938). Newer histochemical techniques together with studies of connections have added greatly to our understanding of how the pulvinar complex is divided into nuclei. Here we are not concerned with the anterior pulvinar, which has connections with areas of somatosensory cortex (Kaas, 2004b), the medial pulvinar with widespread connections to multisensory and other areas of cortex, or the dorsomedial nucleus of the lateral pulvinar with prefrontal and inferior parietal connections (Fig. 2). Instead, we focus on the ventrolateral nucleus of the lateral pulvinar, and nuclei of the inferior pulvinar, as these nuclei relate to occipital and temporal areas of the dorsal and ventral streams of visual processing. Connection patterns with retinotopically organized areas of visual cortex indicate that PIvl and PIcl contain precise representations of the contralateral visual hemifield, while PIcm and possibly other nuclei of PI have crude representations. The representations are aligned in parallel with the upper field represented ventral to that of the lower field (Fig. 3).
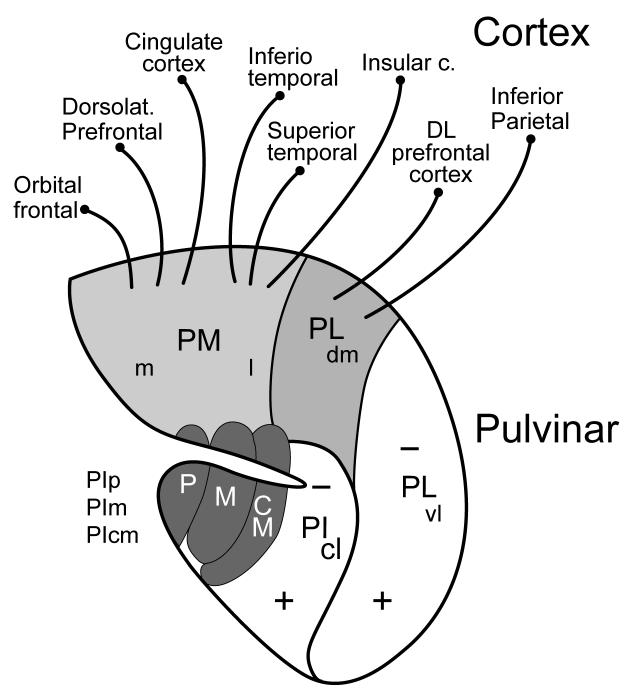
Figure 2.
Subdivisions and connections of the pulvinar complex in macaque monkeys. The outline is from a coronal section through the complex with lateral to the right. The medial pulvinar (PM), with medial (m) and lateral (l) divisions, and the dorsomedial nucleus of the lateral pulvinar (PLdm) have connections with dorsolateral (DL) prefrontal cortex and other parietal, frontal, and temporal areas, and are not considered further here. The ventrolateral nucleus of the lateral pulvinar (Plvl) and the central lateral nucleus of the inferior pulvinar (PIcl) contain large retinotopic representations of the contralateral visual hemifield (+, upper field; - lower field), and interconnect with areas more devoted to the ventral stream of visual processing, while the posterior (p), medial (m), and central medial (cm) nuclei of the inferior pulvinar (PIp, PIm, and PIcm) interconnect with areas of the dorsal stream. See Gutierrez et al. (2000) for a detailed account of the connections of PM and PLdm. See Stepniewska (2003) for review.
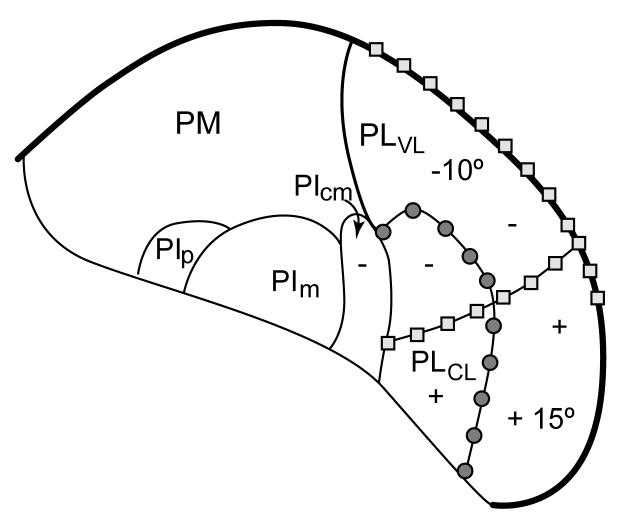
Figure 3.
The retinotopy of nuclei of the inferior and lateral pulvinar. Squares mark the representation of the zero horizontal meridian, while circles mark the vertical meridian. The locations of -10° and +15° on PLvl mark approximate locations representing 10° in the lower field and 15° in the upper field, respectively. (+, upper field; - lower field) Coronal brain section with lateral to the right. Based on unpublished studies of Lyon and Kaas and Adams et al. (2000).
Early efforts to subdivide the inferior pulvinar of monkeys into functionally distinct areas based on differences in histology and connections (Lin and Kaas, 1980) led to a proposed large central nucleus (PIc), a smaller medial nucleus (PIm) and a yet smaller posterior nucleus (PIp). We have retained this early terminology here, but more extensive, histochemical studies have led to a further division of PIc into medial (PIcm) and lateral (PIcl) nuclei (Stepniewska and Kaas, 1997). A marked histochemical difference between these two nuclei, for example, is apparent in brain sections processed for acetylcholinesterase (Fig. 4). In addition, Cusick et al. (1993) have identified a slender subdivision of PIcl along the lateral geniculate nucleus as the lateral shell of the inferior pulvinar (PIls). When a greater range of histological preparations is considered, it is possible to more fully characterize the differences that distinguish these nuclei (Fig. 5). Overall, the evidence is good for four or five divisions of the inferior pulvinar complex in both New World and Old World monkeys (also see Cusick, et al., 1993; Gutierrez, et al., 1995; Gutierrez and Cusick, 1997; Gray, et al., 1999; Adams, et al., 2000; Gutierrez, et al., 2000; O’Brien, et al., 2001; Weller, et al., 2002; Lyon, 2007).
Figure 4.
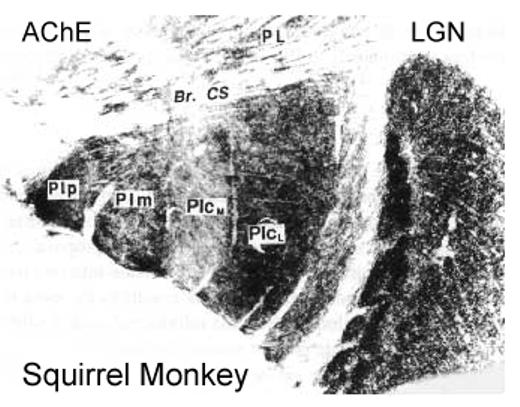
The inferior pulvinar of a squirrel monkey in a coronal section (lateral to the right) processed for acetylcholinesterase (AchE). The posterior (PIp), medial (PIm), central medial (PIcm) and central lateral nuclei (PIcl) of the inferior pulvinar are identified. A lighter lateral zone in PIcl may correspond to a “shell” (s) division of the inferior pulvinar proposed by Gutierrez et al. (1995). The dorsal lateral geniculate nucleus (LGN) is on the right, and the lateral pulvinar (PL) above. Br. CS, brachium of the superior colliculus. Based on Stepniewska and Kaas, 1997.
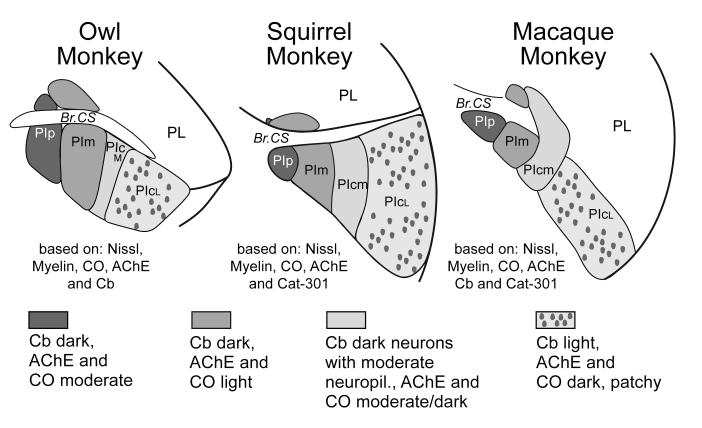
Figure 5.
Histological subdivisions of the inferior pulvinar in New World owl and squirrel monkeys and Old World macaque monkeys. The subdivisions are based on the results of a number of studies including Nissl and myelin stains, and reactions for acetylcholinesterase (AchE), Calbindin (Cb), cytochrome oxidase (CO) and the antigen for the antibody Cat-301. The figure has been modified from Stepniewska and Kaas (1979).
Pulvinar connections with cortical areas of the ventral stream
The central lateral nucleus of the inferior pulvinar, PIcl, and the ventral lateral nucleus of the lateral pulvinar, PLvl, project to three large, retinotopically organized areas; V1, V2, and caudal DL (DLc or V4). While V1 and V2 contribute to both the dorsal and ventral streams of visual processing, the bulk of their output goes to the ventral stream area DLc (V4) either directly or indirectly via relays in V2 and V3 (Casagrande and Kaas, 1994; Roe, 2004). Thus, the predominant influence of PIcl and PLvl would seem to be on processing in the ventral stream.
An example of some of the evidence that PLvl and PIcl project in topographic patterns to V1 of monkeys is shown in Figure 6. Note that injections of different tracers in two retinotopic locations in V1 of a macaque monkey labeled neurons in two locations in both nuclei, providing evidence for parallel representations of the contralateral visual hemifield in the two nuclei. Similar results were obtained in other macaque monkeys, New World marmosets, owl monkeys, and squirrel monkeys, and even prosimian galagos in a series of experiments involving V1 projections. However, only the cortical connections stemming from these injections have been described in detail (Lyon and Kaas, 2001; 2002a; 2002b; 2002c). Similar patterns of projections from these parts of the pulvinar to V1 of monkeys have been described more extensively elsewhere (e.g., Gutierrez and Cusick, 1997; Adams, et al., 2000).
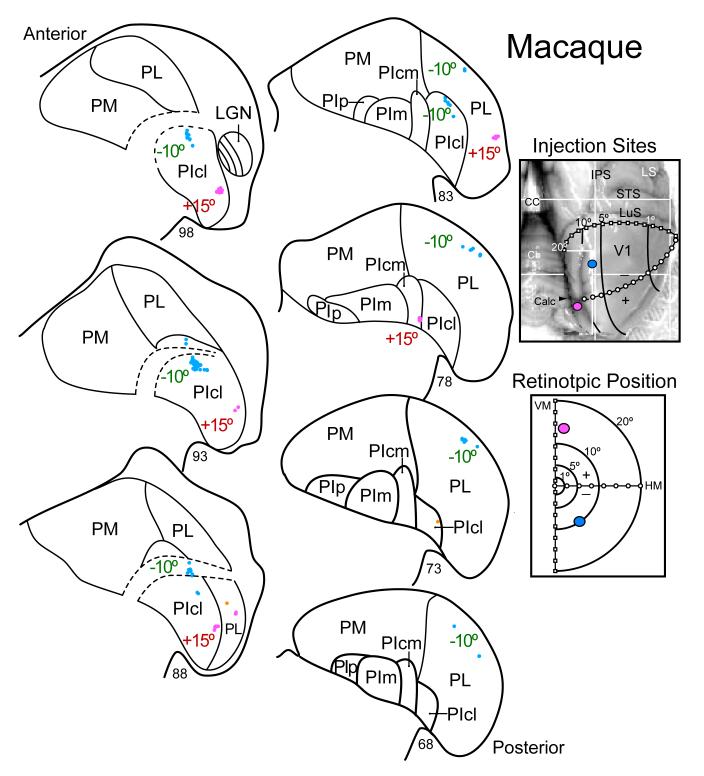
Figure 6.
Evidence for topographic patterns of connections of primary visual cortex (V1) with the ventral lateral nucleus of the lateral pulvinar (PL) and the central lateral nucleus of the inferior pulvinar (PIcl) of a macaque monkey. Injection of different tracers (see Lyon and Kaas, 2002c for details) were placed in locations above (-10°) and below (+15°) the calcarine fissure in V1 that labeled neurons in -10° and +15° locations in PL and PIcl. The box on the upper right shows the locations of the two injection sites on a caudodorsal view of the right cerebral hemisphere. CC, corpus callosum; Cb, cerebellum; Calc, calcarine sulcus; LuS, lunate sulcus; STS, superior temporal sulcus; IPS, intraparietal sulcus; LS, lateral sulcus. The box on the lower right shows the retinotopic locations of the injections in a schematic of the contralateral visual hemifield. On the left, nuclei of the pulvinar complex are outlined on an anterior to posterior numbered series of coronal brain sections. Dots indicate the locations of labeled cells. Connections as in previous figures.
In a similar manner, projections to V2 from the pulvinar are largely from retinotopically matched locations in PLvl and PIcl, with a smaller number of projecting neurons in PIcm. One example of V2 projecting neurons in the pulvinar is shown for a marmoset in Figure 7. For comparison, note that a second injection in auditory cortex (A1) labeled neurons in the medial geniculate nucleus, as expected, and an injection in MST labeled neurons in PIm (see below). Similar results have been described after V2 injections in marmosets by Kaske et al. (1991). In macaques, the dominant inputs to V2 are from PLvl and PIcl, although PIcm and PIm appear to contribute as well (Adams, et al., 2000; O’Brien, et al., 2002; Shipp, 2003). The fewer PIcm and PIm projections to V2 may relate to the modular organization of V2, where two types of modules, the thin stripes and interstripes, project to DL (V4) of the ventral stream while a third type of module, the thick stripes, project to MT of the dorsal stream (see Roe, 2004 for review). Nevertheless, the bulk of the projecting neurons appear to be in PLvl and PIcl, where overlapping populations of neurons project to retinotopically matched locations in V1 and V2, and roughly ten percent of the neurons in PLvl and PIcl project to both V1 and V2 (Kennedy and Bullier, 1985).
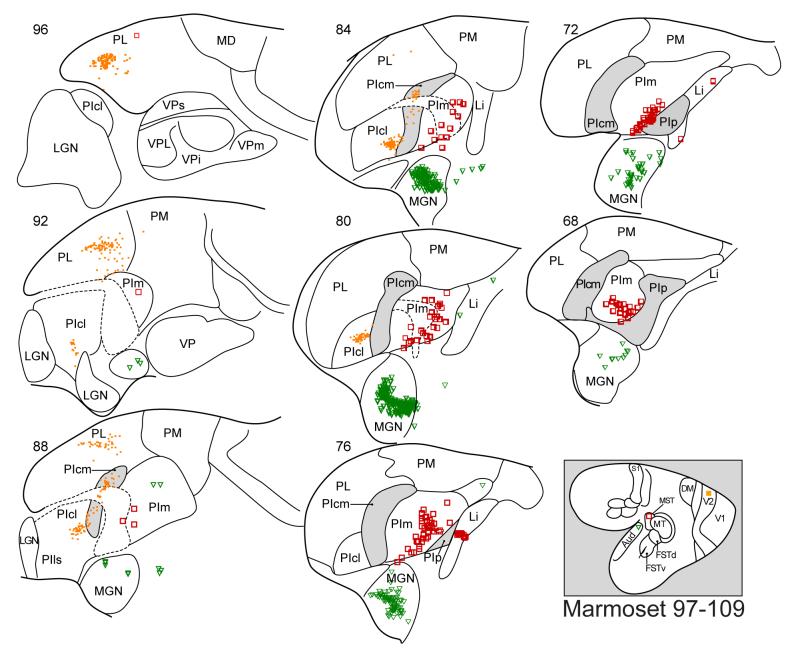
Figure 7.
Thalamic neurons labeled by an injection in V2, MST, or primary auditory cortex (A1) of a marmoset. Thalamic neurons labeled by each injection are indicated by a different symbol (V2, filled squares; MST, open squares, A1, triangles. Conventions are as in previous figures.
The pulvinar connections of DL/V4 depend on whether rostral or caudal DL is involved. Injections of tracers in DLc (caudal V4) in owl monkeys, squirrel monkeys, and macaque monkeys, labeled large numbers of neurons in PLvl and PIcl, while injections in DLr (rostral V4) labeled more neurons in PIcm and PIp (Weller, et al., 2002). In macaques, Adams et al. (2000) previously reported that the regions of PLvl and PIcl projected densely to V4 (their injections largely involved caudal V4). As some projections also came from PIcm, and PIp, there probably was some involvement of rostral V4. These findings are consistent with those of a number of studies describing the connections of caudal DL/V4 with the pulvinar (Lin and Kaas, 1980; Dick, et al., 1991; Soares, et al., 2001; O’Brien, et al., 2002; Shipp, 2003). In contrast, injections in rostral DL (DLr or V4r) of the dorsal stream labeled neurons mainly in PIcm (Cusick et al., 1993; Gray et al., 1999).
We are presently uncertain if V3 resembles V2 by being more involved in the ventral than the dorsal stream. To some extent, inputs from V1 and V2 suggest that V3 resembles V1 and V2 in having a role in both streams. While the pulvinar and cortical connections of V3 are not fully established because it has been difficult to define the borders of V3, injections in caudal DM (Beck and Kaas, 1998b), in cortex next to V2 that now appears to be largely V3, labeled large numbers of neurons in PLvl and PIcl, although some labeled neurons were in PIcm and PIp. Conversely, the results of other studies suggest a greater contribution of projections to V3d from the medial nuclei and the lateral shell of the inferior pulvinar (Shipp, 2001). Furthermore, recent work using transynaptic tracers suggests that V3 shares a common projection from the pulvinar with dorsal stream area MT (Lyon et al., 2005).
Higher-level components of the ventral stream include the several subdivisions of inferior temporal (IT) cortex (Figure 1). In macaques, Baleydier and Morel (1992) concluded that the caudal division of IT cortex, TEO, receives major inputs from the lateral pulvinar (PLvl), while more rostral IT cortex, TE, has dense inputs from the medial pulvinar. Similar results were reported by Bazier, et al. (1993) and Webster et al. (1993), with some additional involvement of PIcl with TEO injections. In squirrel monkeys, injections in a caudal IT region, ITc (comparable to TEO), labeled neurons in PLvl and PIcl, but also in PIp (Steele and Weller, 1993). The neurons labeled in PIp may have resulted from the injection also involving rostral IT, where injections labeled PIp, PLdm, and PM. Thus, the connections of the caudal part of inferior temporal cortex are with parts of the pulvinar that also project to V1, V2, and caudal DL.
Pulvinar connections with cortical areas of the dorsal stream
The middle temporal area, MT, is the earliest visual area that is clearly in the dorsal stream. Patterns of interconnections also place MT in the center of a constellation of visual areas of the dorsal stream, MST, MTc, FSTd, and FSTv. MT receives inputs from V1 and V2 that are dominated by the magnocellular retinogeniculo-cortical pathway, and has interconnections with MST, MTc, FSTd, and DLr, and to posterior parietal visuomotor areas (e.g., Krubitzer and Kaas, 1990). Pulvinar inputs to MT have long been known to originate in PIm (Lin and Kaas, 1980). Figure 8 shows an example of thalamic label after an MT injection in a marmoset. Most of the neurons labeled by the MT injection were in PIm, as expected, but a few were in other subdivisions of the inferior pulvinar. In squirrel monkeys, Cusick, et al. (1993) also found dense label in PIm after MT injections, but some label was in PIcm and PIcl (the authors used a slightly different terminology). Similar results were obtained in both New and Old World monkeys by Stepniewska et al. (2000). In macaque monkeys, Adams et al., (2000) reported dense projections to MT from PIm, while PIcm and PIp contributed a few projections. Results from a number of earlier studies in which PIm was not recognized, are nevertheless consistent with the observations above (e.g., Maunsell and Van Essen, 1983; Ungerleider, et al., 1984). Thus, across New and Old World monkeys, the bulk of pulvinar projections to MT are from PIm, while a few are from PIcm and PIp. Interestingly, MT also receives inputs from the region of the lateral shell of the inferior pulvinar (PIls) adjacent to the lateral geniculate nucleus (Fig. 8).
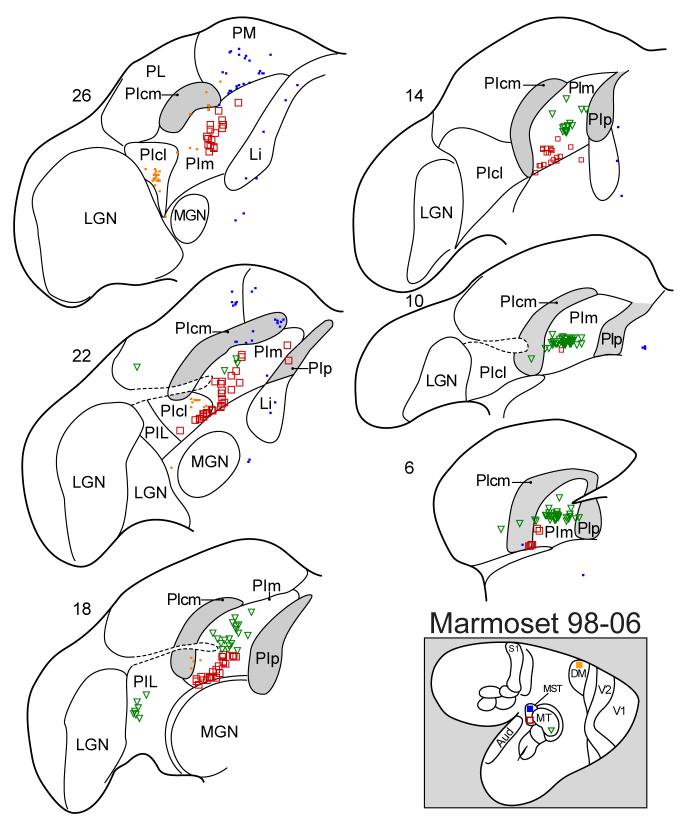
Figure 8.
Thalamic neurons labeled by an injection in MT (green triangles), the rostral DM border (orange squares), the rostral border of MST (blue squares), or MST (open red squares). Conventions as in previous figures.
MST is a dorsal stream visual area with dense inputs from MT. The pulvinar connections of MST appear to be very similar to those of MT. Thus, injections in MST and MT of a marmoset both label neurons largely in PIm (Fig. 8). In addition, the injection in the ventral part of MST, likely to represent upper field vision, labeled neurons more ventral in PIm than the injection in the middle part of MT representing vision nearer the horizontal meridian. Thus, the connection patterns conform to the proposed retinotopy of PIm (Fig. 3). In another marmoset, an injection more dorsal in MST labeled neurons more dorsal in PIm and over a longer extent of PIm (Fig. 7). Another injection along the rostral border of MST, involving mainly cortex rostral to MST, labeled some neurons in PIm, but mostly neurons more dorsal in PM and PLdm. A similar injection in MST of a macaque monkey labeled neurons in the medial portion of PI (PIm), and in the adjoining medial pulvinar (Boussaud, et al., 1992). Overall, the results support the conclusion that PIm is the major source of the pulvinar projections to MST in both marmosets and macaques.
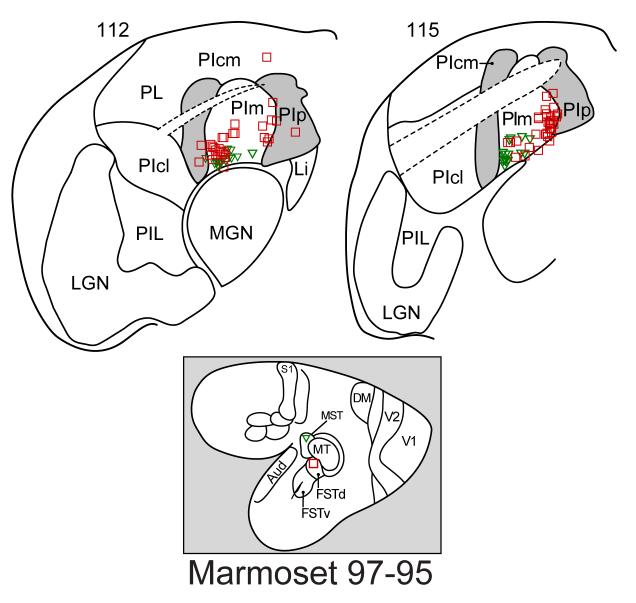
In New World monkeys, we have divided the FST region into a dorsal area, FSTd, with dense interconnections with MT, and a ventral area, FSTv, with dense interconnections with MTc (Kaas and Morel, 1993). This difference in cortical connectivity suggests that the pulvinar connections with FSTd will be more like those of MT than those of FSTv. Figure 9 shows an example of connections after an FSTd injection in a marmoset. The figure also portrays results from an MST injection in the same marmoset. Note that both the MST and the FSTd injections labeled neurons mainly in PIm, as was the case for MT injections, although a few FSTd projecting neurons were in PIcm and PIp. In another marmoset (not shown), an injection largely involved FSTv, but possibly also some of IT. In this case, the labeled neurons were mainly in PIp, with other labeled neurons in PIcm and PIm, suggesting that there may be different patterns of inferior pulvinar inputs to FSTd and FSTv. In macaques, FST injections labeled neurons in the ventrolateral part of the medial pulvinar near the brachium of the superior colliculus (Boussaud et al., 1992). As current histological studies indicate that PIcm, PIm, and PIp all include some pulvinar territory dorsal to the brachium, it seems likely that some or most of the labeled neurons were in the inferior pulvinar. We suggest that the major pulvinar connections of FSTd are with PIm, and that those of FSTv include PIcm and PIp.
Figure 9.
Thalamic connections labeled by injections in FSTd and MST. Labeled neurons are largely in PIm.
The fourth area of the constellation is MTc (Kaas and Morel, 1993). This area forms a narrow belt around MT that includes the area termed V4t in macaques (Ungerleider and Desimone, 1986), but is more extensive as MTc represents both the dorsal and ventral hemifields. Connections of MTc include those with MT and FSTv. Because of the narrowness of the field, it is difficult to confine injections to MTc. However, Weller et al. (2002) described the pulvinar connections that were labeled by injections that were largely restricted to MTc, but also included some of MT or DLr. The results suggest that pulvinar projections to MTc include those from PIm, PIp, and PIcm, as well as some from PIcl and PLdm.
Finally, the dorsomedial area, DM, has connections with posterior parietal cortex, MT, and MST, providing evidence that DM functions as part of the dorsal stream (e.g., Beck and Kaas, 1998a; 1999). The thalamic connections of DM in New World and Old World monkeys described by Beck and Kaas (1998a; 1999) were concentrated in PIm, PIcm and PIcl, while also including PLvl and PLdm. While some of the DM injections likely involved dorsal V3, the dominant pulvinar inputs to DM were those from dorsal stream nuclei of the inferior pulvinar.
The relay of superior colliculus and retinal inputs to visual areas of the dorsal stream
The superior colliculus projects densely to PIp and PIcm, and sparsely to PIcl of the inferior pulvinar of primates (Lin and Kaas, 1979; Stepniewska, et al., 2000). A few projections terminate in PLvl. In addition, some inputs from the superior colliculus project to the region of the lateral shell (PIls) next to the LGN. The terminations in PIp and PIcm originate in a special class of superior colliculus neurons that express the neurotransmitter, substance P. As a result, PIp and PIcm are immunoreactive for substance P. The tectorecipient zone of the lateral posterior nucleus (i.e., pulvinar) of cats is also immunoreactive for substance P (Hutsler and Chalupa, 1991). Thus, substance P may be a marker of one class of superior colliculus inputs to the pulvinar across mammals. In cats, the substance P terminals in the pulvinar are large and terminate on cells that project to cortex (Kelly, et al., 2003), suggesting that these superior colliculus inputs drive pulvinar neurons that could in turn drive cortical neurons. In primates, superior colliculus inputs to the inferior pulvinar could drive neurons that project to MST, FST, MTc, DLr, and DM, providing an extrageniculate source of visual information to visual cortex. Presently, it is uncertain if MTc, FST, MST or DLr of the dorsal stream remains responsive to visual stimuli after the inactivation of primary visual cortex (V1). However, Girard, et al. (1991) reported that some neurons in area V3a (DM) of macaque monkeys remained responsive to visual stimuli after the inactivation of V1. There is also longstanding evidence for the activation of MT via the superior colliculus after V1 lesions in macaques (Rodman, et al., 1989). More recent results in owl monkeys have not supported this view, at least for New World monkeys (Kaas and Krubitzer, 1992; Collins, et al., 2003; Collins, et al., 2005; however see Rosa, et al., 2000). Much of the MT activation via the superior colliculus would be indirect and possibly weak, depending on MTc, FST, or MST inputs. However, a relay from the superior colliculus to MT via the lateral pulvinar shell might provide a more direct pathway (Lyon et al., 2005; Lyon, 2007).
In cats, the neurons in the tectorecipient zone that project to cortex appear to be involved in the integration of motion signals (Merabet, et al., 1998), and the MT constellation of dorsal stream areas also appears to be involved in processing motion signals (Britten, 2003).
Finally, a few projections of the retina terminate directly in the pulvinar complex. Most of these projections appear to terminate in PIm (O’Brien, et al., 2001) providing a very direct extrageniculate pathway to MT. In addition, the koniocells of the LGN project to MT (Stepniewska, et al., 2000; Sincich, et al., 2004; Nassi et al., 2006). Thus, MT and other dorsal stream areas are likely to be at least modulated by visual stimuli by pulvinar and LGN pathways that circumvent V1 cortex.
Conclusions
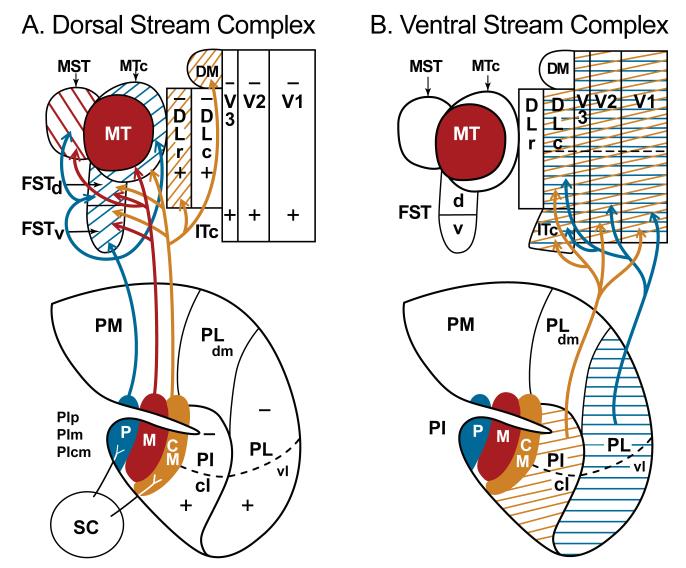
We divide early visual areas in primates into a dorsal stream complex and a complex that is more involved in ventral stream processing (Fig. 10). The ventral stream complex includes the three earliest areas (V1, V2, and V3) that contribute to both the dorsal and ventral streams. Nevertheless, collectively, V1, V2, and V3 provide the dominant activating inputs to the caudal dorsolateral area (DLc or V4), which provides the driving inputs to a series of inferior temporal cortical areas of the ventral stream. The dorsal stream complex includes the key area, MT (which also receives its main cortical inputs from areas V1, V2 and V3), as well as MST, MTc, FSTd and FSTv as its satellites. Interconnections with these areas indicate that DM and DLr also belong to the dorsal stream. Collectively, these dorsal stream areas provide driving outputs to visuomotor areas of posterior parietal cortex.
Two major divisions of the pulvinar complex constitute the visual pulvinar, the inferior pulvinar (PI) and the lateral pulvinar (PL). The medial pulvinar has mixed, largely multisensory functions while the anterior (oral) pulvinar has somatosensory functions.
The lateral pulvinar includes at least two nuclei, the ventrolateral nucleus, PLvl, and the dorsomedial nucleus, PLdm. PLvl has interconnections with V1, V2, DLc and probably V3, thereby providing major inputs to the ventral stream. Other connections are with the most dorsal part of inferior temporal cortex of the ventral stream. Different parts of PLdm have connections with higher-order areas of inferior parietal and prefrontal cortex.
The inferior pulvinar has four distinct nuclei, PIp, PIm, PIcm and PIcl, as well as a lateral shell, PIls. Connections with V1, V2, and PLc associate PIcl with the ventral stream. PIp, PIm, and PIcm are associated with the dorsal stream.
PIp and PIcm receive dense inputs from the superior colliculus. These projection zones express substance P, a marker of superior colliculus inputs to the caudal thalamus in cats and rodents. More sparse inputs from the superior colliculus terminate in PIcl, PIls, and in PL. Overall, information from the superior colliculus appears to have a greater influence on dorsal stream processing via PIp and PIcm than on ventral stream processing. However, PIm, the pulvinar nucleus that targets MT does not receive significant superior colliculus inputs.
The MT satellites, MST, FSTd, FSTv, and MTc receive inputs from PIm and PIp, while PIcm projects to FSTr, FSTd, DLr and DM.
Most of the pulvinar inputs to visual cortex are likely to be modulating. However, some may be driving. The major driving inputs to the visual pulvinar are from visual cortex, although PIp and PIcm may receive driving inputs from the superior colliculus. Each pulvinar nucleus receives inputs from several visual areas of either the dorsal or ventral stream, and projects back to areas of one stream or the other, providing visual information in the feedback and feedforward categories.
While most of visual cortex appears to depend on a lateral geniculate relay to primary visual cortex for driving activation, some dorsal stream areas (especially MST, FSTd, and MTc) may be driven by superior colliculus inputs relayed by PIp and PIcm. MT has long been considered one of these areas, but recent recording evidence from MT after V1 lesions in owl monkeys, argues against this conclusion. DM (V3a) is another dorsal stream area where evidence exists for activation that does not depend on V1.
Figure 10.
Nuclei of the pulvinar related to visual areas of the dorsal and ventral streams. A. Arrows indicate connections between nuclei of the inferior pulvinar and cortical areas of the dorsal stream. Note also, the superior colliculus inputs to posterior (p) and central medial (cm) nuclei of the inferior pulvinar (PI). B. Arrows indicate connections between visual areas of the ventral stream and the central lateral nucleus of the inferior pulvinar (PIcl) and the ventrolateral nucleus of the lateral pulvinar (PL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prepared for: Brain Research Special Issue Reflecting on Golgi and Cajal
References
- Adams AD, Hof PR, Gattass R, Webster MJ, Ungerleider LG. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J. Comp. Neurol. 2000;419:377–393. doi: 10.1002/(sici)1096-9861(20000410)419:3<377::aid-cne9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH. A representation of a visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus) Brain Res. 1971;31:85–105. doi: 10.1016/0006-8993(71)90635-4. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH. A crescent-shaped cortical visual area surrounding the middle temporal area (MT) in the owl monkey (Aotus trivirgatus) Brain Res. 1974;81:199–213. doi: 10.1016/0006-8993(74)90936-6. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH. The dorsomedial cortical visual area: A third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus) Brain Res. 1975;100:473–487. doi: 10.1016/0006-8993(75)90153-5. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Desimone R, Ungerleider LG. Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Vis. Neurosci. 1993;10:59–72. doi: 10.1017/s0952523800003229. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Morel A. Segregated thalamocortical pathways to inferior parietal and inferotemporal cortex in macaque monkey. Vis. Neurosci. 1992;8:391–405. doi: 10.1017/s0952523800004922. [DOI] [PubMed] [Google Scholar]
- Beck PD, Kaas JH. Cortical connections of the dorsomedial visual area in new world owl monkeys (Aotus trivirgatus) and squirrel monkeys (Saimiri sciureus) J. Comp. Neurol. 1998a;400:18–34. doi: 10.1002/(sici)1096-9861(19981012)400:1<18::aid-cne2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Beck PD, Kaas JH. Thalamic connections of the dorsomedial visual area in primates. J. Comp. Neurol. 1998b;396:381–398. [PubMed] [Google Scholar]
- Beck PD, Kaas JH. Cortical connections of the dorsomedial visual area in Old World macaque monkeys. J. Comp. Neurol. 1999;406:487–502. [PubMed] [Google Scholar]
- Boussaud D, Desimone R, Ungerleider LA. Subcortical connections of visual areas MST and FST in macaques. Vis. Neurosci. 1992;9:291–301. doi: 10.1017/s0952523800010701. [DOI] [PubMed] [Google Scholar]
- Britten KH. The middle temporal visual area: motion processing and the link to perception. In: Chalupa LM, editor. The Visual Neurosciences. MIT Press; Cambridge: 2003. pp. 1203–1216. [Google Scholar]
- Cajal SRY. Histology of the Nervous System. Oxford Univ Press; Oxford: 1911. English translation by N. Swanson and L.W. Swanson, 1995. [Google Scholar]
- Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland K, editors. Cerebral Cortex. Vol. 10. Plenum Press; New York: 1994. pp. 201–259. [Google Scholar]
- Cavada C. The visual parietal areas in the macaque monkey: current structural knowledge and ignorance. NeuroImage. 2001;14:521–526. doi: 10.1006/nimg.2001.0818. [DOI] [PubMed] [Google Scholar]
- Collins CE, Lyon DC, Kaas JH. Responses of neurons in the middle temporal visual area after long-standing lesions of the primary visual cortex in adult New World monkeys. J. Neurosci. 2003;23:2251–2264. doi: 10.1523/JNEUROSCI.23-06-02251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CE, Xu X, Khaytin I, Kaskan PM, Casagrande VA, Kaas JH. Optical imaging of visually evoked responses in the middle temporal area after deactivation of primary visual cortex in adult primates. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5594–5599. doi: 10.1073/pnas.0501762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick CG, Kaas JH. Cortical connections of area 18 and dorsolateral visual cortex in squirrel monkeys. Vis. Neurosci. 1988;1:211–237. doi: 10.1017/s0952523800001486. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Scripter JL, Darensbourg JG, Weber JT. Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual areas, MT and DLr. J. Comp. Neurol. 1993;336:1–30. doi: 10.1002/cne.903360102. [DOI] [PubMed] [Google Scholar]
- Dick A, Kaske A, Creutzfeldt OD. Topographical and topological organization of the thalamocortical projection to the striate and prestriate cortex in the marmoset (Callithrix jacchus) Exp. Brain Res. 1991;84:233–253. doi: 10.1007/BF00231444. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Girard P, Salin P-A, Bullier J. Visual activity in areas V3a and V3 during reversible inactivation of area V1 in the macaque monkey. J. Neurophysiol. 1991;66:1493–1503. doi: 10.1152/jn.1991.66.5.1493. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr. Opin. Neurobiol. 2004;14:203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gray D, Gutierrez C, Cusick C. Neurochemical organization of inferior pulvinar complex in squirrel monkeys and macaques revealed by acetylcholinesterase histochemistry, calbindin and Cat-301 immunostaining, and Wisteria floribunda agglutinin binding. J. Comp. Neurol. 1999;409:452–468. doi: 10.1002/(sici)1096-9861(19990705)409:3<452::aid-cne9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Yaun A, Cusick CG. Neurochemical subdivisions of the inferior pulvinar in macaque monkeys. J. Comp. Neurol. 1995;363:545–562. doi: 10.1002/cne.903630404. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Cuskic CG. Area V1 in macaque monkeys projects to multiple histochemically defined subdivisions of the inferior pulvinar complex. Brain Res. 1997;765:349–356. doi: 10.1016/s0006-8993(97)00696-3. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Cola MG, Seltzer B, Cusick C. Neurochemical and connectional organization of the dorsal pulvinar complex in monkeys. J. Comp. Neurol. 2000;419:61–86. doi: 10.1002/(sici)1096-9861(20000327)419:1<61::aid-cne4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Chalupa LM. Substance P immunoreactivity identifies a projection from the cat’s superior colliculus to the principal tectorecipient zone of the lateral posterior nucleus. J. Comp. Neurol. 1991;312:379–390. doi: 10.1002/cne.903120306. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Early Visual Areas: V1, V2, V3, DM, DL, and MT. In: Kaas JH, Collins CE, editors. The Primate Visual System. CRC Press; Boca Raton: 2004a. pp. 139–159. [Google Scholar]
- Kaas JH. Somatosensory System. In: Paxinos G, Mai JK, editors. The Human Nervous System. Elsevier Academic Press; New York: 2004b. pp. 1059–1092. [Google Scholar]
- Kaas JH, Krubitzer LA. Area 17 lesions deactivate area MT in owl monkeys. Vis. Neurosci. 1992;9:399–407. doi: 10.1017/s0952523800010804. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Lyon DC. Visual cortex organization in primates: theories of V3 and adjoining visual areas. Prog. Brain Res. 2001;134:285–295. doi: 10.1016/s0079-6123(01)34019-0. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Morel A. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal and ventral subdivisions of FST. J. Neurosci. 1993;13:534–546. doi: 10.1523/JNEUROSCI.13-02-00534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaske A, Dick A, Creutzfeldt OD. The local domain for divergence of subcortical afferents to striate and extrastriate cortex in the common marmoset (Callithrix jacchus): a multiple labeling study. Exp. Brain Res. 1991;84:254–265. doi: 10.1007/BF00231445. [DOI] [PubMed] [Google Scholar]
- Kelly LR, Li J, Carden WB, Bickford ME. Ultrastructure and synaptic targets of tectothalamic terminals in the cat lateral posterior nucleus. J. Comp. Neurol. 2003;464:472–486. doi: 10.1002/cne.10800. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Bullier J. A double-labeling investigation of the afferent connectivity to cortical areas V1 and V2 of the macaque monkey. J. Neurosci. 1985;5:2815–2830. doi: 10.1523/JNEUROSCI.05-10-02815.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. Cortical connections of MT in four species of primates: Areal, modular, and retinotopic patterns. Vis. Neurosci. 1990;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The dorsomedial visual area of Owl monkeys: connections, myeloarchitecture, and homologies in other primates. J. Comp. Neurol. 1993;334:497–528. doi: 10.1002/cne.903340402. [DOI] [PubMed] [Google Scholar]
- Lin CS, Kaas JH. The inferior pulvinar complex in owl monkeys: architectonic subdivisions and patterns of input from the superior colliculus and subdivisions of visual cortex. J. Comp. Neurol. 1979;187:655–678. doi: 10.1002/cne.901870403. [DOI] [PubMed] [Google Scholar]
- Lin CS, Kaas JH. Projections from the medial nucleus of the inferior pulvinar complex to the middle temporal area of the visual cortex. Neurosci. 1980;5:2219–2228. doi: 10.1016/0306-4522(80)90138-4. [DOI] [PubMed] [Google Scholar]
- Lyon DC. The evolution of visual cortex and visual systems. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems. Vol. 3. Elsevier; London: 2007. pp. 267–306. [Google Scholar]
- Lyon DC, Kaas JH. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J. Neurosci. 2001;21:249–261. doi: 10.1523/JNEUROSCI.21-01-00249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav. Evol. 2002a;59:114–129. doi: 10.1159/000064159. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. J. Comp. Neurol. 2002b;449:281–297. doi: 10.1002/cne.10297. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron. 2002c;33:453–461. doi: 10.1016/s0896-6273(02)00580-9. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Xu X, Casagrande VA, Stefansic JD, Shima D, Kaas JH. Optical imaging reveals retinotopic organization of dorsal V3 in New World owl monkeys. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15735–15742. doi: 10.1073/pnas.242600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DC, Jain N, Kaas JH. The visual pulvinar in tree shrews II. Projections of four nuclei to areas of visual cortex. J. Comp. Neurol. 2003;467:607–627. doi: 10.1002/cne.10940. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Nassi JJ, Callaway EM. Primate dorsal stream visual areas receive disynaptic inputs from the superior colliculus. Soc Neurosci Abstrs. 2005:31. [Google Scholar]
- Maunsell JHR, Van Essen DC. The connections of the middle temporal Visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet L, Desautels A, Minville K, Casanova C. Motion integration in a thalamic visual nucleus. Nature. 1998;396:265–268. doi: 10.1038/24382. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Lyon DC, Callaway EM. The parvocellular LGN provides a robust disynaptic input to the visual motion area MT. Neuron. 2006;50:319–327. doi: 10.1016/j.neuron.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien BJ, Abel PL, Olavarria JF. The retinal input to calbindin-D28k-defined subdivisions in macaque inferior pulvinar. Neurosci. Lett. 2001;312:145–148. doi: 10.1016/s0304-3940(01)02220-0. [DOI] [PubMed] [Google Scholar]
- O’Brien BJ, Abel PL, Olavarria JF. Connections of Calbindin-D28k-defined subdivisions in inferior pulvinar with visual areas V2, V4, and MT in macaque monkeys. Thal. Rel. Sys. 2002;28:1–14. [Google Scholar]
- Olszewski J. The Thalamus of the Macaca mulatta, an Atlas for Use with the Stereotaxic Instrument. Karger Press; Basel: 1952. [Google Scholar]
- Rizzolatti G, Fogossi L, Gallese V. Parietal cortex: from sight to action. Curr. Opin. Neurobiol. 1997;7:562–567. doi: 10.1016/s0959-4388(97)80037-2. [DOI] [PubMed] [Google Scholar]
- Rodman HR, Gross CG, Albright TD. Afferent basis of visual response properties in area MT of the macaque: I. Effects of striate cortex removal. J. Neurosci. 1989;9:2033–2050. doi: 10.1523/JNEUROSCI.09-06-02033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AW. Modular complexity of area V2 in the macaque monkeys. In: Kaas JH, Collins CE, editors. The Primate Visual System. CRC Press; Boca Raton: 2004. pp. 109–138. [Google Scholar]
- Rosa MG, Elston GN. Visuotopic organisation and neuronal response selectivity for direction of motion in visual areas of the caudal temporal lobe of the marmoset monkey (Callithrix jacchus): middle temporal area, middle temporal crescent, and surrounding cortex. J Comp Neurol. 1998;393:505–527. [PubMed] [Google Scholar]
- Rosa MGP, Tweedale R, Elston GN. Visual responses of neurons in the middle temporal area of New World monkeys after lesions of striate cortex. J. Neurosci. 2000;20:5552–5563. doi: 10.1523/JNEUROSCI.20-14-05552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus. Academic Press; San Diego: 2001. [Google Scholar]
- Shipp S. Corticopulvinar connections of areas V5, V4, and V3 in macaque monkeys: a dual model of retinal and cortical topographies. J. Comp. Neurol. 2001;439:469–490. doi: 10.1002/cne.1363. [DOI] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico-pulvinar connections. Phil. Trans. B Roy. Soc. London. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nat. Neurosci. 2004;7:1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Soares JGM, Gattass R, Souza APB, Rosa MG, Fiorani M, Jr., Brandas BL. Connectional and neurochemical subdivisions of the pulvinar in cebus monkeys. Vis. Neurosci. 2001;18:25–41. doi: 10.1017/s0952523801181034. [DOI] [PubMed] [Google Scholar]
- Steele GE, Weller RE. Subcortical connections of inferior temporal cortex in squirrel monkeys. Vis. Neurosci. 1993;10:563–583. doi: 10.1017/s0952523800004776. [DOI] [PubMed] [Google Scholar]
- Stepniewska I. The pulvinar complex. In: Kaas JH, Collins CE, editors. The Primate Visual System. CRC Press; Boca Raton: 2003. pp. 53–80. [Google Scholar]
- Stepniewska I, Kaas JH. Architectonic subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 1997;14:1043–1060. doi: 10.1017/s0952523800011767. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Qi HX, Kaas JH. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 2000;17:529–549. doi: 10.1017/s0952523800174048. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Collins CE, Kaas JH. Connection patterns of dorsolateral visual cortex in macaques suggest the dorsal and ventral boundaries of V4. Cereb. Cortex. 2004;15:809–822. doi: 10.1093/cercor/bhh182. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. J. Comp. Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. What and where in the human brain. Curr. Opin. Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DG, Goodale MA, Mansfield RJQ, editors. Analysis of Visual Behavior. MIT Press; Cambridge: 1982. pp. 549–586. [Google Scholar]
- Ungerleider LG, Desimone R, Galkin TW, Mishkin M. Subcortical projections of area MT in the macaque. J. Comp. Neurol. 1984;223:368–386. doi: 10.1002/cne.902230304. [DOI] [PubMed] [Google Scholar]
- Wagor E, Lin CS, Kaas JH. Some cortical projections of the dorsomedial visual area (DM) of association cortex in the owl monkey, Aotus trivirgatus. J. Comp. Neurol. 1975;163:227–250. doi: 10.1002/cne.901630208. [DOI] [PubMed] [Google Scholar]
- Walker AE. The Primate Thalamus, Univ. Chicago Press; Chicago: 1938. [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Subcortical connections of inferior temporal areas TE and TEO in macaque monkeys. J. Comp. Neurol. 1993;335:73–91. doi: 10.1002/cne.903350106. [DOI] [PubMed] [Google Scholar]
- Weller RE, Steele GE, Kaas JH. Pulvinar and other subcortical connections of dorsolateral visual cortex in monkeys. J. Comp. Neurol. 2002;450:217–242. doi: 10.1002/cne.10298. [DOI] [PubMed] [Google Scholar]
- Zeki SM. The third visual complex of rhesus monkey prestriate cortex. J. Physiol. 1978;277:245–272. doi: 10.1113/jphysiol.1978.sp012271. [DOI] [PMC free article] [PubMed] [Google Scholar]