Abstract
Several studies have suggested dissociations between neural circuits underlying the expression of appetitive (i.e. sexual motivation) and consummatory components (i.e., copulatory behavior) of vertebrate male sexual behavior. The medial preoptic area (mPOA) clearly controls the expression of male copulation but, according to a number of experiments, is not necessarily implicated in the expression of appetitive sexual behavior. In rats for example, lesions to the mPOA eliminate male-typical copulatory behavior but have more subtle or no obvious effects on measures of sexual motivation. Rats with such lesions still pursue and attempt to mount females. They also acquire and perform learned instrumental responses to gain access to females. However, recent lesions studies and measures of the expression of the immediate early gene c-fos demonstrate that, in quail, sub-regions of the mPOA, in particular of its sexually dimorphic component the medial preoptic nucleus, can be specifically linked with either the expression of appetitive or consummatory sexual behavior. In particular more rostral regions can be linked to appetitive components while more caudal regions are involved in consummatory behavior. This functional sub-region variation is associated with neurochemical and hodological specializations (i.e. differences in chemical phenotype of the cells or in their connectivity), especially those related to the actions of androgens in relation to the activation of male sexual behavior, that are also present in rodents and other species. It could thus reflect general principles about POA organization and function in the vertebrate brain.
Keywords: Sexual behavior, Sexual motivation, Mesencephalic central gray, Medial preoptic nucleus, Appetitive sexual behavior, FOS
1. Introduction
The analysis the neural bases of behavior represents one of the most daunting tasks for neuroscientists. This endeavor can be approached in a reductionist manner by studying a relatively simple behavioral response (e.g. the gill withdrawal reflex in Aplysia; [83]) or a simple organisms such as the nematode worm Ceanorhabditis elegans that has only 302 neurons whose pattern of connectivity has been thoroughly mapped out [71,151]. These reductionist approaches have been very useful to the field of behavioral neuroscience but the ultimate goal of the behavioral neuroscience research program is to understand highly structured behaviors that are produced by complex organisms including humans. Therefore it is important that experimental analyses also be performed on a variety of behaviors exhibiting a range of complexity in vertebrates. The study of the neural mechanisms of sexual behavior is a particularly appealing topic in this regard. In non-human animals, it is a stereotyped, species-typical behavior that clearly has inherited components but also learned components. One important feature of this behavioral system is that it involves social interactions between partners so that gametes may be successfully transferred and ultimately the result of these interactions determines the reproductive success of a given individual. Furthermore, this behavior is finely controlled by the action of sex steroid hormones in the brain and therefore the identification of the brain sites of steroid action provides a valuable first step toward identifying the neural architecture of the vertebrate brain that controls this behavior.
The brain sites that are the target of sex steroids were first recognized by in vivo autoradiographic studies in the seventies [85,107,108,132] and at approximately the same time, studies based on the stereotaxic implantation of steroids directly into the brain clearly identified the medial preoptic area as a key site for the action of testosterone on the activation of male sexual behavior [34,89]. These studies along with previous lesion studies that demonstrated that damage to the preoptic area (POA) greatly attenuated male sexual behavior [87] provided converging evidence for the central role played by the POA in the neural circuit controlling male sexual behavior and for its role as a key site of hormone action in the activation of this behavior.
Descriptions of male sexual behavior in non-human animals have distinguished between two different phases, a highly variable sequence of behaviors that involves attracting and courting a female followed by the highly stereotyped copulatory sequence [38]. This initial variable phase is often referred to as the appetitive phase while the highly stereotyped copulatory phase is often referred to as the consummatory phase [31,67,141]. Studies of the (neuro)endocrine controls of male sexual behavior have for understandable reasons focused to a large extent on the consummatory aspects of the behavior and it is only more recently that due consideration has been given to the analysis of the appetitive phases of this behavioral sequence. Investigations in rats by Barrry Everitt and his colleagues suggesting a double dissociation between brain regions controlling appetitive and consummatory sexual behavior in rats [67,68] were especially important in stimulating recent work on circuit specializations related to the control of male sexual behavior. Sophisticated behavioral tests have now been designed to analyze in a reasonably independent manner these two aspects of sexual behavior [29,62,67,110,140]. Studies on appetitive male sexual behavior in a diverse set of species are particularly important from a clinical perspective. Patterns of male sexual performance are often stereotypic and can be species-specific in nature. Generalizations from non-human animals is sometimes difficult [112]. In the case of humans, significant advances have been made by urologists concerning the peripheral mechanisms that control penile erections and an entire class of new compounds are available (i.e., the phosphodiesterase type 5 [PDE5] inhibitors such as Sildenafil citrate, Tadalafil and Vardenafil) that act peripherally and have greatly improved sexual performance problems resulting from erectile dysfunction [14]. In contrast to our knowledge about the peripheral mechanisms regulating sexual performance, we know relatively little about the central or peripheral control of male sexual motivation. Anomalies related to male sexual motivation underlies several serious social pathologies so attention to this problem is of potential great clinical significance.
We present here a selective review of the literature concerning the identification of preoptic sub-regions implicated in the activation of appetitive and consummatory components of male sexual behavior by testosterone. Our focus is largely on work in Japanese quail that has exploited the advantages exhibited by this species for the investigation of testosterone action in relation to the activation of male sexual behavior [17,31]. We think that our conclusions are of general significance so corresponding data in other vertebrates when available are also summarized.
2. The preoptic area as an integration center for the control of motivated behaviors
The medial preoptic area (mPOA), a brain region at the junction of the telencephalon and diencephalon but clearly associated with the anterior hypothalamus based on functional considerations, is bi-directionally connected to a large number of brain regions [129]. In particular, the mPOA receives, directly or indirectly, inputs from most if not all sensory modalities and is therefore ideally organized to integrate information from the environment and adjust responses made by the organism to environmental inputs [43,44,80,94,100,123,126,130,135]. The mPOA, in general, is a key center controlling many autonomic functions (e.g. thermoregulation, thirst or hunger) as well as male sexual and maternal behavior (see [117,129] for a list of references). The role of the mPOA in the control of male copulatory behavior in particular has been the subject of intense investigation. Many of these behaviors and physiological activities linked to mPOA function can be placed under the umbrella of the term “motivated” behaviors and thus the preoptic area is often identified as a particularly important brain area for the control of motivation. Motivation in the sense we use it in this review refers to a marked behavioral change in response to a constant stimulus. This definition or variations of it have long been popular with behavioral neuroscientist [109,152]. We do not think it useful to consider the preoptic area as a center for a particular motivational state but rather that it is an important part of several neural pathways that regulate behaviors that are considered to be motivated behaviors based on variation in their response properties to a constant stimulus.
Lesions of the mPOA impair copulation in male rats and in a large number of other mammalian species as well as in all species of birds, reptiles, amphibians and fishes that have been investigated (see [36,80,81] for review). Conversely, a large number of studies in a variety of species indicate that stereotaxic implants of testosterone in the mPOA activate most if not all aspects of male copulatory behavior [32,80]. The implication of the mPOA in the control of male sexual behavior is also attested by experiments demonstrating that performance of this behavior increases neuronal activity in this brain region as assessed by an increase in 2-deoxyglucose incorporation [60], cytochrome oxidase activity [122] or increased transcription of immediate early genes such as c-fos or egr-1 (also know as Zenk in the avian literature) [49,56,76,93,118,134,153].
3. The preoptic neuronal circuit controlling male sexual behavior in vertebrates
Although many lines of evidence implicate the preoptic area in the control of male-typical copulatory behavior, the relative importance of specific cell groups within this region for behavioral control is still not well understood [80,81]. In rats, the medial preoptic nucleus (MPN) is the most clearly defined cell group in the mPOA based on cytoarchitectural criteria [129]. It is obviously essential for male copulatory behavior based on lesion and testosterone implant studies [36,80,81]. The expression of the immediate early gene c-fos in this nucleus following performance of the behavior [80] also supports this view. However, these studies did not determine whether neurons in the preoptic region involved in copulatory behavior are limited to the MPN. Neurochemical studies and analysis of connectivity have revealed clearly defined sub-regions within this nucleus (medial, central and lateral parts; e.g., [41,128,129]) but the specific implication of these sub-regions in the control of different aspects of male sexual behavior is still not well understood. A central subdivision of the MPN in rats that is larger in volume in males than in females was shown to be sexually differentiated by the action of steroids during embryonic life (the sexually dimorphic nucleus of the mPOA or SDN-POA; [41,72]). Several experiments were therefore designed to investigate whether this cell group plays a role in the control of copulatory behavior but, despite the fact that large mPOA/MPN lesions severely disrupt copulatory behavior (as reviewed previously), more discrete lesions specifically destroying the SDN-POA do not seem to affect copulatory behavioral expression in the male [9] (see [143] for review). Transient behavioral deficits were however observed following lesions of the SDN-POA in sexually inexperienced rats [58] and similar lesions also disrupted the expression of male-typical behavior in females [144]. Taken together, available data suggest that relatively large lesions of the mPOA (involving at least parts of the MPN) are required to eliminate copulatory behavior in male rats but no critical sub-group of neurons has been identified as being particularly more important than any other subgroup.
Studies in other species have revealed discrete neuronal groups in the POA that can be linked to the control of male sexual behavior. In gerbils (Meriones unguiculatus) for example, two distinct neuronal aggregates in the mPOA are apparent in Nissl-stained sections, one in the medial and one in the lateral POA, are significantly larger in males than in females [52]. These sexually dimorphic areas (SDA), which can also be identified by the presence of sex steroid receptors [55] and by a denser acetylcholinesterase immunoreactivity [53] seem to be specifically implicated in the control of male sexual behavior in that lesions of both the medial and the lateral SDA produce severe disruptions of male copulatory behavior as compared to lesions in other parts of the mPOA [54]. The lateral and medial SDA are thus part of a neural circuit that is critical for the control of male sexual behavior.
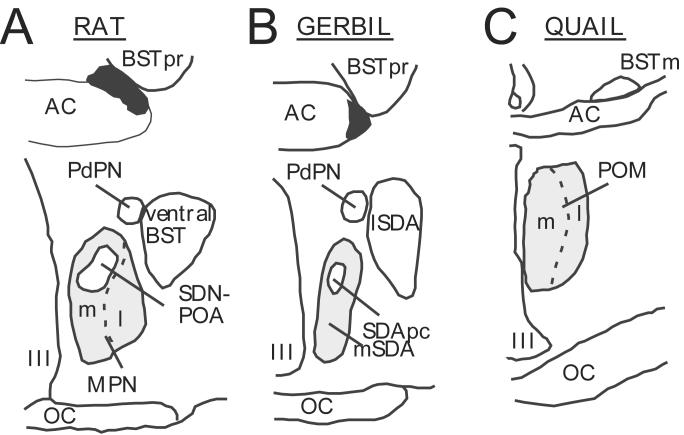
The medial SDA in gerbils has long been considered to be homologous to the medial part of the rat MPN, consistent with the fact that cell-body lesions of the rat MPN eliminate mounting behavior [66,74,90]. The SDA pars compacta, a sexually differentiated smaller group of cells located within the medial SDA that is organized by sex steroids during ontogeny, would then be equivalent to the rat SDN-POA [69,156]. In contrast, the rat equivalent of the gerbil lateral SDA had not been identified. As illustrated in figure 1, a recent study based on the analysis of connectivity and of behavioral effects of discrete cell-body lesions indicates that the gerbil lateral SDA should be considered as homologous to the ventral bed nucleus of the stria terminalis [69].
Figure 1.
Schematic dimorphic nuclei identified in the rat, gerbil and Japanese quail brain. The homologies between rat and gerbil are presented according to the recent work of Finn and Yahr [69] and panels A and B are redrawn from a figure published in that study. The quail medial preoptic nucleus (POM) appears to be homologous to the MPN of rats based on arguments that are presented in the text. Abbreviations: AC: anterior commissure; BST: bed nucleus of the stria terminalis; BSTm: bed nucleus of the stria terminalis, medial part; BSTpr: bed nucleus of the stria terminalis principal nucleus; l: lateral; lSDA: lateral part of the sexually dimorphic area; m: medial; MPN: medial preoptic nucleus (in rats); mSDA: ; OC: optic chiasma; PdPN: posterodorsal preoptic nucleus; POM: medial preoptic nucleus (in quail); SDApc: sexually dimorphic area, pars compacta; SDN-POA: sexually dimorphic nucleus of the preoptic area; III: third ventricle.
Studies utilizing an avian species, the Japanese quail (Coturnix japonica) have similarly led to the identification of a preoptic group of neurons involved in the activation by testosterone of male copulatory behavior (see [17,31,103] for review). Quail display several features that facilitate the identification of the neural circuitry controlling copulation. The volume of the medial preoptic nucleus (POM; illustrated in Fig.1 and 2A) in this species is larger in males than in females [146] and interestingly co-varies with circulating levels of testosterone: it is reduced by castration or exposure to short daylengths (simulating winter conditions of low testosterone concentrations) and increases following treatment with exogenous testosterone or exposure to long days which increase testicular activity [101,139].
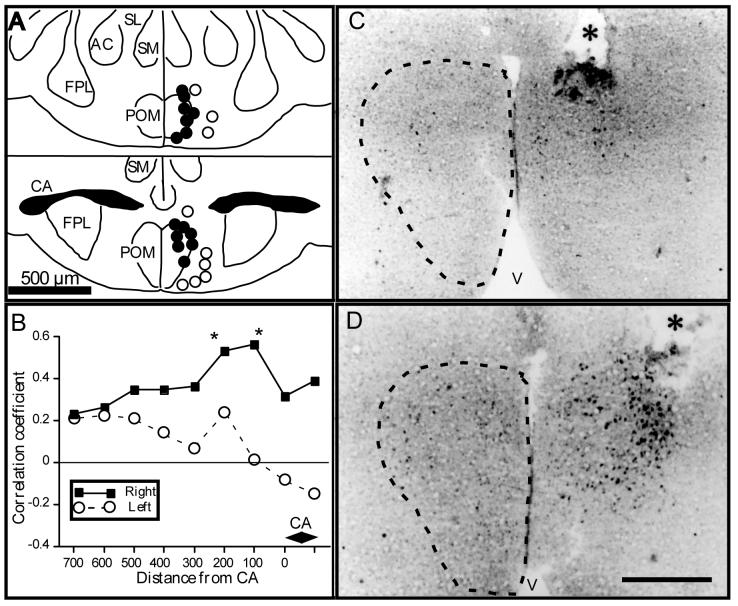
Figure 2.
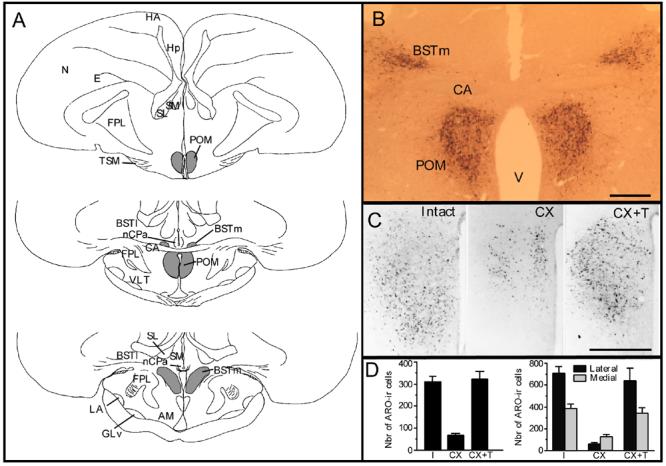
In Japanese quail, the medial preoptic nucleus (POM) is sexually dimorphic and its boundaries are specifically outlined by a dense cluster of aromatase-immunoreactive (ARO-ir) cells. The volume of POM and the number of ARO-ir cells in this nucleus represent a signature of testosterone action in the brain. A. Schematic presentation of the anatomical localization of the POM, which extends through most of the rostro-caudal extent of the preoptic area. At a level just caudal to the anterior commissure (CA), the POM merges with the medial part of the bed nucleus of the stria terminalis (BSTm). The distribution of ARO-ir cells in this brain area (illustrated in gray tone) overlaps exactly with the boundaries of POM and BSTm. Sections are arranged in a rostral to caudal order from top to bottom. B. Photomicrograph illustrating a section through the preoptic area at the level of the anterior commissure that was stained by immunocytochemistry for aromatase. The two groups of ARO-ir cells outlining the POM and BSTm are clearly visible. C. Photomicrographs of the left POM stained by immunocytochemistry for aromatase respectively in a sexually mature gonadally intact male (Intact), in a castrated male (CX) and in a castrated male treated with testosterone (CX+T). D. Quantification of the ARO-ir cells in the POM. In the entire POM, the number of ARO-ir cells is decreased by castration and restored by a treatment with exogenous testosterone (left graph; redrawn from [75]). Separate counts reveal that effects of castration and testosterone are more prominent in the lateral than in the medial part of the nucleus (right graph; redrawn from [12]). Note that data in the two parts of this panel come from different studies in which quantification was performed by different methods in sample fields of different sizes. Absolute numbers of ARO-ir cells thus cannot be directly compared and attention should be paid only to relative changes induced by castration and testosterone treatment. Magnification bar in B-C= 500 μm.
Abbreviations: AM: nucleus anterior medialis hypothalami; BSTl: bed nucleus of the stria terminalis, lateral part; BSTm: bed nucleus of the stria terminalis, medial part; CA: commissura anterior; E: entopallium; FPL: lateral forebrain bundle; GLv: nucleus geniculatus lateralis, pars ventralis; HA: hyperpallium apicale; Hp: hippocampus; LA: nucleus lateralis anterior thalami; N: nidopallium; nCPa; POM: nucleus preopticus medialis; SL: nucleus septalis lateralis; SM: nucleus sepatalis medialis; TSM: tractus septopallio-mesencephalicus; V: third ventricle; VLT: nucleus ventrolateralis thalami.
The size of neurons in the lateral part of the POM is similarly controlled by plasma testosterone concentrations [102] being larger in the presence than in the absence of testosterone. Theses lateral cells therefore tend to be larger in males than in females [102] since males on average have higher circulating concentrations of testosterone in the blood [18]. The volume of the entire POM and the size of neurons in the lateral part of this nucleus thus provide a clear morphological signature of testosterone action in the brain contrary to what is observed for sexually dimorphic brain areas in the preoptic region in other species such as the SDN-POA of rats [11,73,143] but similar to observations reported concerning the gerbil SDA [52,53,55] or the rat MPN [41,42]. Interestingly, the effects of testosterone on the neuronal size are more pronounced in the lateral part of the POM than in the medial part of the nucleus adjacent to the third ventricle [102]. This anatomical specificity of testosterone effects correlates with functional properties of these sub-regions of the POM (see below).
There are reasons to support the hypothesis that the quail POM may be homologous to the rat MPN or gerbil medial SDA. For example, the physiological regulation of the morphology of these structures and aspects of their neurochemical signature are quite similar. The sex differences in the volume of these structures that have been observed in these species are influenced by the endocrine state of the subjects in adulthood and do not simply reflect organizational effects of steroids as is the case for volumetric sex differences in the more restricted parts of these nuclei identified as the SDN-POA in rat of the SDA pars compacta in gerbils (see above). The fact that the rat MPN and the quail POM are specifically labeled by a dense expression of aromatase (identified by in situ hybridization in rat and by in situ hybridization and immunocytochemistry in quail) further support this hypothesized homology [13,70,119,147]. Based on this criterion, the sheep sexually dimorphic nucleus of the mPOA would also be homologous [127].
As discussed below, heterogeneity has also been described within the quail POM with the lateral portion of the nucleus displaying specialized neurochemical features and differential responses to testosterone as compared to the medial part of the nucleus. At present, however, there is no information supporting the idea that the medial and lateral parts of the POM correspond to the medial and lateral SDN in rats. Similarly no specialized subregion of the POM has to this date been identified that might correspond to the rat SDN or to the gerbil SDA pars compacta.
In quail like in many other birds and mammals [32,80], the activation by testosterone of male copulatory behavior first requires the aromatization of the androgen into an estrogen such as 17β-estradiol (E2) within the preoptic area [4,19,21,32,149,150]. The enzyme P450arom or aromatase which catalyzes this transformation can, for reasons that are still partly unexplained, be easily identified by immunocytochemistry in the quail brain [20,70] while this is still very difficult if not impossible in the mPOA of mammalian species including rodents. Because aromatase is a limiting step in the activation of sexual behavior by testosterone [32] and because aromatase expression is itself regulated by testosterone [30], immunocytochemical visualization of this enzyme provides a cellular marker of testosterone action in the brain in relation to the expression of copulatory behavior (Fig. 2B-D). As will be discussed extensively in subsequent sections of this review, aromatase-immunoreactive (ARO-ir) neurons specifically label the quail POM and thus provide an unambiguous neurochemical marker for this cellular population thus facilitating its experimental analysis. Studies of the neuroendocrine control of quail male sexual behavior that have exploited this feature of its neuroendocrine system are summarized in the next sections of this review and compared with similar data in mammals (when available) to generalize the conclusions drawn from the quail studies.
4. The quail POM as a model system illustrating the effects of testosterone action on male copulatory behavior
A suite of studies utilizing converging approaches clearly demonstrate that the sexually dimorphic POM, identified as a dense neuronal aggregate in Nissl stained brain tissue or as a dense group of ARO-ir cells, is a necessary and sufficient site of testosterone action related to the activation of male copulatory behavior when subjects are exposed to appropriate sexual stimuli (e.g. a sexually receptive female)[22]. These data, however, do not exclude the possibility that steroid hormones do act in other brain sites to promote behavior expression under physiological conditions [17].
Electrolytic lesions aimed at the mPOA inhibit copulatory behavior activated in castrated males by treatment with exogenous testosterone only if they overlap with a substantial portion of the POM. Lesions located elsewhere in the POA do not affect the behavior [22]. Conversely, stereotaxic implants filled with testosterone activate all aspects of copulatory behavior in castrated male quail if their tip is located within the boundaries of the POM but not if it is located in the adjacent POA [22,23] Implants that are only a few hundred microns lateral to POM have no behavioral effects indicating the rather precise neuroanatomical specificity of testosterone action in relation to the activation of male copulatory behavior (See Fig 3A).
Figure 3.
Stereotaxic implants of testosterone in the POM activate male copulatory behavior in male Japanese quail. A. Schematic drawings illustrating the position of implants that did or did not activate male-typical sexual behavior. Implants restore copulatory behavior in castrated male quail only if located within the limits of the POM but not in adjacent structures of the medial preoptic area. The panel shows two coronal sections located at the level of the anterior commissure (CA: bottom) and 180 μm more rostrally (top). Filled circles represent implants that restored at least mount attempts in castrates, open circles represent implants that were behaviorally ineffective. Implants that are only 100-200 μm lateral to POM are ineffective. AC: nucleus accumbens (now renamed nucleus striae terminalis lateralis; see [113]); CA: commissura anterior; FPL: fasciculus prosencephali lateralis; POM: nucleus preopticus medialis; SL: nucleus septalis lateralis; SM: nucleus septalis medialis. Redrawn from [22]. B. Testosterone implants in the POM also induce the expression of aromatase that can be detected by the quantification of the numbers of ARO-ir cells on the implantation side. The intensity of sexual behavior activated by the implants (frequency of mount attempts during the last two behavioral tests before sacrifice) correlates best with the induction of aromatase expression (numbers of ARO-ir cells) in sections located just rostrally (100-200 μm) to the anterior commissure (CA). The figure represents the correlations (Pearson's product-moment coefficient) between individual measures of sexual behavior and numbers of ARO-ir cells in the left or right POM (counted in one 20 μm-thick section every 100 μm). The testosterone implant was always positioned on the right side. Coefficients that are significantly different from zero are indicated by an asterisk (Redrawn from [23]). C-D. Photomicrographs illustrating the POM as observed in sections stained by immunocytochemistry for aromatase in two castrated quail that received a stereotaxic implant filled with cholesterol (C) or with testosterone (D) in the dorso-lateral part of the nucleus on the right side of the brain. The induction of aromatase by testosterone as evidenced by an increase in the number of ARO-ir cells in clearly visible in panel D. The tip of the implants is clearly visible and labeled by an asterisk. The dotted line indicates the limits of the POM. Magnification bar= 500 μm (modified from [23]).
The dependence of male copulatory behavior on testosterone aromatization within the POM [19,21,150] combined with the control of aromatase expression by testosterone in this same brain structure could then be used to define more precisely the anatomical sites of steroid action on copulatory behavior. In quail, aromatase-immunoreactive (ARO-ir) neurons are a specific marker of the nuclear boundaries of the POM [20,27] and the number of these ARO-ir cells is strongly (4-5 fold) increased by testosterone [24] (Fig 2 C-D). This induction of ARO-ir cells was thus used to map precisely the areas that are stimulated by the stereotaxic implantation of testosterone in the POA and these data were correlated with the behavior of individual subjects in order to identify the parts of the POA where testosterone action is critical for the activation of copulatory behavior [23]. This analysis indicated that the intensity of the behavior activated by testosterone implants correlates best with the induction of ARO-ir cells in the caudal part of the POM (just rostral to the anterior commissure; See Fig 3B-D). This caudal part of the POM is also the region of the dimorphic nucleus where electrolytic lesions and destruction of ARO-ir cells induce the most marked behavioral deficits (see [23] for detail of these analyses). This same caudal part of the POM is also the region of the nucleus where the largest number of c-fos-immunoreactive cells is observed in birds that were allowed to express copulatory behavior before brain collection [93]. Lesion studies, testosterone implant experiments and investigations of c-fos expression thus all converge to indicate that the caudal part of the POM, just rostral to the anterior commissure, is critically implicated in the activation of male copulatory behavior.
5. Neurochemical and hodological heterogeneity of the medial preoptic nucleus
Interestingly, the caudal POM displays several neurochemical and hodological (i.e., based on connectivity) specializations that are likely to be related functionally to its prominent role in the control of male-typical copulatory behavior. The caudal POM is not only the part of the nucleus that contains the largest number of ARO-ir cells but it is also the region where testosterone has the most dramatic effect on these cells. Detailed analysis of the induction by testosterone of ARO-ir cells revealed that if two weeks of treatment with testosterone increases the number ARO-ir cells throughout the rostro-caudal extent of the POM, the magnitude of the effects is 3-5 times larger in the caudal as compared to the rostral POM [27]. This induction of ARO-ir cell numbers is more prominent in the lateral as compared to the medial part of the caudal POM (Fig. 2D; [12,27]). Furthermore the size of the ARO-ir neurons is increased by testosterone more in the caudo-lateral as compared to the caudo-medial POM, suggesting that testosterone is acting in a more prominent manner particularly in this subregion of the nucleus POM [12]. Finally, as already mentioned, immunocytochemical studies indicate that the induction of the FOS protein following expression of male copulatory behavior is more pronounced in the caudal than in the rostral part of the POM [93].
Tract-tracting combined with aromatase immunocytochemistry also revealed that ARO-ir cells in the POM, and in particular its lateral portion, project densely to the periaqueductal central gray (PAG; often labeled in avian brain atlases as the substantia grisea centralis or GCt; see [37,84,86,131]). These projections originate predominantly from the lateral part of the POM as demonstrated by the fact that retrograde tracing identifies a larger number of ARO-ir cells containing tracer injected in the PAG in the lateral than in the medial POM (Fig 4A-B) [1].
Figure 4.
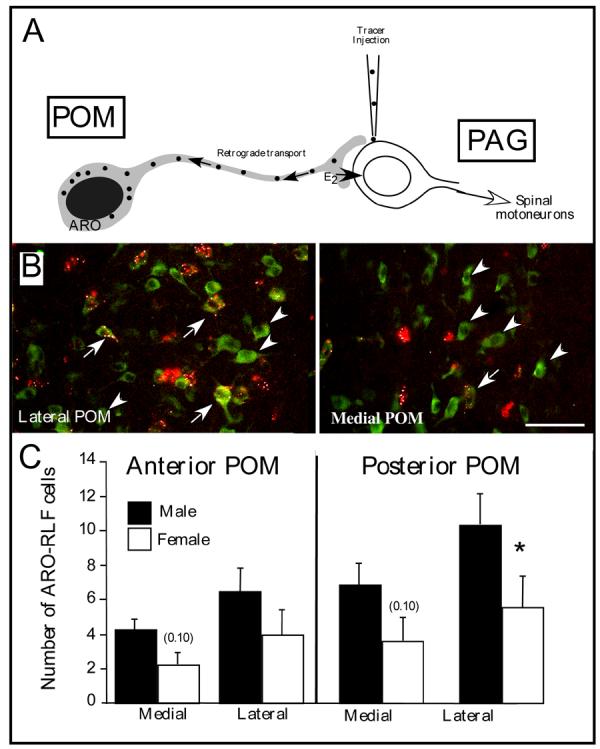
Aromatase-immunoreactive cells located in the medial preoptic nucleus (POM) send dense projection the premotor mesencephalic periacqueductal gray (PAG), this projection is denser in males than in females and this sex difference is more prominent in the caudal than in the rostral part of the nucleus. Retrogradely labeled cells are also more numerous in the lateral than in the medial part of the nucleus A. Schematic representation of the POM-PAG projection and of the experimental design used to study this connection. A retrograde tracer (fluorescent latex beads) was injected in PAG, retrogradely transported to POM neurons that project to PAG and then identified in sections through the POM that were also stained by immunocytochemistry for aromatase. B. Representative photomicrographs through the lateral (left) or medial (right) POM illustrating the presence of retrogradely transported latex beads (red) that had been injected in PAG in aromatase-immunoreactive cells (green). In the medial POM a large fraction of ARO-ir cells project to the PAG, this proportion is smaller in the lateral POM. Arrows point to ARO-ir cells containing red latex bed that project to the PAG, arrowheads indicate ARO-ir cells that do not project to PAG C. Number (mean±SEM) of retrogradely fluorescent (RLF) aromataseimmunoreactive (ARO-ir) A cells counted in 4 different locations in the POM (medial and lateral field at a rostral and caudal level) in male (black bars) and female (hatched bars) quail illustrating the effects of the position within POM (medial vs. lateral and rostral vs. caudal) on the number of ARO-RLF cells and the presence of larger numbers in males as compared to females. Post hoc MANOVA confirmed that the sex difference is significant only in the lateral caudal POM (*=p<0.05) but statistical tendencies (p<0.10) are also observed in the medial POM at both rostrocaudal levels. Redrawn from [48].
Female quail never exhibit male-typical copulatory behavior even after a treatment with testosterone at doses that are behaviorally effective in males [3,18]. The mechanisms underlying this behavioral insensitivity of females to the action of testosterone have not been identified to this date [26]. We recently studied whether a male-biased projection of ARO-ir cells in the POM to the PAG could be responsible for the differential behavioral effects of testosterone in the two sexes. In rodents, it is indeed well established that the PAG is a premotor nucleus and that the projection from the mPOA to the PAG plays an important role in the control of reproductive behavior [80,96,97]. We showed in quail by retrograde tracing combined with aromatase immuncytochemistry that males have more aromatase-immunoreactive neurons projecting to the PAG than females and this difference was most prominent in the caudo-lateral part of the nucleus (Fig. 4C) [48]. These data therefore support the notion that sex differences in POM-PAG connectivity are causally linked to the sex difference in the behavioral response to testosterone and, in connection with the purpose of the present review, that the caudo-lateral part of the POM is preferentially implicated in the control of this behavior.
6. The medial preoptic area is also implicated in the control of appetitive aspects of male sexual behavior
As mentioned previously, most research investigating the neuroendocrine controls of male sexual behavior has focused on the copulatory sequence sensu stricto. This is understandable in that this sequence can usually be readily elicited in captive conditions and is easily quantifiable. However, there is much more to male sexual behavior than just copulation itself. Courtship and related preparatory behaviors that put males in a position to copulate are important as well. Several different schemes have been proposed to describe these differences [36,98,112]. We find the ethological perspective on these distinctions to be especially useful for considering the organization of male sexual behavior in non-human animals. Early in the history of the field, ethologists proposed a distinction between appetitive and consummatory aspects of rewarding behaviors such as sexual interactions [57,78,92]. According to the ethological scheme, appetitive aspects of a behavioral sequence represent variable behavioral responses made by an animal when searching and trying to interact with the appropriate stimuli that would lead to the expression of the more stereotyped so-called consummatory response [15,92,142]. The consummatory response is generally one that results in a functional outcome such as when copulatory behavior results in the formation of a zygote or drinking results in the cessation of thirst [78]. A general claim is that engaging in consummatory behavior reduces a motivation state [78,141]. This distinction between appetitive and consummatory behaviors is related to other behavioral classification schemes such as the distinction between motivation and performance that is often employed when discussing male sexual behavior in non-human animals as well as in humans (See [67,80,112] for a detailed discussion). The importance of making this distinction for research programs focused on the neuroendocrine bases of sexual behavior was first articulated by F.A. Beach based on his studies of rodent sexual behavior [38].
The use of such a dichotomy is potentially problematic for the analysis of complex sequences of behavior because one stage of the sequence can often be considered as consummatory in relation to preceding events but be appetitive in relation to events that will follow. For example, mounts and intromission could be viewed as consummatory acts following anogenital investigations and pursuit of the female but could as well be the appetitive aspects of the ejaculatory response (see [80,141] for a more detailed discussion). These concepts should thus be used cautiously but the distinction between appetitive sexual behavior (ASB) and consummatory sexual behavior (CSB) continues to be useful to both ethologists and behavioral neuroendocrinologists in the elucidation of neural / neuroendocrine mechanisms mediating a range of behaviors, including sexual behavior. Certain behaviors such as courtship displays are clearly necessary for and distinct from copulation itself and though both are under the control of testosterone and its metabolites, the neural circuits that implement these different aspects of the behavior will by necessity be distinct to some degree [67].
In both rats and quail, the two species in which most research on this topic has been carried out, the expression of ASB is markedly inhibited if not completely suppressed by castration and recovery is observed following treatment with exogenous testosterone (e.g. [25,29,67]). In both species, the action of testosterone on ASB also seems to be mediated by its aromatization into an estrogen. For example, Roselli and collaborators [120] demonstrated that treatment of rats with the aromatase inhibitor Fadrozole™ markedly decreases a widely accepted measure of ASB, the number of level changes in a bi-level apparatus in which a male can freely pursue a female [110]. Aromatase-knock out mice also show major deficits in measures of partner preference and sexual motivation [16]. Similarly in quail, measures of ASB (see below) appear to be testosterone-dependent and the action of testosterone seems to be mediated by its aromatization into an estrogen [25,28]. Available evidence thus supports the notion that both ASB and CSB depend for their activation on similar if not identical endocrine stimuli. This can easily be understood from an ultimate causation point of view: natural selection would favor the control by similar hormones of behaviors that must by nature be expressed in sequence to ensure successful reproduction [5].
Interestingly, however, several studies have suggested dissociations between neural circuits underlying the expression of appetitive (i.e. motivational) and consummatory components (i.e., copulatory behavior) of male sexual behavior. In rats for example, lesions to the mPOA eliminate male-typical copulatory behavior [77,80] but have more discrete or no effects on some measures of sexual motivation. Rats with such lesions still pursue and attempt to mount females [35]; they also perform learned instrumental responses to gain access to females [67,68]. Furthermore, these lesions do not inhibit measures of sexual arousal such as penile erections in response to remote cues from estrous females [90]. In contrast, it was found that lesions to the basolateral amygdala inhibit noncontact erections and the ability of males to acquire learned responses that are rewarded with access to females [67,68,90]. These observations lead to the idea of a double dissociation between brain areas mediating copulatory behavior on the one hand (the mPOA) and appetitive sexual behavior / sexual arousal / sexual motivation on another hand (amygdala, bed nucleus striae terminalis; see [66-68]). This notion was also supported by a number of pharmacological studies that revealed selective effects on consummatory sexual behavior of manipulations of the preoptic opioid system but left relatively intact measures of appetitive sexual behavior (see [68] for a review).
However, there is also clear evidence that the mPOA plays some role in the control of these other appetitive aspects of sexual behavior. Lesions of the mPOA diminish preference for a female partner in rats and ferrets [7,65,105,106], decrease pursuit of the female by male rats [104], and decrease anticipatory erections and anogenital investigations in marmosets [91]. Furthermore, in vivo dialysis experiments have revealed that sexual interactions progressively increase the level of extracellular dopamine in the mPOA of male rats and this increase begins as soon as the male is introduced to the female and initiates pursuits and anogenital investigations, i.e., several minutes before the beginning of copulatory interactions (consummatory responses) sensu stricto [79]. Pharmacological manipulations of the dopaminergic system in the mPOA similarly confirm its involvement in the control of appetitive sexual response [111]. It has, for example, been shown that microinjections of a dopaminergic antagonist within the POA decrease measures of sexual motivation such as the preference of a male for the female arm in a maze [95,148]. In summary, it appears that mPOA lesions diminish but do not eliminate sexual motivation suggesting that the mPOA plays a role in the control of this aspect of sexual behavior but this role is less apparent than is the case for copulatory behavior.
7. Sub-regions of the medial preoptic nucleus are differentially implicated in the control of appetitive and consummatory sexual behavior in quail
The quail has also been used in several experiments to investigate the brain areas involved in the control of ASB and CSB. In addition to the advantages discussed above that are related to the anatomically discrete signature of testosterone action in the mPOA, behavioral studies have identified in quail two easily measurable responses that provide sensitive indexes of ASB while being relatively independent of the copulatory sequence itself.
It was first discovered by Domjan and colleagues [61,62] (see [64] for a review) that when a male copulates with a female for a single time in an arena there is a marked change in his behavior. After copulating with a female, the male will stand in front of a window that provides him with visual access to the female for most of the day while he was ignoring this window before copulation [61,62]. This robust response, called learned social proximity response, is reliably produced in the laboratory, easily quantifiable and provides a useful way to investigate the neuroendocrine mechanisms regulating male appetitive sexual behavior [31]. This response is however learned only if the male birds perform copulatory behavior in the testing chamber and, as a consequence, cannot be studied completely independently of the occurrence of CSB.
A second procedure to assess ASB has been more recently developed based on studies conducted in the laboratory of Elizabeth Adkins-Regan at Cornell University [140]. Male quail produce a meringue-like foam that is transferred to females during copulation and enhances the probability that sperm will fertilize the eggs [50,51]. This foam is produced by rhythmic movements of a sexually dimorphic striated cloacal sphincter muscle that is interdigitated with the proctodeal gland [125]. These movements are greatly facilitated in males, including sexually naive males, by the simple view of a female [125,140]. These rhythmic cloacal sphincter movements are facilitated nearly 20-fold in castrated males treated with testosterone and provided with visual access to a female as compared to castrated males receiving no testosterone [29]. These cloacal sphincter muscle movements are reminiscent of non-contact erections described in mammalian species [121] in that they involve muscle movements that control effector organs associated with consummatory aspects of male sexual behavior in response to sensory cues emitted by a female.
Expression of both the learned social proximity response and rhythmic cloacal sphincter movements are testosterone-dependent. These responses are not expressed in castrated males and are restored to rates observed in intact sexually mature males by treatments with exogenous testosterone [6,25,29,63]. As mentioned above, effects of testosterone on these two measures of ASB require aromatization of the androgen [28,136]. The localization of brain aromatase can thus provide insight into areas that may be part of the neural circuit regulating these behaviors. Since the POM contains the largest number of ARO-ir cells, experiments were performed to test the possible implication of this brain area in the control of ASB.
7.1. Effects of stereotaxic implants of testosterone in POM
In a series of studies, ASB and CSB were measured in castrated male quail that had received testosterone-implants directed at the POM [114]. Behavioral tests assessing the acquisition of the learned social proximity response were performed and immediately followed by a 5 min period during which the males had free access to the females and could express CSB. Only a few birds displayed the full copulatory sequence ending in cloacal contact movements, but these were all males who had testosterone implants directly located in the POM or just outside of POM (less than 200-300 μm). Additionally, males bearing testosterone implants in POM displayed a higher rate of mount attempts and cloacal contact movements as compared to birds with empty implants when observed in a small test arena.
Overall, these males treated with testosterone in the POM also displayed a higher percentage of time exhibiting the social proximity response than birds in the other groups (control empty implant or testosterone implant outside POM) although this difference was not statistically significant. As already mentioned above, the learned social proximity response does not develop in birds that fail or are not allowed to copulate with the female [25] and this functional link therefore prevented a thorough assessment of the effect of testosterone implants in the POM on this response because only a fraction of the testosterone implanted birds achieved copulation. However a separate analysis of the behavior of the birds that copulated revealed that these same birds also displayed a significant elevation in time spent in front of and looking through the window, while testosterone-treated birds that failed to copulate showed very low levels of these response, similar to birds with empty implants. Testosterone implants in the POM also increased the average number of rhythmic cloacal sphincter movements produced by the males in response to the visual presentation of a female although this effect was only observed in a small number of subjects and associated with a large between subject variability; it was thus not statistically significant [114].
7.2. Effects of POM lesions
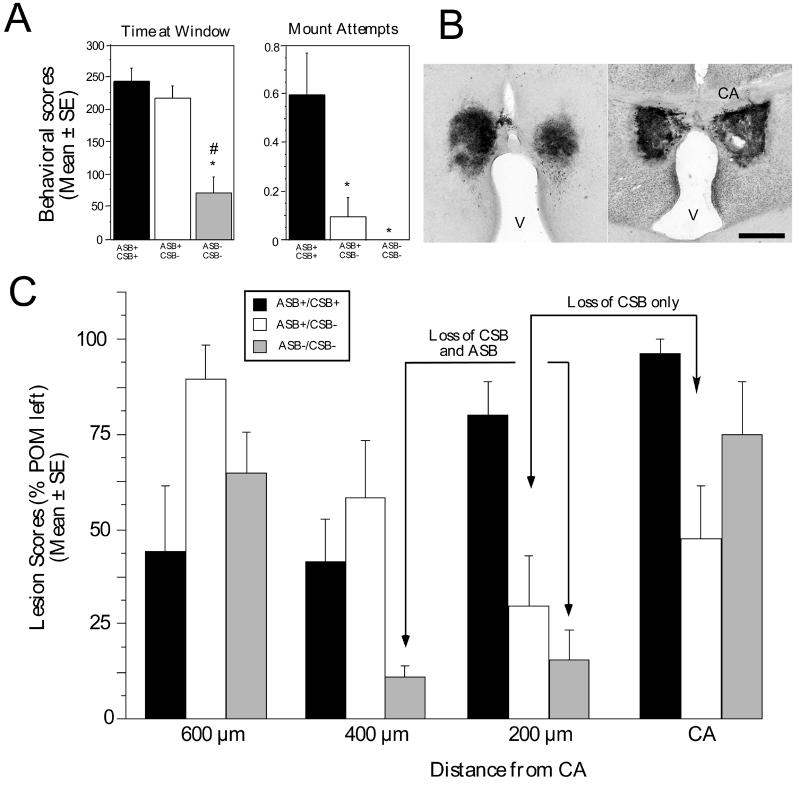
Another series of studies analyzed the effects of discrete electrolytic lesions aimed at POM on the expression of both CSB and ASB. In parallel to their well-documented effects on CSB that were reproduced in this experiment, these lesions of the POM decreased the expression of the learned social proximity response although the effect had, as an average, a lower amplitude than the inhibition of CSB even if the inhibition was statistically significant [29]. As an average, the time spent watching the female in front the window in the two chamber apparatus decreased by about 30-40% in birds bearing POM lesions [29]. Closer inspection of the data revealed, however, that this modest decrease of the proximity response was associated with a high degree of individual variability. While expression of CSB was almost completely suppressed in all birds bearing a POM lesion, the decrease in learned social proximity response ranged in individual birds from no change to a nearly complete suppression. This allowed for the definition of three statistically different groups of subjects: birds displaying a weak consummatory sexual behavior and an apparently normal appetitive sexual behavior (ASB+/CSB+; n=5), birds with a complete suppression of CSB but with an apparently normal appetitive sexual behavior (ASB+/CSB−; n=7) and birds with a complete suppression of both components of sexual behavior (ASB−/CSB−; n=4).
POM lesions were then re-constructed based both on Nissl-stained sections and on the pattern of immunoreactive aromatase remaining after the lesion and the specific location of the individual lesions was correlated with the magnitude of the deficit affecting expression of the learned social proximity response. These analyses revealed that lesions at the level of or just rostral (200 μm) to the anterior commissure were associated with decreased expression of CSB, while slightly more rostral lesions were specifically associated with inhibition of the measures of ASB [29].
Birds in the ASB+/CSB+ group possessed a lesion destroying primarily the rostral part of the POM (600-400 μm rostral to anterior commissure). In contrast, birds in which CSB had been completely suppressed (ASB+/CSB− and ASB−/CSB−) had an extensive lesion of the more caudal POM. These last two groups were however clearly separable: the inhibition of ASB (group ASB−/CSB−) was specifically associated with a large lesion in the anterior POM, 400 μm rostral to the anterior commissure that was not observed in the other group (compare lesion scores for level 400 μm in the ASB+/CSB− and the ASB−/CSB− groups; see Fig. 5C) (see [29] for detail). In addition, these POM lesions almost completely abolished the expression of rhythmic cloacal sphincter movements induced in castrate males treated with exogenous testosterone by the view of a female [29].
Figure 5.
Electrolytic lesions affecting the POM just rostral to the anterior commissure inhibit male consummatory sexual behavior (CSB), more rostral lesions also inhibit appetitive sexual behavior (ASB). A. Behavioral scores (time at window during the learned social proximity test [ASB] or mount attempt frequencies [CSB]) computed for three subgroups of birds bearing a POM lesion defined by the presence of appetitive sexual behavior and a low level of consummatory sexual behavior (ASB+/CSB+), or by the complete absence of consummatory behavior associated or not with an inhibition of appetitive sexual behavior (ASB−/CSB− and ASB+/CSB− respectively). *=p < 0.05 by comparison with ASB+/CSB+ subgroup ; #=p < 0.05 by comparison with ASB+/CSB− subgroup). B. Photomicrographs of sections stained by immunocytochemistry for aromatase illustrating a bilateral electrolytic lesion in the rostral part of the POM (left) and in the caudal part of the nucleus at the level of the anterior commissure (CA). Magnification bar= 500 μm, V: third ventricle. C. Average lesions scores observed in the POM at four rostro-caudal levels (identified by reference to the commissural anterior, CA) in the three subgroups of birds defined by their behavior as described in panel A. Lesions were scored on a 5 point scale with 0= no POM left (complete lesion) and 4= 100 % of POM left (no lesion at this level). A nearly complete loss of CSB is observed in birds bearing large lesions at the CA level or 200 μm more rostrally. The most important deficits in ASB are observed in birds bearing large lesions 200 or 400 μm rostral to CA. Redrawn from data in [29].
Taken together, available evidence based on electrolytic lesions or stereotaxic implantation of testosterone in the brain therefore supports the notion that in quail like in rats, the mPOA (in quail, the POM specifically) is implicated to some degree in the control of both consummatory and appetitive sexual behavior. A finer analysis additionally revealed that there are specialization in the sub-regions of the POM that participate to the activation of these two components. This conclusion was confirmed by a completely independent experimental approach.
8. Studies of the immediate early gene c-fos
A study recently investigated via the detection of immediate early gene expression the neural sites that are activated by the expression of appetitive and consummatory aspects of male sexual behavior in Japanese quail [137]. Castrated males treated with testosterone were allowed to interact freely with a female and express the full sequence of copulatory sexual behavior (CSB group), or to express rhythmic cloacal sphincter movements in response to the visual presentation of a female (ASB group) or were simply handled and immediately returned to their home cage as a control manipulation. Behavioral tests lasted 5 min after which the birds were returned to their home cage. Ninety min later, brains were collected and stained by immunocytochemistry for the FOS protein, the produced of the immediate early gene c-fos. An increase in FOS expression was observed throughout the rostro-caudal extent of the medial preoptic nucleus (POM) in males of the CSB group, whereas the view of a female and expression of rhythmic cloacal sphincter movements (ASB group) induced an increased FOS expression in the rostral POM only (See Fig. 6). These data thus provide additional support to the idea that the POM is implicated in the expression of both ASB and CSB but that there is a partial anatomical dissociation within this nucleus between sub-regions involved in the control of each aspect of male sexual behavior. They also provide independent support to the notion that the rostral and caudal POM differentially control the expression of ASB and CSB in quail.
Figure 6.
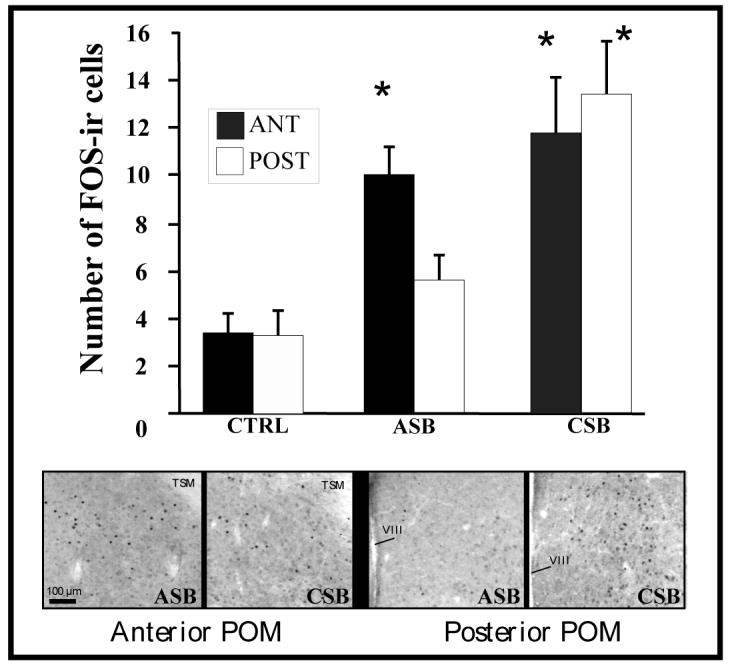
Performance of consummatory sexual behavior (CSB) induces FOS expression throughout the medial preoptic nucleus (POM) while performance of appetitive sexual behavior (ASB; production rhythmic cloacal sphincter movements in response to the view of a female) induces FOS in the anterior POM only. The bar graph represents the number of FOSimmunoreactive cells counted in sections through the anterior (ANT) or posterior (POST) POM in birds that had expressed either ASB or CSB 90 min before sacrifice or had been handled as a control (CTRL) manipulation. *= significantly different from the control situation. The lower part of the panel shows photomicrographs through representative sections in the ASB and CSB groups. Redrawn from data in [137].
9. Is this specialization of the medial preoptic area a general phenomenon among vertebrate species?
9.1. Functional studies
Detailed information on the potential separation between parts of the mPOA specifically involved in the control of ASB and CSB is not available for other vertebrate species, including rodents, but scattered evidence suggests that a similar sort of specialization within the mPOA might exist in a variety of species. For example, it has been shown that in rats, like in quail, that lesions of the caudal POA and anterior hypothalamus block the expression of copulatory behavior but more rostral lesions in the POA had little or no effect [145]. Detailed statistical analysis of these data also indicated that at the individual level, the deficits in male copulatory behavior correlate significantly with the extent of the lesion in the caudal POA but not with the lesion in the rostral POA. In hamsters (Mesocricetus auratus), studies of FOS expression in the brain indicated that pheromones alone are able to activate neurons in the mPOA in the absence of copulatory interaction with females suggesting that stimuli encountered in the appetitive phase of male sexual behavior are processed, at least in part, in the mPOA [134]. This anatomical separation is also consistent with findings in a songbird, the house sparrow (Passer domesticus) demonstrating that female-directed song, an appetitive behavior that precedes copulation, relates positively to the induction of the immediate early genes c-fos and zenk in the rostral POM but not the caudal part of this nucleus [115].
Studies in rats have also shown that contextual cues that have been associated with the female elicit c-fos expression in several brain regions including the ventral and dorsal striatum and the basolateral amygdala but not in the mPOA [68]. This is reminiscent of recent studies in quail demonstrating a lack of c-fos activation in the POM after presentation of an arbitrary stimulus that had been repeatedly paired to female presentation despite the fact that this conditioned stimulus was able to elicit the expression of a form of appetitive sexual behavior (rhythmic cloacal sphincter movements) and induced changes (a decrease in this case) in c-fos expression in two other brain regions, the nucleus taeniae of the amygdala and hippocampus (see [138] for additional discussion). It will therefore be important to test in male rats appetitive responses elicited by stimuli directly associated with the female to properly assess the role of the mPOA in the production of appetitive responses.
9.2. Anatomical studies
Neurochemical or hodological properties of sub-regions of the mPOA that would support their specific involvement in the control of ASB versus CSB remain largely unexplored at present. One must recall, however, that in quail, the caudal POM expresses a higher number of aromataseimmunoreactive cells than its rostral part [27], that these caudal cells are more affected by testosterone [27], that following expression of copulatory behavior more FOS-expressing cells are found in the caudal than in the rostral POM [93] and finally that the sex difference affecting the number of aromatase-immunoreactive of the POM projecting to the PAG is more prominent in the caudal part of the nucleus [48].
Such differences between the rostral and caudal mPOA are apparently also present in rats. Lisciotto and Morrell, for example, showed that that the caudal part of medial preoptic nucleus of male rats contains more testosterone-target neurons (as identified by in vivo autoradiography with tritiated testosterone) and that more of these testosterone-target neurons project to the mesencephalic PAG in males than in females while such a sex difference was not observed in the rostral MPN [88]. This sex difference in neurochemically defined connectivity is reminiscent of the similar difference recently reported in quail (see above; [48]) and could play a key role in the control of male copulatory behavior since the PAG represents a premotor nucleus receiving afferents from a variety of preoptic and hypothalamic sites and projecting to the spinal neurons controlling muscles implicated in sexual behavior [96,97].
The PAG exhibits a topographic organization that has been described as columnar in nature [33]. These columns correspond to well known physiological responses mediated by the PAG such as defensive reactions, analgesia, and autonomic regulation [33]. There also appears to be a sexual column based primarily on studies of the neurochemical control of lordosis in female rats [124]. No column specifically dedicated to the control of male sexual behavior has been identified to date, but the origins of projections from the mPOA to the PAG in male rats appear to be topographically organized. Injection of a retrograde tracer in PAG indeed labeled cells throughout most of the of the medial preoptic nucleus but the distribution of labeled cells shifted medially to laterally along the rostral to caudal axis of the medial preoptic area. Rostrally, there was selective retrograde labeling in the central and lateral divisions of medial preoptic nucleus, whereas caudally, labeled cells were primarily located only in the lateral subdivision of medial preoptic nucleus [116]. Anterograde tracing from the mPOA to the PAG also labeled specific columns in this latter nucleus. [116]. Additional experiments demonstrated that stimulation of the mPOA activates c-fos expression in a topographically organized manner within the PAG, thus strongly suggesting the existence of functional columns in this nucleus, presumably linked to functional subdivisions in the mPOA. Neurons activated in PAG also project to the medulla where they presumably participate to the activation of motor aspects of male sexual behavior [117].
Although the present review focuses on the role the POA in the control of consummatory or appetitive aspects of male sexual behavior, it is important to emphasize that this behavior is activated by the action of steroids at multiple anatomical levels of a complex neural circuit. In quail, steroid-sensitive areas such as the medial part of the bed nucleus striae terminalis and parts of the arcopallium including the nucleus taeniae of the amygdala are also clearly involved as indicated by a number of studies based on targeted electrolytic lesions and on the analysis of c-fos expression [2,29,49,137,140]. This is also clearly the case and documented in more detail in other species such as rodents in which areas such as the parts of the amygdala play a critical role in the integration of chemosensory and hormonal cues mediating mating behavior [99,154,155]. As mentioned before, the work of Everitt and collaborators additionally implicates parts of the basolateral amygdala in the control of ASB in rats [67,68]. Many other brain regions including the entire olfactory pathways (main and accessory), the bed nucleus of the stria terminalis, or the mesencephalic periaqueductal central gray are also critically involved. It is beyond the scope of the present review to analyze the entire circuit implicated in the control of male sexual behavior which has in any case been extensively described in a number of recent reviews [36,80,81]. We would like to stress here that the present discussion of the functional specificity of subregions within the POA is completely compatible with the well-supported claim that this brain region is only one among many brain areas that play a key role in the control of this behavior.
10. Summary and concluding statements
Appetitive and consummatory aspects of male sexual behavior must obviously be expressed in a coordinated manner in order to produce a functionally adapted behavioral sequence that can result in fertilization of the female and successful reproduction. It is therefore not unexpected that these two aspects of the sexual behavior are under the control of similar if not identical endocrine stimuli. Studies carried out mostly in rodents suggested, however, the presence of a double dissociation between brain areas controlling these two aspects of male sexual behavior: the medial preoptic area was implicated specifically the control of copulatory behavior while sexual motivation seemed to be controlled by the basolateral amygdala. This dissociation may relate to the methods that were used to assess appetitive sexual behavior (males working to gain access to female in an operant conditioning procedure with a second-order sexual reinforcer; potential confusion between motivation and motor ability or ability to remember learned associations; see [80]). A more detailed analysis of the behavioral effects of lesions or pharmacological manipulations of specific sub-regions of the mPOA in rats would be useful in order to reveal effects on appetitive aspects of male sexual behavior.
Studies in Japanese quail have indicated that the preoptic area participates in the control of both components even if research completed so far does not exclude the possibility that other brain areas could also play a very important and specific role. Quite interestingly, studies based on completely independent approaches (lesion and quantification of c-fos expression) have provided converging evidence for an anatomical separation of the cell groups preferentially implicated in the control of the appetitive and consummatory aspects of sexual behavior. The appetitive component would be controlled by a sub-region of the POM rostral to the region controlling consummatory aspects. This functional separation also seems to correspond to neurochemical and hodological specializations within the preoptic area. Scattered information further suggests that such a functional and neurochemical specialization may also be present in several mammalian species. Systematic studies investigating such potential regional differences within the mPOA are required at this time to confirm this hypothesis.
The differences between subregions of the quail mPOA that appear to be differentially involved in the control of ASB and CSB may seem to be somewhat subtle at least based on the analyses completed to date. It is therefore reasonable to question how such relatively minor neuroanatomical or neurochemical differences could be responsible for the emergence of qualitatively different behaviors such as those expressed during the appetitive or consummatory phases of the male sexual behavior sequence. As already mentioned above, the fact that ASB and CSB occur in a sequence makes it obvious that they must be functionally coordinated. It is thus no surprise that there is some overlap between the brain areas and neurochemical mechanisms controlling their expression. At the same time, it is important to recognize that the preoptic area is highly heterogeneous so that local differences that appear quantitative in nature, based on our relatively imprecise neuroanatomical analyses might, in fact, represent qualitative differences at the cellular level. As already articulated before in a slightly different context, the presence of 40,000 molecules of estrogen receptor in a microdissected brain region may reflect existence of 10 neurons with 4,000 molecules of receptor each or of one neuron with 22,000 and 9 neurons with only 2,000 receptor molecules. If the neuron with a high number of receptors is part of the circuit mediating a given behavior, a very different functional outcome can be expected in these two scenarios, depending on whether the key neuron has 22,000 or 4,000 receptor molecules [39].
Similarly, the different responses to testosterone of neurons in various parts of the POM, the gradients in the numbers of ARO-ir cells and their different projections to the PAG, even if they only appear as minor quantitative regional differences, could reflect at the cellular and/or molecular level qualitative differences affecting specific subsets of neurons. If some of these subsets are specifically implicated in the control of ASB or CSB, they could thus generate qualitatively different responses. It is obviously difficult to identify in an exhaustive manner the specific neurons in the POM that are part of the male sexual behavior circuit and due to the plasticity of the vertebrate brain this task might be impossible to achieve (unlike the situation existing in simpler invertebrate models such as the Aplysia or Caenorhabditis elegans mentioned in the introduction of this paper). It is therefore at the present time impossible to determine whether the neurochemical differences of admittedly limited amplitude that have been identified between subregions of the POM reflect all-or-none differences in a subset of functionally specialized neurons that are critical for sexual behavior or minor quantitative differences spreading through broader regions of the nucleus. Based on available information, we favor, however, the first of these interpretations, which would explain why the mPOA is able to control such a broad diversity of responses including sexual or maternal behavior and many autonomic functions, including thermoregulation, thirst or hunger.
It is also important to emphasize here that the use of a variety of animal models exhibiting a variety of biological features that provide practical advantages (clearly defined appetitive or consummatory components of behavior, easily definable dimorphic or neurochemically identified structures in the POA, …) has been instrumental in making progress in the analysis of the relationships between specific aspects of behavior and neuroanatomical/ neurochemical features of the preoptic area. Homologies between sexually dimorphic nuclei in various animal species including rats, hamsters, guinea pig, gerbils or quail and the dimorphic structures of the preoptic area in humans and monkeys [8,40,45-47,133] remain unclear at present and information coming from different species is still likely to improve our understanding of the neural bases of behavior in primates including humans.
Functional neuroanatomical studies have often provided essential clues about physiological mechanisms controlling behavior [10,59,82,157]. A topographic specialization of the preoptic region related to the control of specific components of male sexual behavior could be an important tool in elucidating neural processes preferentially controlling different aspects of male sexual behavior.
Acknowledgements
The preparation of this review and the experimental work described was supported by grants from the NIMH (Grant number RO1 MH50388) to GFB and from the Belgian Fonds de la Recherche Fondamentale Collective (Grant number 2.4562.05) to JB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Absil P, Riters LV, Balthazart J. Preoptic aromatase cells project to the mesencephalic central gray in the male Japanese quail (Coturnix japonica) Horm. Behav. 2001 doi: 10.1006/hbeh.2001.1702. [DOI] [PubMed] [Google Scholar]
- 2.Absil P, Braquenier JB, Balthazart J, Ball GF. Effects of lesions of nucleus taeniae on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Brain Behav. Evol. 2002;60:13–35. doi: 10.1159/000064119. [DOI] [PubMed] [Google Scholar]
- 3.Adkins EK. Hormonal basis of sexual differentiation in the Japanese quail. J. Comp. Physiol. Psychol. 1975;89:61–71. doi: 10.1037/h0076406. [DOI] [PubMed] [Google Scholar]
- 4.Adkins EK, Boop JJ, Koutnik DL, Morris JB, Pniewski EE. Further evidence that androgen aromatization is essential for the activation of copulation in male quail. Physiol. Behav. 1980;24:441–446. doi: 10.1016/0031-9384(80)90233-4. [DOI] [PubMed] [Google Scholar]
- 5.Adkins-Regan E. Hormones and animal social behavior. Princeton University Press; Princeton, NJ: 2005. p. 411. [Google Scholar]
- 6.Adkins-Regan E, Leung CH. Hormonal and social modulation of cloacal muscle activity in female Japanese quail. Physiol. Behav. 2006;87:82–87. doi: 10.1016/j.physbeh.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Alekseyenko OV, Waters P, Zhou H, Baum MJ. Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. Physiol. Behav. 2007;90:438–449. doi: 10.1016/j.physbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J. Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res. Bull. 1983;10:147–154. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- 10.Arnold AP, Nottebohm F, Pfaff DW. Hormone concentrating cells in vocal control areas of the brain of the zebra finch (Poephila guttata) J. Comp. Neurol. 1976;165:487–512. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- 11.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Ann. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 12.Aste N, Panzica GC, Aimar P, Viglietti-Panzica C, Harada N, Foidart A, Balthazart J. Morphometric studies demonstrate that aromatase-immunoreactive cells are the main target of androgens and estrogens in the quail medial preoptic nucleus. Exp. Brain Res. 1994;101:241–252. doi: 10.1007/BF00228744. [DOI] [PubMed] [Google Scholar]
- 13.Aste N, Panzica GC, Viglietti-Panzica C, Harada N, Balthazart J. Distribution and effects of testosterone on aromatase mRNA in the quail forebrain: A non-radioactive in situ hybridization study. J. Chem. Neuroanat. 1998;14:103–115. doi: 10.1016/s0891-0618(97)10023-0. [DOI] [PubMed] [Google Scholar]
- 14.Aversa A, Bruzziches R, Pili M, Spear G. Phosphodiesterase 5 inhibitors in the treatment of erectile dysfunction. Curr. Pharm. Des. 2006;12:3467–3484. doi: 10.2174/138161206778343046. [DOI] [PubMed] [Google Scholar]
- 15.Baerends GP. Ethology. In: Atkinson RC, Herrnstein RJ, Lindzey G, Luce RD, editors. Steven's handbook of experimental psychology. 2nd Edition. Vol. 1. Wiley; New York: 1988. pp. 765–830. Learning and cognition. [Google Scholar]
- 16.Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (Cyp19) gene in male mice. Horm. Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- 17.Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol. Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J, Schumacher M, Ottinger MA. Sexual differences in the Japanese quail: behavior, morphology and intracellular metabolism of testosterone. Gen. Comp. Endocrinol. 1983;51:191–207. doi: 10.1016/0016-6480(83)90072-2. [DOI] [PubMed] [Google Scholar]
- 19.Balthazart J, Evrard L, Surlemont C. Effects of the non-steroidal aromatase inhibitor, R76713 on testosterone-induced sexual behavior in the Japanese quail (Coturnix coturnix japonica) Horm. Behav. 1990;24:510–531. doi: 10.1016/0018-506x(90)90039-z. [DOI] [PubMed] [Google Scholar]
- 20.Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N. Distribution of aromatase in the brain of the Japanese quail, ring dove, and zebra finch: An immunocytochemical study. J. Comp. Neurol. 1990;301:276–288. doi: 10.1002/cne.903010210. [DOI] [PubMed] [Google Scholar]
- 21.Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol. Behav. 1990;48:599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- 22.Balthazart J, Surlemont C. Copulatory behavior is controlled by the sexually dimorphic nucleus of the quail POA. Brain Res. Bull. 1990;25:7–14. doi: 10.1016/0361-9230(90)90246-v. [DOI] [PubMed] [Google Scholar]
- 23.Balthazart J, Surlemont C, Harada N. Aromatase as a cellular marker of testosterone action in the preoptic area. Physiol. Behav. 1992;51:395–409. doi: 10.1016/0031-9384(92)90158-x. [DOI] [PubMed] [Google Scholar]
- 24.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J. Steroid Biochem. Mol. Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 25.Balthazart J, Reid J, Absil P, Foidart A, Ball GF. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behav. Neurosci. 1995;109:485–501. [PubMed] [Google Scholar]
- 26.Balthazart J, Tlemçani O, Ball GF. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm. Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- 27.Balthazart J, Tlemçani O, Harada N. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J. Chem. Neuroanat. 1996;11:147–171. doi: 10.1016/0891-0618(96)00149-4. [DOI] [PubMed] [Google Scholar]
- 28.Balthazart J, Castagna C, Ball GF. Aromatase inhibition blocks the activation and sexual differentiation of appetitive male sexual behavior in Japanese quail. Behav. Neurosci. 1997;111:381–397. [PubMed] [Google Scholar]
- 29.Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J. Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) Trends Neurosci. 1998;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- 31.Balthazart J, Ball GF. The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Ann. Rev. Sex Res. 1998;9:96–176. [PubMed] [Google Scholar]
- 32.Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol. Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 34.Barfield RJ. Activation of copulatory behavior by androgen implanted into the preoptic area of the male fowl. Horm. Behav. 1969;1:37–52. [Google Scholar]
- 35.Baum MJ. Reassessing the role of medial preoptic area/anterior hypothalamic neurons in appetitive aspects of masculine sexual behavior. In: Bancroft J, editor. The pharmacology of sexual function and dysfunction. Elsevier Science; Amsterdam: 1995. pp. 133–139. [Google Scholar]
- 36.Baum MJ. Neuroendocrinology of sexual behavior in the male. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. MIT Press; Cambridge, Mass: 2002. pp. 153–203. [Google Scholar]
- 37.Baylé JD, Ramade F, Oliver J. Stereotaxic topography of the brain of the quail. J. Physiol.(Paris) 1974;68:219–241. [PubMed] [Google Scholar]
- 38.Beach FA. Characteristics of masculine “sex drive”. Nebraska Symposium on Motivation. 1956;4:1–32. [Google Scholar]
- 39.Blaustein JD, Olster DH. Gonadal steroid hormone receptors and social behaviors. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology. Vol. 3. Springer Verlag; Berlin: 1989. pp. 31–104. [Google Scholar]
- 40.Bleier R, Byne W, Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J. Comp. Neurol. 1982;212:118–130. doi: 10.1002/cne.902120203. [DOI] [PubMed] [Google Scholar]
- 41.Bloch GJ, Gorski RA. Cytoarchitectonic analysis of the SDN-POA of the intact and gonadectomized rat. J. Comp. Neurol. 1988;275:604–612. doi: 10.1002/cne.902750408. [DOI] [PubMed] [Google Scholar]
- 42.Bloch GJ, Gorski RA. Estrogen/progesterone treatment in adulthood affects the size of several components of the medial preoptic area in the male rat. J. Comp. Neurol. 1988;275:613–622. doi: 10.1002/cne.902750409. [DOI] [PubMed] [Google Scholar]
- 43.Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 2000;5:S157–161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- 44.Buijs RM, Scheer FA, Kreier F, Yi C, Bos N, Goncharuk VD, Kalsbeek A. Organization of circadian functions: interaction with the body. Prog. Brain Res. 2006;153:553–579. doi: 10.1016/S0079-6123(06)53020-1. [DOI] [PubMed] [Google Scholar]
- 45.Byne W. Medial preoptic and anterior hypothalamic regions of the rhesus monkey: cytoarchitectonic comparison with the human and evidence for sexual dimorphism. Brain Res. 1998;793:346–350. doi: 10.1016/s0006-8993(98)00275-3. [DOI] [PubMed] [Google Scholar]
- 46.Byne W, Lasco MS, Kemether E, Shinwari A, Edgar MA, Morgello S, Jones LB, Tobet S. The interstitial nuclei of the human anterior hypothalamus: an investigation of sexual variation in volume and cell size, number and density. Brain Res. 2000;856:254–258. doi: 10.1016/s0006-8993(99)02458-0. [DOI] [PubMed] [Google Scholar]
- 47.Byne W, Tobet S, Mattiace LA, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB. The interstitial nuclei of the human anterior hypothalamus: An investigation of variation with sex, sexual orientation, and HIV status. Horm. Behav. 2001;40:86–92. doi: 10.1006/hbeh.2001.1680. [DOI] [PubMed] [Google Scholar]
- 48.Carere C, Ball GF, Balthazart J. Sex differences in projections from preoptic area aromatase cells to the periaqueductal gray in Japanese quail. J. Comp. Neurol. 2007;500:894–907. doi: 10.1002/cne.21210. [DOI] [PubMed] [Google Scholar]
- 49.Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- 50.Cheng KM, Hickman AR, Nichols CR. Role of the proctodeal gland foam of male Japanese quail in natural copulations. The Auk. 1989;106:279–285. [Google Scholar]
- 51.Cheng KM, McIntyre RF, Hickman AR. Proctodeal gland foam enhances competitive fertilization in domestic Japanese quail. The Auk. 1989;106:286–291. [Google Scholar]
- 52.Commins D, Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the mongolian gerbil brain. J. Comp. Neurol. 1984;224:132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- 53.Commins D, Yahr P. Acetylcholinesterase activity in the sexually dimorphic area of the gerbil brain: sex differences and influences of adult gonadal steroids. J. Comp. Neurol. 1984;224:123–131. doi: 10.1002/cne.902240111. [DOI] [PubMed] [Google Scholar]
- 54.Commins D, Yahr P. Lesions of the sexually dimorphic area disrupt mating and marking in male gerbils. Brain Res. Bull. 1984;13:185–193. doi: 10.1016/0361-9230(84)90020-0. [DOI] [PubMed] [Google Scholar]
- 55.Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J. Comp. Neurol. 1985;231:473–489. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- 56.Coolen LM, Peters HJPW, Veening JG. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: A sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- 57.Craig W. Appetites and aversions as constituents of instincts. Proc. Natl. Acad. Sci USA. 1917;3:685–688. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, Van De Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res. Bull. 1989;23:483–492. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- 59.De Vries GJ, Simerly RB. Anatomy, development, and function of sexually dimorphic neuroal circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4. Academoc press; San Diego, CA: 2002. pp. 137–191. [Google Scholar]
- 60.Dermon CR, Stamatakis A, Tlemçani O, Balthazart J. Performance of appetitive or consummatory components of male sexual behavior is mediated by different brain areas: A 2-deoxyglucose autoradiographic study. Neuroscience. 1999;94:1261–1277. doi: 10.1016/s0306-4522(99)00318-8. [DOI] [PubMed] [Google Scholar]
- 61.Domjan M, Hall S. Sexual dimorphism in the social proximity behavior of Japanese quail (Coturnix coturnix japonica) J. Comp. Psychol. 1986;100:68–71. [PubMed] [Google Scholar]
- 62.Domjan M, Hall S. Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): male behavior. J. Comp. Psychol. 1986;100:59–67. [PubMed] [Google Scholar]
- 63.Domjan M. Photoperiodic and endocrine control of social proximity behavior in male Japanese quail (Coturnix coturnix japonica) Behav. Neurosci. 1987;101:385–392. doi: 10.1037//0735-7044.101.3.385. [DOI] [PubMed] [Google Scholar]
- 64.Domjan M. Going wild in the laboratory: Learning about species-typical cues. In: Medin D, editor. The Psychology of Learning and Motivation. Vol. 38. Academic Press; San Diego: 1998. pp. 155–186. [Google Scholar]
- 65.Edwards DA, Einhorn LC. Preoptic and midbrain control of sexual motivation. Physiol. Behav. 1986;37:329–335. doi: 10.1016/0031-9384(86)90242-8. [DOI] [PubMed] [Google Scholar]
- 66.Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): Effects of preoptic area lesions, castration and testosterone. J. Comp. Psychol. 1987;101:407–419. [PubMed] [Google Scholar]
- 67.Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses in male rats. Neurosci. Biobehav. Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 68.Everitt BJ. Neuroendocrine mechanisms underlying appetitive and consummatory elements of masculine sexual behavior. In: Bancroft J, editor. The pharmacology of sexual function and dysfunction. Elsevier; Amsterdam: 1995. pp. 15–31. [Google Scholar]
- 69.Finn PD, Yahr P. Projection from the ventral bed nucleus of the stria terminalis to the retrorubral field in rats and the effects of cells in these areas on mating in male rats versus gerbils. Horm. Behav. 2005;47:123–138. doi: 10.1016/j.yhbeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical reexamination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J. Chem. Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 71.Franks CJ, Holden-Dye L, Bull K, Luedtke S, Walker RJ. Anatomy, physiology and pharmacology of Caenorhabditis elegans pharynx: a model to define gene function in a simple neural system. Invert. Neurosci. 2006;6:105–122. doi: 10.1007/s10158-006-0023-1. [DOI] [PubMed] [Google Scholar]
- 72.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 73.Gorski RA. Sexual differentiation of the brain: mechanisms and implications for neuroscience. From message to mind. In: Easter SSJ, Barald KF, Carlson BM, editors. Sinauer Associates Inc; Sunderland, MA: 1987. pp. 256–271. [Google Scholar]
- 74.Hansen S, Köhler C, Goldstein M, Steinbusch HWM. Effects of ibotenic acid-induced neuronal degeneration in the medial preoptic area and lateral hypothalamic area on sexual behaviour in the male rat. Brain Res. 1982;239:213–232. doi: 10.1016/0006-8993(82)90843-5. [DOI] [PubMed] [Google Scholar]
- 75.Harada N, Abe-Dohmae S, Loeffen R, Foidart A, Balthazart J. Synergism between androgens and estrogens in the induction of aromatase and its messenger RNA in the brain. Brain Res. 1993;622:243–256. doi: 10.1016/0006-8993(93)90825-8. [DOI] [PubMed] [Google Scholar]
- 76.Heeb MM, Yahr P. c-Fos immunoreactivity in the sexually dimorphic area of the hypothalamus andrelated brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neuroscience. 1996;72:1049–1071. doi: 10.1016/0306-4522(95)00602-8. [DOI] [PubMed] [Google Scholar]
- 77.Heimer L, Larsson K. Impairment of mating behavior in male rats following lesions in the preoptic-anterior hypothalamic continuum. Brain Res. 1966;3:248–263. [Google Scholar]
- 78.Hinde RA. Animal behaviour. 2nd Edition, 0 edn. McGraw-Hill; New York: 1970. p. 0. [Google Scholar]
- 79.Hull EM, Du JF, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: Implications for sexual motivation and hormonal control of copulation. J. Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 2. Academic Press; San Diego, CA: 2002. pp. 1–137. [Google Scholar]
- 81.Hull EM, Wood RI, McKenna KE. The neurobiology of male sexual behavior. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd edition Academic Press; San Diego, New York: 2005. pp. 1729–1824. [Google Scholar]
- 82.Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Curr. Opin. Neurobiol. 2000;10:784–789. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 83.Kandel ER. Cellular mechanisms of learning and the biological basis of individuality Principles of neural science. In: Kandel ER, Schwartz JH, Jessel TM, editors. Elsevier science publishing Co., inc.; New York - Amsterdam - London - Tokyo: 1991. pp. 1009–1031. [Google Scholar]
- 84.Karten HJ, Hodos W. A stereotaxic atlas of the brain of the pigeon (Columba livia) John Hopkins; Baltimore: 1967. p. 0. [Google Scholar]
- 85.Kim YS, Stumpf WE, Sar M, Martinez-Vargas MC. Estrogen and androgen target cells in the brain of fishes, reptiles, and birds: phylogeny and ontogeny. Amer. Zool. 1978;18:425–433. [Google Scholar]
- 86.Kuenzel WJ, Masson M. A stereotaxic atlas of the brain of the chick (Gallus domesticus) The Johns Hopkins University Press; Baltimore: 1988. pp. 1–166. [Google Scholar]
- 87.Larsson K, Heimer L. Mating behaviour of male rats after lesions in the preoptic area. Nature. 1964;202:413–414. doi: 10.1038/202413a0. [DOI] [PubMed] [Google Scholar]
- 88.Lisciotto CA, Morrell JI. Sex differences in the distribution and projections of testosterone target neurons in the medial preoptic area and the bed nucleus of the stria terminalis of rats. Horm. Behav. 1994;28:492–502. doi: 10.1006/hbeh.1994.1047. [DOI] [PubMed] [Google Scholar]
- 89.Lisk RD. Neural localization for androgen activation of copulatory behavior in the male rat. Endocrinology. 1967;80:754–761. doi: 10.1210/endo-80-4-754. [DOI] [PubMed] [Google Scholar]
- 90.Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: Differential effects on copulatory behavior and noncontact erection in male rats. J. Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lloyd SA, Dixson AF. Effects of hypothalamic lesions upon the sexual and social behaviour of the male common marmoset (Callithrix jacchus) Brain Res. 1988;463:317–329. doi: 10.1016/0006-8993(88)90405-2. [DOI] [PubMed] [Google Scholar]
- 92.Lorenz K. The comparative method in studying innate behavior patterns. Symp. Soc. Exp. Biol. 1950;4:221–268. [Google Scholar]
- 93.Meddle SL, King VM, Follett BK, Wingfield JC, Ramenofsky M, Foidart A, Balthazart J. Copulation activates Fos-like immunoreactivity in the male quail forebrain. Behav. Brain Res. 1997;85:143–159. doi: 10.1016/s0166-4328(97)87581-x. [DOI] [PubMed] [Google Scholar]
- 94.Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The Physiology of reproduction. Vol. 2. Raven Press; New York: 1994. pp. 3–105. [Google Scholar]
- 95.Moses J, Loucks JA, Watson HL, Matuszewich L, Hull EM. Dopaminergic drugs in the medial preoptic area and nucleus accumbens: Effects on motor activity, sexual motivation, and sexual performance. Pharmacol. Biochem. Behav. 1995;51:681–686. doi: 10.1016/0091-3057(94)00437-n. [DOI] [PubMed] [Google Scholar]
- 96.Murphy AZ, Rizvi TA, Ennis M, Shipley MT. The organization of preopticmedullary circuits in the male rat: Evidence for interconnectivity of neural structures involved in reproductive behavior, antinociception and cardiovascular regulation. Neuroscience. 1999;91:1103–1116. doi: 10.1016/s0306-4522(98)00677-0. [DOI] [PubMed] [Google Scholar]
- 97.Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: A potential circuit for the initiation of male sexual behavior. J. Comp. Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- 98.Nelson RJ. An introduction to behavioral endocrinology. 2 edn. Sinauer Associates, Inc.; Sunderland, Massachussets; 2000. p. 0. [Google Scholar]
- 99.Newman SW. The medial extended amygdala in male reproductive behavior - A node in the mammalian social behavior network. Ann. N.Y. Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 100.Numan M, Insel TR. The neurobiology of parental behavior. Springer Verlag; New York: 2003. [Google Scholar]
- 101.Panzica GC, Viglietti-Panzica C, Calcagni M, Anselmetti GC, Schumacher M, Balthazart J. Sexual differentiation and hormonal control of the sexually dimorphic preoptic medial nucleus in quail. Brain Res. 1987;416:59–68. doi: 10.1016/0006-8993(87)91496-x. [DOI] [PubMed] [Google Scholar]
- 102.Panzica GC, Viglietti-Panzica C, Sanchez F, Sante P, Balthazart J. Effects of testosterone on a selected neuronal population within the preoptic sexually dimorphic nucleus of the Japanese quail. J. Comp. Neurol. 1991;303:443–456. doi: 10.1002/cne.903030310. [DOI] [PubMed] [Google Scholar]
- 103.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front. Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- 104.Paredes RG, Highland L, Karam P. Socio-sexual behavior in male rats after lesions of the medial preoptic area: Evidence for reduced sexual motivation. Brain Res. 1993;618:271–276. doi: 10.1016/0006-8993(93)91275-w. [DOI] [PubMed] [Google Scholar]
- 105.Paredes RG, Baum MJ. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area anterior hypothalamus. J. Neurosci. 1995;15:6619–6630. doi: 10.1523/JNEUROSCI.15-10-06619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paredes RG, Tzschentke T, Nakach N. Lesions of the medial preoptic area anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res. 1998;813:1–8. doi: 10.1016/s0006-8993(98)00914-7. [DOI] [PubMed] [Google Scholar]
- 107.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J. Comp. Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 108.Pfaff DW. The neuroanatomy of sex hormone receptors in the vertebrate brain. In: Anand Kumar TC, editor. Neuroendocrine regulation of fertility. Karger; Basel: 1976. pp. 30–45. [Google Scholar]
- 109.Pfaff DW. Neurobiological and molecular mechanisms of sexual motivation. The MIT Press; Cambridge, Mass: 1999. Drive; pp. 1–312. [Google Scholar]
- 110.Pfaus JG, Mendelson SD, Phillips AG. A correlational and factor analysis of anticipatory and consummatory measures of sexual behavior in the male rat. Psychoneuroendocrinology. 1990;15:329–340. doi: 10.1016/0306-4530(90)90058-h. [DOI] [PubMed] [Google Scholar]
- 111.Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav. Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- 112.Pfaus JG, Beach Frank A. Award - Homologies of animal and human sexual behaviors. Horm. Behav. 1996;30:187–200. doi: 10.1006/hbeh.1996.0024. [DOI] [PubMed] [Google Scholar]
- 113.Reiner AD, Perkel J, Bruce L, Butler A, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, C. S, E.D. J. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Riters LV, Absil P, Balthazart J. Effects of brain testosterone implants on appetitive and consummatory components of male sexual behavior in Japanese quail. Brain Res. Bull. 1998;47:69–79. doi: 10.1016/s0361-9230(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 115.Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav. Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 116.Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J. Comp. Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- 117.Rizvi TA, Murphy AZ, Ennis M, Behbehani MM, Shipley MT. Medial preoptic area afferents to periaqueductal gray medullo-output neurons: a combined Fos and tract tracing study. J. Neurosci. 1996;16:333–344. doi: 10.1523/JNEUROSCI.16-01-00333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robertson GS, Pfaus JG, Atkinson LJ, Matsumura H, Phillips AG, Fibiger HC. Sexual behavior increases c-fos expression in the forebrain of the male rat. Brain Res. 1991;564:352–357. doi: 10.1016/0006-8993(91)91477-i. [DOI] [PubMed] [Google Scholar]
- 119.Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol. Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- 120.Roselli CE, Cross E, Poonyagariyagorn HK, Stadelman HL. Role of aromatization in anticipatory and consummatory aspects of sexual behavior in male rats. Horm. Behav. 2003;44:146–151. doi: 10.1016/s0018-506x(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 121.Sachs BD. Placing erection in context: The reflexogenic-psychogenic dichotomy reconsidered. Neurosci. Biobehav. Rev. 1995;19:211–224. doi: 10.1016/0149-7634(94)00063-7. [DOI] [PubMed] [Google Scholar]
- 122.Sakata JT, Crews D. Cytochrome oxidase activity in the preoptic area correlates with differences in sexual behavior of intact and castrated male leopard geckos (Eublepharis macularius) Behav. Neurosci. 2004;118:857–862. doi: 10.1037/0735-7044.118.4.857. [DOI] [PubMed] [Google Scholar]
- 123.Satinoff E. Neural integration of thermoregulatory responses. In: Cara LD, editor. Limbic and autonomic system research. Plenum Press; New York: 1974. pp. 41–83. [Google Scholar]
- 124.Schwartz-Giblin S, McCarthy MM. A sexual column in the PAG? Trends Neurosci. 1995;18:129–120. doi: 10.1016/0166-2236(95)93889-6. [DOI] [PubMed] [Google Scholar]
- 125.Seiwert CM, Adkins-Regan E. The foam production system of the male Japanese quail: Characterization of structure and function. Brain Behav. Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- 126.Sewards TV, Sewards MA. Fear and power-dominance drive motivation: neural representations and pathways mediating sensory and mnemonic inputs, and outputs to premotor structures. Neurosci. Biobehav. Rev. 2002;26:323–344. doi: 10.1016/s0149-7634(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 127.Sharma TP, Blache D, Roselli CE, Martin GB. Distribution of aromatase activity in brain and peripheral tissues of male sheep: effect of nutrition. Reprod. Fertil. Dev. 2004;16:709–715. doi: 10.1071/rd04018. [DOI] [PubMed] [Google Scholar]
- 128.Simerly RB, Swanson LW, Gorski RA. Demonstration of a sexual dimorphism in the distribution of serotonin immunoreactive fibers in the medial preoptic nucleus in the rat. J. Comp. Neurol. 1984;225:151–166. doi: 10.1002/cne.902250202. [DOI] [PubMed] [Google Scholar]
- 129.Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J. Comp. Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- 130.Sinnamon HM. Preoptic and hypothalamic neurons and the initiation of locomotion in the anesthetized rat. Prog. Neurobiol. 1993;41:323–344. doi: 10.1016/0301-0082(93)90003-b. [DOI] [PubMed] [Google Scholar]
- 131.Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J. Comp. Neurol. 1974;156:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- 132.Stumpf WE, Sar M. Anatomical distribution of estrogen, androgen, progestin, corticoid and thyroid hormone target sites in the brain of mammals: phylogeny and ontogeny. Amer. Zool. 1978;18:435–445. [Google Scholar]
- 133.Swaab DF, Chung WCJ, Kruijver FPM, Hofman MA, Ishunina TA. Structural and functional sex differences in the human hypothalamus. Horm. Behav. 2001;40:93–98. doi: 10.1006/hbeh.2001.1682. [DOI] [PubMed] [Google Scholar]
- 134.Swann JM. Gonadal steroids regulate behavioral responses to pheromones by actions on a subdivision of the medial preoptic nucleus. Brain Res. 1997;750:189–194. doi: 10.1016/s0006-8993(96)01348-0. [DOI] [PubMed] [Google Scholar]
- 135.Swanson LW, Mogenson GJ. Neural mechanisms for the functional coupling of autonomic, endocrine and somatomotor response in adaptive behavior. Brain Res. 1981;3:1–34. doi: 10.1016/0165-0173(81)90010-2. [DOI] [PubMed] [Google Scholar]
- 136.Taziaux M, Cornil CA, Balthazart J. Aromatase inhibition blocks the expression of sexually-motivated cloacal gland movements in male quail. Behav. Processes. 2004;67:461–469. doi: 10.1016/j.beproc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 137.Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, Balthazart J. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behavior in Japanese quail. Eur. J. Neurosci. 2006;23:1869–1887. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- 138.Taziaux M, Lopez J, Cornil CA, Balthazart J, Holloway KS. Differential c-fos expression in the brain of male Japanese quail following exposure to stimuli that predict or do not predict the arrival of a female. Eur. J. Neurosci. 2007;25:2835–2846. doi: 10.1111/j.1460-9568.2007.05542.x. [DOI] [PubMed] [Google Scholar]
- 139.Thompson RR, Adkins-Regan E. Photoperiod affects the morphology of a sexually dimorphic nucleus within the preoptic area of male Japanese quail. Brain Res. 1994;667:201–208. doi: 10.1016/0006-8993(94)91497-4. [DOI] [PubMed] [Google Scholar]
- 140.Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): A comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav. Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- 141.Timberlake W, Silva KM. Appetitive behavior in ethology, psychology, and behavior systems. In: Thompson NS, editor. Perspectives in Ethology, Volume 11: Behavioral Design. Plenum Press; New York: 1995. pp. 211–253. [Google Scholar]
- 142.Tinbergen N. The study of instinct. Clarendon Press; Oxford: 1951. p. 0. [Google Scholar]
- 143.Tobet SA, Fox TO. Sex differences in neuronal morphology influenced hormonally throughout life. In: Gerall AA, Moltz H, Ward IL, editors. Handbook of behavioral neurobiology. Vol 11. Sexual differentiation. Plenum Press; New York: 1992. pp. 41–83. [Google Scholar]
- 144.Turkenburg JL, Swaab DF, Endert E, Louwerse AL, Van De Poll NE. Effects of lesions of the sexually dimorphic nucleus on sexual behavior of testosterone-treated female wistar rats. Brain Res. Bull. 1988;21:215–224. doi: 10.1016/0361-9230(88)90234-1. [DOI] [PubMed] [Google Scholar]
- 145.Van De Poll NE, Van Dis H. The effect of medial preoptic-anterior hypothalamic lesions on bisexual behavior of the male rat. Brain Res. Bull. 1979;4:505–511. doi: 10.1016/0361-9230(79)90035-2. [DOI] [PubMed] [Google Scholar]
- 146.Viglietti-Panzica C, Panzica GC, Fiori MG, Calcagni M, Anselmetti GC, Balthazart J. A sexually dimorphic nucleus in the quail preoptic area. Neurosci. Ltrs. 1986;64:129–134. doi: 10.1016/0304-3940(86)90087-x. [DOI] [PubMed] [Google Scholar]
- 147.Voigt C, Ball GF, Balthazart J. Neuroanatomical specificity of sex differences in expression of aromatase mRNA in the quail brain. J. Chem. Neuroanat. 2007;33:75–86. doi: 10.1016/j.jchemneu.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 148.Warner RK, Thompson JT, Markowski VP, Loucks JA, Bazzett TJ, Eaton RC, Hull EM. Microinjection of the dopamine antagonist cis-flupenthixol into the MPOA impairs copulation, penile reflexes and sexual motivation in male rats. Brain Res. 1991;540:177–182. doi: 10.1016/0006-8993(91)90505-p. [DOI] [PubMed] [Google Scholar]
- 149.Watson JT, Adkins-Regan E. Activation of sexual behavior by implantation of testosterone propionate and estradiol benzoate into the preoptic area of the male Japanese quail (Coturnix japonica) Horm. Behav. 1989;23:251–268. doi: 10.1016/0018-506x(89)90065-2. [DOI] [PubMed] [Google Scholar]
- 150.Watson JT, Adkins-Regan E. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm.Behav. 1989;23:432–447. doi: 10.1016/0018-506x(89)90055-x. [DOI] [PubMed] [Google Scholar]
- 151.Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 152.Whalen RE, Simon NG. Biological motivation. Ann. Rev. Psychol. 1984;35:257–276. doi: 10.1146/annurev.ps.35.020184.001353. [DOI] [PubMed] [Google Scholar]
- 153.Wood RI, Newman SW. Mating activates androgen receptor-containing neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1993;614:65–77. doi: 10.1016/0006-8993(93)91019-o. [DOI] [PubMed] [Google Scholar]
- 154.Wood RI, Newman SW. The medial amygdaloid nucleus and medial preoptic area mediate steroidal control of sexual behavior in the male Syrian hamster. Horm. Behav. 1995;29:338–353. doi: 10.1006/hbeh.1995.1024. [DOI] [PubMed] [Google Scholar]
- 155.Wood RI, Newman SW. Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. J. Neurosci. 1995;15:7261–7269. doi: 10.1523/JNEUROSCI.15-11-07261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yahr P. Pars compacta of the sexually dimorphic area of the gerbil hypothalamus: postnatal ages at which development responds to testosterone. Behav. Neural. Biol. 1988;49:118–124. doi: 10.1016/s0163-1047(88)91312-x. [DOI] [PubMed] [Google Scholar]
- 157.Young LJ, Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]