Abstract
Sphingosine-1-Phosphate (S1P) is a bioactive lipid that signals through a family of five G protein-coupled receptors, termed S1P1–5. S1P stimulates growth and invasiveness of glioma cells, and high expression levels of the enzyme that forms S1P, sphingosine kinase-1, correlate with short survival of glioma patients. In this study we examined the mechanism of S1P stimulation of glioma cell proliferation and invasion by either overexpressing or knocking down, by RNA interference, S1P receptor expression in glioma cell lines. S1P1, S1P2 and S1P3 all contribute positively to S1P-stimulated glioma cell proliferation, with S1P1 being the major contributor. Stimulation of glioma cell proliferation by these receptors correlated with activation of ERK MAP kinase. S1P5 blocks glioma cell proliferation, and inhibits ERK activation. S1P1 and S1P3 enhance glioma cell migration and invasion. S1P2 inhibits migration through Rho activation, Rho kinase signaling and stress fiber formation, but unexpectedly, enhances glioma cell invasiveness by stimulating cell adhesion. S1P2 also potently enhances expression of the matricellular protein CCN1/Cyr61, which has been implicated in tumor cell adhesion, and invasion as well as tumor angiogenesis. A neutralizing antibody to CCN1 blocked S1P2-stimulated glioma invasion. Thus, while S1P2 decreases glioma cell motility, it may enhance invasion through induction of proteins that modulate glioma cell interaction with the extracellular matrix.
Keywords: sphingolipids, proliferation, invasion, CCN1, glioblastoma multiforme
Introduction
Sphingosine-1-phosphate (S1P) is a bioactive lipid that regulates cellular proliferation, migration, survival, cytoskeletal rearrangement, and angiogenesis [1–3]. S1P signals both intracellularly as a second messenger [4] and through five cell surface, G protein-coupled receptors (GPCRs) of the EDG family, S1P1/EDG-1, S1P2/EDG-5, S1P3/EDG-3, S1P4/EDG-6 and S1P5/EDG-8 [3]. Our group has been exploring the roles played by S1P in the growth and invasiveness of glioblastoma multiforme (GBM) cells. GBM is the most commonly occurring primary brain tumor in adults and is highly malignant, displaying aggressive growth and invasion into surrounding brain tissue [5] leading to a median life expectancy of only 10–12 months following diagnosis [6]. A better understanding of the molecular regulation of GBM cell growth and invasion will be necessary to develop effective molecular-based therapies.
We have shown that S1P is mitogenic for [7], and enhances motility and invasiveness of GBM cell lines [8]. As an indication of the importance of this signaling system for in vivo GBM, high expression levels of the enzyme which forms S1P, sphingosine kinase-1 (SphK1), in GBM tissue correlate with a more than 3 fold shorter survival time of GBM patients [9]. Furthermore, knockdown of SphK1 or SphK2 expression using RNA interference decreases GBM cell proliferation by preventing entry of cells in the cell cycle [9].
The mitogenic and invasive effects of S1P on GBM cells are at least partially mediated through its GPCRs, since both responses are sensitive to pertussis toxin [7], which specifically inhibits signaling through the Gi/o family of G proteins. Moreover, S1P induction of both mitogenesis and invasiveness of GBM cells occurs at nanomolar concentrations, consistent with the affinities of S1P for its receptors [7]. GBM cell lines [8] and GBM tissue [9] commonly express three S1P receptors, S1P1, S1P2 and S1P3. S1P4, which is primarily expressed in cells of hematopoietic origin [10], has not been detected in gliomas. S1P5 is expressed in normal brain in oligodendrocytes [11], however we have detected only very low levels of S1P5 expression in a limited number of GBM cases and several glioma cell lines (unpublished observation).
Each S1P receptor subtype activates a unique set of G proteins with varying preferences [3, 12]. Therefore, the individual influence of each receptor subtype on S1P regulation of GBM cell behavior may depend on the amounts of the individual S1P receptors expressed. In this study, the effects of S1P through individual receptor subtypes on glioma cell growth, migration, invasion, and adhesion were examined by overexpressing individual S1P receptors in glioma cells with low endogenous receptor levels, and knocking down individual S1P receptor expression by RNA interference in glioma cells with high levels of expression. The results show that S1P1, S1P2 and S1P3 all contribute to glioma cell growth and invasion through distinct, but overlapping, mechanisms. Conversely, S1P5 inhibits these cellular activities when overexpressed in glioma cells. Furthermore, the S1P2 receptor subtype displays a novel enhancement of S1P-stimulated invasion while decreasing migration. The S1P2-stimulated invasiveness in these glioma cell lines correlates with enhanced cell adhesion and is mediated, at least partially, by the matricellular protein CCN1/Cyr61. Thus, this study begins to define the roles of S1P in glioma cells through its individual receptors.
Materials and methods
Materials
Cell culture medium and fetal bovine serum were purchased from Mediatech (Herndon, VA). Fatty acid-free BSA was purchased from Sigma (St Louis, MO). S1P was from Avanti Polar Lipids (Alabaster, AL). Gelatin was purchased from BioRad (Hercules, CA). Matrigel was from Becton Dickinson (Palo Alto, CA). Assay-On-Demand gene expression assays and real time PCR reagents were purchased from Applied Biosystems (Foster City, CA). Antibodies to CCN1, phosphoERK and phosphoAKT were from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture
All glioma cell lines used in this study were maintained in Eagle’s minimum essential medium (EMEM) containing 10% fetal bovine serum, non-essential amino acids and sodium pyruvate. The cells were grown at 37°C in 95% air, 5% CO2. Cultures were passaged approximately once per week at a ratio of 1:12.
Generation of stably-transfected glioma cell lines
For S1P receptor overexpression, U-118 MG cells were transfected with pcDNA3.0 plasmid (Invitrogen, Carlsbad CA) containing the open reading frame of S1P receptor subtype 1, 2, 3, or 5. To down regulate S1P receptors by RNA interference, U-373 MG cells were transfected with the pSilencer 3.1-H1 hygro plasmid (Ambion, Austin TX) containing inserts designed to express short hairpin (sh)RNAs targeting individual S1P receptors. To determine effective target sequences for RNA interference, three sequences were chosen for each receptor using the small interfering (si)RNA Target Finder algorithm at the Ambion web site. siRNA oligonucleotides were synthesized using the Silencer siRNA construction kit (Ambion), and were transfected into U-373 MG cells using siPORT-lipid transfection reagent (Ambion) according to manufacturer’s instructions. Two days later RNA was extracted and RT-PCR analysis was used to measure receptor mRNA expression as described below. The most effective sequence for each receptor was synthesized as a DNA oligonucleotide (Integrated DNA Technologies) containing both sense and antisense sequences separated by a loop. The strands were annealed and then ligated into the pSilencer plasmid. Sequences of inserts were as follows: S1P1 -5′-GATCCGCCGGACGATGATATCATAGTTCAAGAGACTATGATAT CATCGTCCGGTTTTTTGGAAA-3′; S1P2 - 5′- GATCCGCACGCACAGCACATAATGCTTCAAGAGAGCATTATGTGCTGTGCGTGTTTT TTGGAAA-3′; S1P3 - 5′-GATCCAGACATCAGAATGTTGACCTTCAAGAGAG GTCAACATTCTGATGTCTTTTTTTGGAAA-3′.
Cells were transfected using Lipofectamine 2000 (Invitrogen), according to manufacturer’s instructions. U-118 MG S1P receptor overexpressing stable cell lines were selected with Geneticin (Invitrogen). U-373 MG cells expressing shRNAs for S1P receptors were selected with Hygromycin (Invitrogen). Individual colonies were isolated, grown, and S1P receptor expression was quantitated by RT-PCR analysis as described below.
Real time quantitative PCR
Quantitative RT-PCR to measure S1P receptor mRNA expression level was performed as previously described [9]. Briefly, total RNA was extracted using Trizol (Invitrogen) according to manufacturer’s instructions, followed by treatment with DNase1 (Ambion) for 20 min at 37°C. The RNA was purified and concentrated using the Absolutely RNA miniprep kit (Stratagene, LA Jolla CA). The Superscript II First Strand Synthesis System (Invitrogen) was used to synthesize cDNA according to manufacturer’s instructions. RT-PCR analysis was performed using Applied Biosystems Assays-on-Demand gene expression assays and the Taqman Universal PCR Master Mix in the ABI PRISMw 7700 Sequence Detection System. Data was obtained using Sequence Detection System 1.7a software and exported to Microsoft Excel worksheets for analysis. Relative changes in gene expression were calculated as fold differences using the 2−ΔΔT method [13]. These values were then scaled to the S1P1 receptor expression of each individual cell line, after the efficiencies of all amplicons were determined to be approximately equal by the method of Livak et al. [13] and therefore valid for comparison.
Growth curve analysis
U-373 MG and U-118 MG cell lines were plated at 100,000 cells per well in 12 well plates and grown in EMEM with 10% FBS for 24 hours. The cells were then serum-starved for 48 hours and treated with 100 nM S1P. S1P was readded every 24 hours during the course of the experiment. At each indicated time point, cells were trypsinized and counted using a Coulter Z2 Particle Count and Size Analyzer.
Thymidine incorporation assay
Cells were grown to confluence in 24-well plates in EMEM containing 10% FBS, serum-starved for 24 hours and treated without and with 100 nM S1P. After 17 hours, 1 μCi/ml of [3H]thymidine was added, and cells were incubated an additional 6 hours. Cells were washed twice with cold Phosphate-Buffered Saline (PBS), and DNA was precipitated with cold 5% trichloroacetic acid. Trichloroacetic acid-insoluble material was dissolved in 0.25 M NaOH and radioactivity was quantitated in a scintillation counter.
Cell motility assay
Transwell inserts (Corning Costar) with polycarbonate filters containing 8 μm pores were coated with 0.1% gelatin in 0.1% acetic acid and allowed to dry. Cells were plated at 100,000 cells per insert in EMEM containing 0.1% fatty acid-free BSA (E-BSA). The indicated concentration of S1P in E-BSA was placed in the bottom well, and cells were incubated for 36 h at 37°C. The inserts were then fixed in 70% ethanol and stained with Dif-Quik dye (Dade Behring, Newark, DE). Filters were washed and mounted on slides, where non-migrating cells were scraped off. The dye was dissolved from stained, migrated cells in 10% acetic acid and absorbance measured at 635 nm. Results from three separate filters were averaged for each condition tested.
Invasion assays
Transwell inserts were coated with 15 μg Matrigel per insert and allowed to polymerize at 37°C. After rehydration with PBS, the inserts were placed in a Transwell 24-well plate with 100,000 cells per insert of each glioma cell line. The inserts were incubated at 37°C for 72 h in wells containing various concentrations of S1P in E-BSA. After the incubation period, the cells that had migrated through the Matrigel were quantitated as described above for the cell motility assay. In some experiments 20 μg/ml of polyclonal anti-CCN1 antibody (Santa Cruz Biotechnology) or anti pAKT as an irrelevant control polyclonal antibody was added prior to cell plating.
For spheroid assays, spheroids were formed by the inverted drop method as described [14], with minor modifications. Briefly, cells were spotted on the lid of a 100 cm tissue culture dish at a concentration of 50,000 per 100 μl in EMEM and grew inverted on the dish for 3.5 days. The spheroids were then removed and added to 150 μl of polymerized Matrigel in a 24-well plate. This was then covered by an additional 150 μl of Matrigel which was allowed to polymerize for 30 minutes at 37°C. The Matrigel-spheroid-Matrigel sandwich was then supplemented with E-BSA media with or without 100 nM S1P. This media was supplemented with additional S1P approximately every 36 hours. Pictures were taken at indicated time points using both 4x and 10x objectives.
Cell adhesion assay
Wells of a 96 well plate were coated with 2.5 μg Matrigel or 0.1% gelatin in 0.1% acetic acid and allowed to polymerize for one hour at 37°C. Wells were blocked with 3% BSA in PBS for an additional 30 min, and then washed three times with PBS. Glioma cells were starved overnight and then treated for 1 h without or with 100 nM S1P, scraped from flasks in PBS containing 10 mM EDTA and washed in E-BSA. Cells were then resuspended at 5 × 105/ml in E-BSA containing the indicated concentration of S1P, and 50,000 cells per well were allowed to adhere to coated wells for 3 h at 37°C. The supernatant was removed and the cells were fixed in 70% ethanol, and stained in 0.5% crystal violet in 20% MeOH. After washing, a 10% acetic acid solution was used to elute stain, which was then quantitated by measuring the absorbance at 540 nm.
Rac and Rho activation assays
Cells were grown to approximately 75–80% confluence and serum starved overnight, followed by stimulation without or with 100 nM S1P for 10 minutes. Rac activation lysates were obtained by lysing cells in 50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.5 M NaCl, 10 M MgCl2, and 10 μg/ml each of aprotinin and leupeptin). Activated Rac was isolated using the Rac Activation Assay Kit (Upstate Cell Signaling Solutions, Lake Placid NY). Western analysis of isolated, activated Rac was then performed using Rac antibody (Upstate Cell Signaling Solutions) following manufacturer’s protocol. For Rho activation assays, cells were lysed in 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 0.5 M NaCl, and 1% Triton X-100 using the Rho activation Biochem Kit according to manufacturer’s protocol (Cytoskeleton, Denver CO). Immunoprecipitated, activated Rho was then subjected to Western blot analysis using Rho antibody (Cytoskeleton) according to manufacturer’s protocol. One sample was loaded with a non-hydrolysable GTP analog, GTPγS, prior to immunoprecipitation in both assays as a positive control of activation.
Western blotting
Cells were grown to approximately 85–90% confluence before overnight starvation and stimulation without or with 100 nM S1P for the indicated time. Cells were lysed in 0.5 M Hepes (pH 7.5), 150 mM NaCl, 10 mM MgCl2, 0.5 M EDTA, 20% Triton X-100, 10% SDS, 1 M DTT, 0.5% deoxycholate, 0.2 mM PMSF, and 10 μg/ml each of aprotinin and leupeptin. Equal amounts of protein were separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked for 1 h at room temperature in PBS + 0.1% Tween-20 (PBS-T) containing 5% (w/v) non-fat dry milk. Membranes were probed overnight with primary antibodies in blocking solution and washed 5 × 5 min with PBS-T, followed by incubation for 1 h at room temperature with goat anti-mouse IgG or goat anti-rabbit IgG conjugated to horseradish peroxidase (1:10,000 in blocking solution). Membranes were washed as above, followed by incubation in Pierce Super Signal West Pico chemiluminescent substrate. Bands were visualized by exposure to x-ray film.
Analysis of actin cytoskeleton
Cells were plated at 10–15% confluence onto glass coverslips and starved overnight prior to inhibitor pretreatment and stimulation with or without 100 nM S1P for 3 hours. Inhibitor pretreatments were carried out using 200 ng/ml pertussis toxin (Calbiochem, San Diego CA) for 3 hours or 10 μM Y-27632 (Calbiochem) for 30 minutes. Cells were washed twice in PBS and fixed for 5 minutes in 3.7% formaldehyde in PBS. After extensive washing in PBS, cells were permeabilized in 0.1% Triton X-100 for 1 hour at room temperature and washed again before addition of 10 μg/mL Phalloidin–Tetramethylrhodamine B isothiocyanate (TRITC) (Sigma-Aldrich, Oakville, Ontario, Canada). Unbound phalloidin conjugate was removed with extensive washing and glass inserts were mounted on slides for analysis. Filamentous actin was observed using a Zeiss 510 META confocal microscope.
Results
Generation of S1P receptor over- and underexpressing glioma cell lines
U-373 MG cells express similar levels of the S1P receptors, S1P1, S1P2 and S1P3 [8]. In addition, U-373 MG cells respond to S1P treatment with both increased proliferation and motility/invasiveness [7, 8]. Therefore, this cell line was chosen to down regulate individual S1P receptors using RNA interference. In contrast, U-118 MG cells express very low levels of S1P1, S1P2, S1P3 and S1P5 by real time PCR analysis (data not shown), and respond to S1P with only minimally increased proliferation (Fig. 1A, Fig. 2A) or motility (Fig. 5A). Thus, U-118 MG cells were chosen to overexpress S1P receptors.
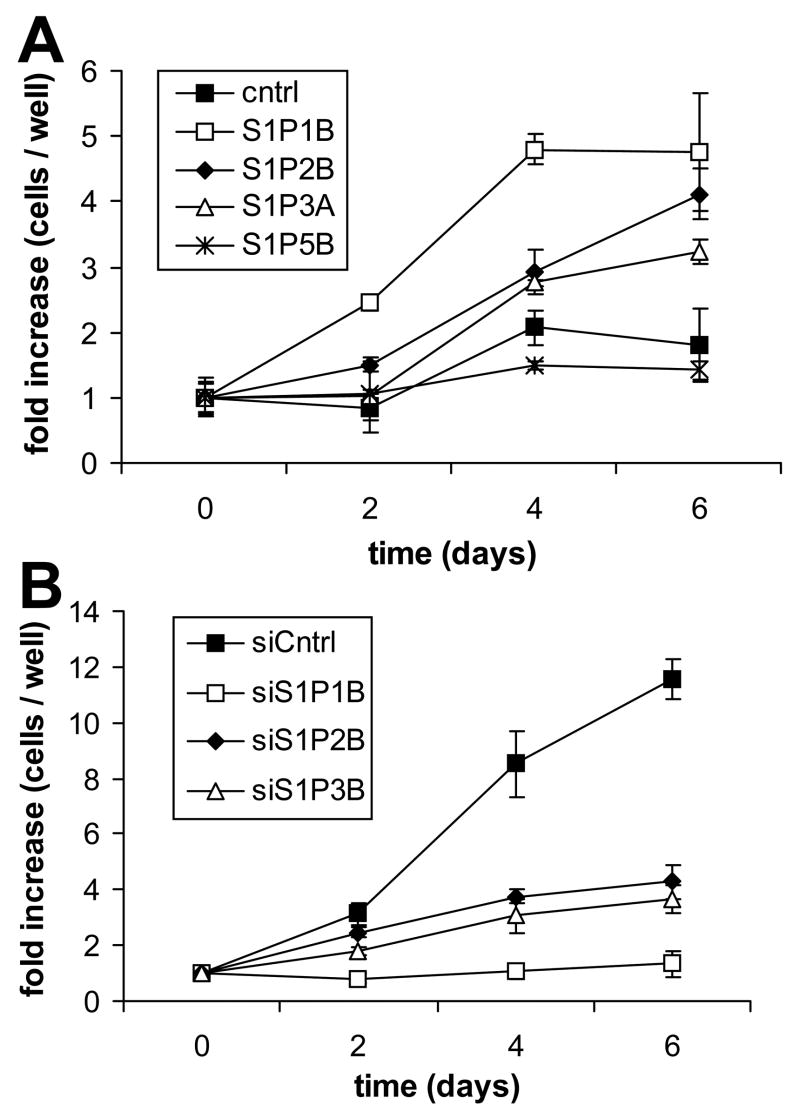
Fig. 1.
Growth curve analysis of S1P receptor over- and underexpressing glioma cell lines. U-118 MG (A) and U-373 MG (B) cell lines were analyzed for growth in the presence of 100 nM S1P as described in Materials and Methods. Data are expressed as fold increase in cell number over time relative to cells present at Day 0, and are means ± standard deviations of triplicate determinations. Two independent experiments provided similar results. Cell lines of similar levels of receptor over or under expression were used in this experiment.
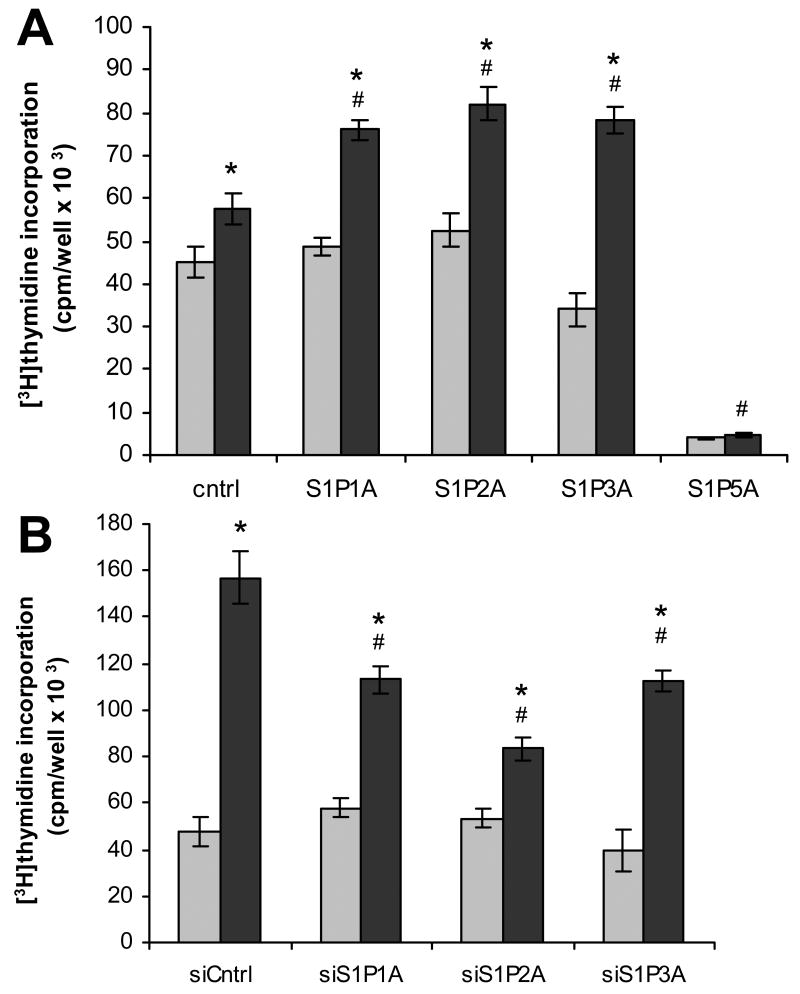
Fig. 2.
S1P effects on DNA synthesis in S1P receptor over- and underexpressing glioma cell lines. U-118 MG (A) and U-373 MG (B) cell lines were treated with (black bars) and without (gray bars) 100 nM S1P and analyzed for DNA synthesis by [3H]thymidine incorporation as described in Materials and Methods. Data are means ± standard deviations of quadruplicate determinations. Two independent experiments provided similar results. A statistically significant difference (p< 0.05) between S1P-treated and untreated samples for a given cell line is represented with * and a statistically significant difference (p< 0.05) between the S1P-treated control and the S1P-treated receptor over- or underexpressing cell line is represented with #.
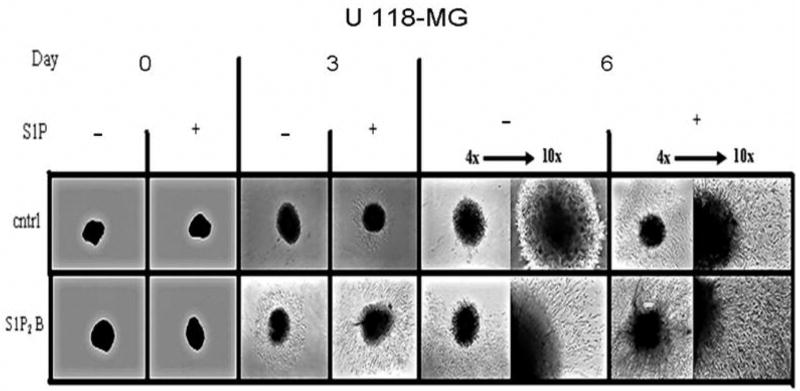
Fig. 5.
S1P2 regulation of invasion in a cell spheroid assay. U-118 MG control and S1P2- overexpressing cell spheroids were embedded in Matrigel, and invasion into the surrounding Matrigel was monitored in the absence of presence of 100 nM S1P. Spheroids were photographed every three days using both 4x and 10x objectives of an inverted phase contrast microscope.
U-373 MG cells were transfected with the pSilencer vectors containing inserts designed to express a random sequence control shRNA (pSil-control) or shRNAs targeting individual S1P receptors. U-118 MG cells were transfected with expression constructs of S1P receptors or empty pcDNA3 expression vector as control. Stable cell lines were selected and screened for decreased or increased S1P receptor expression in comparison to control stable cell lines and untransfected cells by real time RT-PCR analysis. Both the U-118 MG and U-373 MG control cell lines did not have significantly different expression of any S1P receptor subtype in comparison to untransfected counterparts (data not shown). Expression of S1P receptors in control lines was then used as a standard to measure relative S1P receptor expression in each stable over- or underexpressing cell line (Table 1). For one clone of each cell line expression of S1P1, S1P2, S1P3 and S1P5 receptors was measured, to verify that transfections specifically affected only the targeted S1P receptor. The U-118 MG cell lines all overexpressed the desired receptor by 2–6 fold without significant change in the other S1P receptors. The U-373 MG transfected cell lines each had a 3–10 fold decreased expression in the targeted S1P receptor subtype, with no significant effect on expression of the other subtypes. Thus, the stable cell lines created for this study each express specifically altered expression of the targeted S1P receptors.
Table 1.
S1P receptor Expression levels in stably-transfected U-118 MG and U-373 MG cell lines
| Percent expression relative to control cell lines1 | |||||
|---|---|---|---|---|---|
| Cell line | transfection | S1P1 | S1P2 | S1P3 | S1P5 |
| U-118-S1P1A | S1P1 | 682 | 87 | 106 | 116 |
| U-118-S1P1B | S1P1 | 408 | ND2 | ND | ND |
| U-118-S1P2A | S1P2 | 90 | 200 | 97 | 110 |
| U-118-S1P2B | S1P2 | ND | 459 | ND | ND |
| U-118-S1P3A | S1P3 | 90 | 97 | 428 | 112 |
| U-118-S1P5A | S1P5 | 119 | 115 | 88 | 216 |
| U-118-S1P5B | S1P5 | ND | ND | ND | 444 |
| U-373-pSil-1A | siS1P1 | 8 | 118 | 98 | 116 |
| U-373-pSil-1B | siS1P1 | 30 | ND | ND | ND |
| U-373-pSil-2A | siS1P2 | 92 | 33 | 118 | 110 |
| U-373-pSil-2B | siS1P2 | ND | 36 | ND | ND |
| U-373-pSil-3A | siS1P3 | 104 | 120 | 17 | 114 |
| U-373-pSil-3B | siS1P3 | ND | ND | 39 | ND |
Expression was measured by real time quantitative PCR analysis as described in Materials and Methods. Expression levels of targeted receptors are shown in bold type.
Results are percentages relative to expression level in vector-transfected U-118 control cells and pSil-control-transfected U-373 MG cells.
ND = Not determined
S1P receptor-induced effects on glioma cell growth
We have previously shown that S1P stimulates proliferation of U-373 MG cells which express high levels of S1P receptors, but not of U-118 MG cells, which express low S1P receptor levels [7]. Therefore, we first examined the effects of altered S1P receptor expression in these cell lines on cell growth in order to delineate the contributions of the various S1P receptors to S1P-stimulated glioma cell proliferation. The cell clones used expressed similar levels of the various S1P receptors, approximately 4 fold overexpression of each receptor in U-118 MG cell clones and 30 – 39% of control expression levels in U-373 MG cell clones (Table 1). No significant effect of S1P receptor overexpression or underexpression was seen on cell proliferation in either medium containing 10% FBS or in serum-free medium in the absence of added S1P (data not shown). However, in serum-free medium containing 100 nM S1P, overexpression of S1P1, S1P2 or S1P3 in U-118 MG cells resulted in an increase in cell accumulation compared to control cells, which proliferated very slowly in these conditions (Fig. 1A). S1P1 overexpression had the most potent effect, leading to cell numbers that were significantly higher than those seen for S1P2 or S1P3 overexpressers throughout the experiment. Interestingly, S1P5 overexpression completely inhibited S1P-stimulated proliferation of U-118 MG cells (Fig. 1A).
As previously seen for untransfected U-373 MG cells [7], S1P potently stimulated proliferation of pSil-control-transfected U-373 MG cells. Knock down of either S1P1, S1P2 or S1P3 receptor expression in U-373 MG cells significantly inhibited S1P-stimulated growth, as cell counts were lower than those seen for control cells at all measured time points (Fig. 1B). In agreement with the strong effect of S1P1 overexpression on growth of U-118 MG cells, S1P1 knock down inhibited S1P-stimulated U-373 MG cell growth more potently than S1P2 or S1P3 knock down. Thus, S1P1–3 receptor subtypes all contribute to S1P-stimulated growth of glioma cells, with S1P1 being the most potent stimulator of this response, while the S1P5 receptor subtype decreases glioma cell growth. Similar results were obtained using different clones of S1P receptor over- and underexpressing glioma cells (data not shown).
S1P stimulation of DNA synthesis was also examined in these cell lines using [3H]thymidine incorporation. In U-118 MG cells, overexpression of S1P1, S1P2 or S1P3 resulted in a significant increase in S1P-stimulated thymidine incorporation in comparison to vector-transfected control cells (Fig. 2A). DNA synthesis in S1P5-overexpressing U-118 MG cells was not significantly affected by S1P, however basal DNA synthesis was dramatically decreased in comparison to control U-118 MG glioma cells. Consequently, S1P5 overexpression influences both growth and DNA synthesis in a negative manner. S1P-stimulated DNA synthesis in U-373 MG cells was partially inhibited by knock down of each S1P receptor subtype in comparison to the S1P-treated control cells (Fig. 2B). Taken together, these results indicate that S1P utilizes S1P1, S1P2 and S1P3 receptors to increase proliferation of glioma cell lines.
ERK regulation in S1P-stimulated glioma cell lines
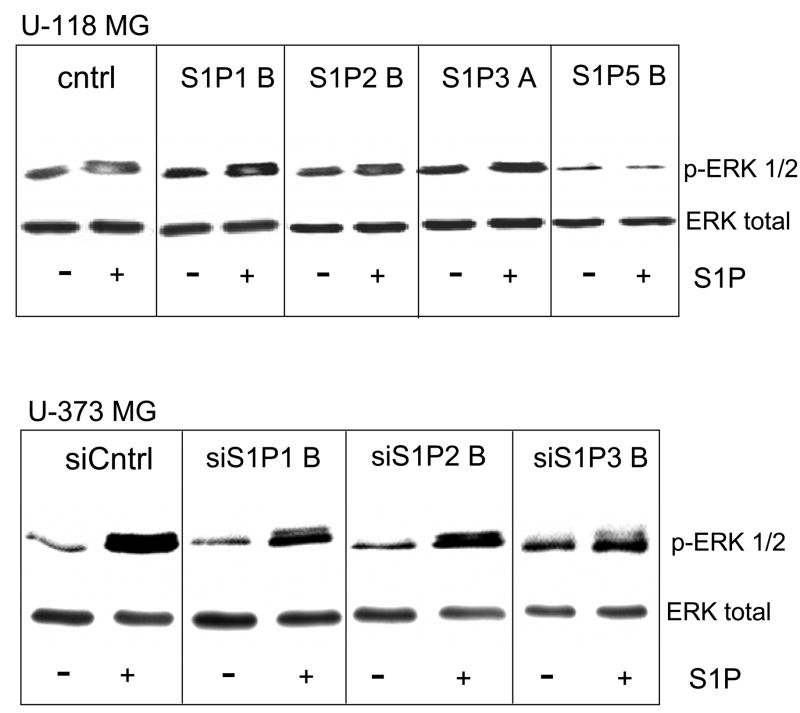
We previously showed that activation of extracellular signal-regulated kinase (ERK) is necessary for S1P-stimulation of glioma cell proliferation [7]. Therefore we examined ERK activation by S1P in glioma cell lines over and under expressing individual S1P receptor subtypes by Western blot analysis using phosphoERK-specific antibodies (Fig. 3). S1P stimulation increased phosphoERK formation in U-118 MG control as well as S1P receptor overexpressing cell lines. However, overexpression of S1P1 or S1P3 enhanced S1P-stimulated ERK activation, with S1P1 being most potent. S1P2 overexpression had no effect in comparison to control. S1P5 overexpression decreased basal levels of phosphoERK and further decreased phosphoERK upon S1P stimulation (Fig. 3A), in agreement with previously published results [15].
Fig. 3.
Erk activation in S1P receptor over- and underexpressing glioma cell lines. U-118 MG and U-373 MG cell lines were treated with or without 100 nM S1P for 10 minutes and ERK activation was measured by Western analysis using a phosphoERK-specific antibody. Blots were stripped of antibodies and reprobed with antibody to ERK2 to show that total ERK2 was present at similar levels in all samples. Two independent experiments provided similar results.
Down regulation of S1P1, S1P2, or S1P3 expression in U-373 MG cells partially decreased S1P stimulation of ERK phosphorylation (Fig. 3B). Thus, S1P appears to regulate ERK activation in glioma cells through S1P1, S1P2 and S1P3, in agreement with the ability of all three receptors to positively influence cell proliferation. In particular, the potency of S1P1 for ERK activation correlates with its strong effect on cell proliferation.
S1P receptor subtype effects on glioma cell migration and invasion
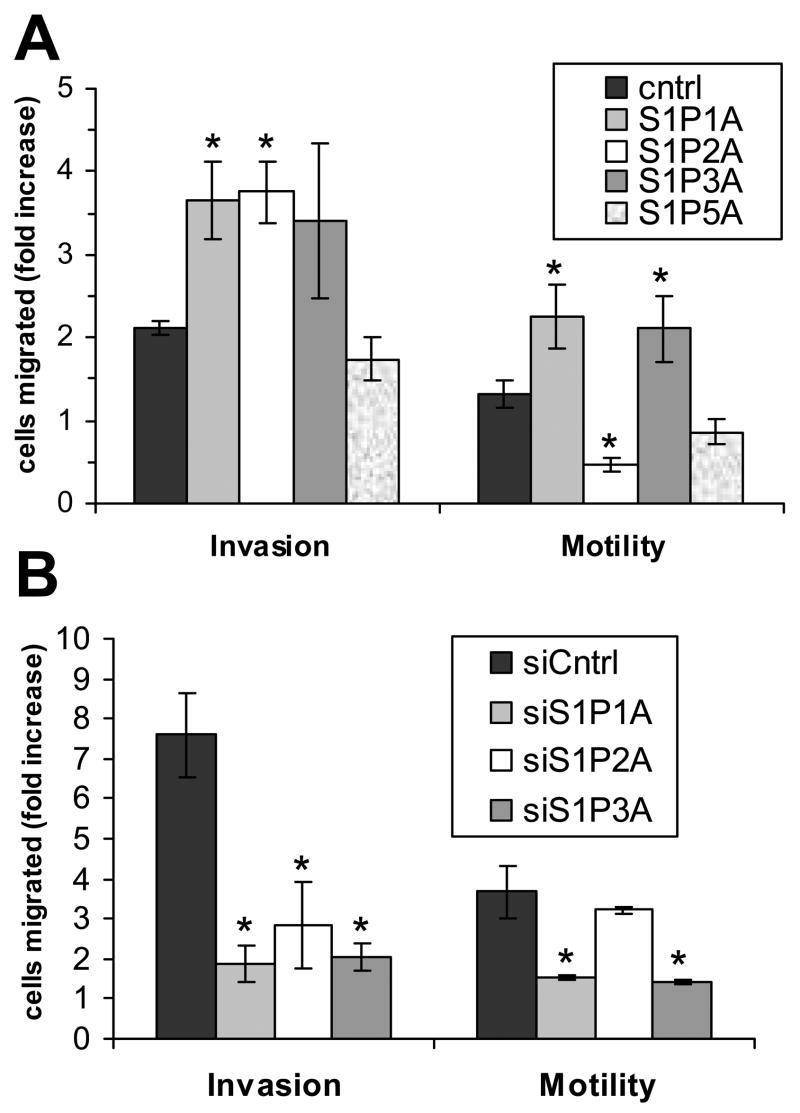
S1P also stimulates migration and invasion of glioma cells [8]. Therefore, we next examined these responses to S1P in our S1P receptor over and underexpressing glioma cells using transwell assays with inserts coated with either a dilute gelatin mixture to measure migration, or Matrigel to assess invasiveness. In U-118 MG cells, S1P1 overexpression significantly increased S1P stimulation of both invasion and migration (Fig. 4A). Similarly, S1P1 down regulation in U-373 MG cells resulted in a significant decrease in S1P-stimulated migration and invasion (Fig. 4B). S1P3 overexpression and down regulation had similar effects in both cell lines (Fig. 4). S1P5 overexpression in U-118 MG cells did not result in a significant change in either motility or invasiveness. Thus, S1P1 and S1P3 contribute to S1P-induced glioma cell motility and invasiveness.
Fig. 4.
Migration and invasion of S1P receptor over- and underexpressing glioma cell lines. U-118 MG (A) and U-373 MG (B) cell lines were analyzed for invasion through Matrigel or migration on gelatin as described in Materials and Methods in the absence and presence of 100 nM S1P. Results are expressed as fold difference in cell movement of S1P-treated cells compared to untreated cells. Data are means ± standard deviations of triplicate determinations. Two independent experiments provided similar results. A statistically significant difference of S1P-treated receptor over- or underexpressing cells relative to control S1P-treated cells (p< 0.05) is represented by *.
As expected, S1P2 overexpression in U-118 MG cells decreased cellular motility, but surprisingly, significantly increased invasiveness. While S1P2 is known to decrease cell motility [16–18], the increase in S1P-stimulated invasiveness due to S1P2 overexpression represents a novel finding. The targeted decrease in S1P2 expression in U-373 MG cells had no effect on cell motility, suggesting that the motility response of U-373 MG cells to S1P is dominated by the pro-migration effects of S1P1 and S1P3. However, S1P2 knock down significantly decreased invasion through Matrigel compared to U-373 MG control cells (Fig. 4B). Thus, S1P stimulation of S1P2 results in a negative effect on glioma cell motility, while positively affecting invasion. Similar results for overexpression of S1P1, S1P2 and S1P3 were obtained using different clones of S1P receptor overexpressing U-118 MG cells (Fig. 8), demonstrating that the changes seen in cell migration and invasion in these cell lines are not due to random changes occurring during cell clone selection, but are instead due to the receptor overexpression.
Fig 8.
Role of Rho kinase in S1P-stimulated motility and invasiveness. U-118-control or S1P receptor overexpressing cells were pretreated with or without Y-27632 for 30 minutes and then examined for S1P regulation of cell motility and invasiveness as described for Figure 4. Results are means ± standard deviations of triplicte determinations. The * indicates a statistically significant difference (p < 0.05) for S1P + Y-27632 compared to S1P alone for a given cell line.
The unexpected results with S1P2-overexpressing cells warranted verification by an alternative method, and therefore, we examined invasion of U-118 MG control and S1P2-overexpressing cells in a spheroid invasion assay. This assay may be more relevant to an in vivo setting than a transwell invasion assay, as the three dimensional spheroid more closely resembles an actual tumor [14]. The cells were grown inverted for several days in order to form a spheroidal mass of cells, which was transplanted into a three dimensional matrix and monitored for invasion into the surrounding Matrigel. The control U-118 MG cell line responded to S1P treatment with moderate invasion, while the untreated control spheroids showed virtually no invasion after six days. Untreated U-118-S1P2 cells were more invasive over time than the untreated control cells. When S1P2-overexpressing U-118 MG cells were treated with S1P, extensive cellular infiltration was observed over time. The aggressiveness was clearly evident at the six day time point where both the distance of invasion and density of invading cells was much greater than the S1P-treated control cells (Fig. 5). Thus, these results confirm that S1P2 overexpression enhances invasiveness of U-118 MG cells.
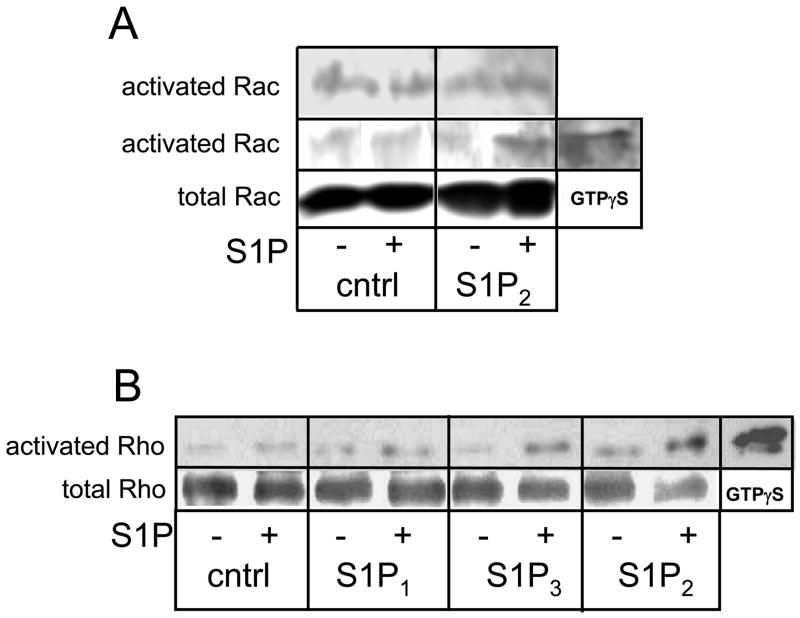
S1P2 has been shown to inhibit cell migration through blocking activation of the small GTPase Rac in several cell types, and this response is mediated through Rho [16–18]. Interestingly, although S1P2 has also been shown to block migration of glioma cells, and to activate Rho, it failed to inhibit Rac activation in these cells [19]. Given the unusual dual effect of S1P2 on migration and invasion in our cells, we wished to determine whether S1P activated Rac and inhibited Rho in this system. S1P treatment failed to decrease levels of activated Rac in S1P2-overexpressing U-118 MG cells (Fig. 6A). As expected, S1P activated Rho in S1P2-overexpressing U-118 MG cells. Thus, our data agree with those of Lepley et al. [19] in that, although S1P2 signaling decreases glioma cell motility and activates Rho in glioma cells, it does not negatively affect Rac activation in glioma cells. S1P3 overexpression also led to activation of Rho in these cells, while S1P1 overexpression did not.
Fig. 6.
Regulation of Rac and Rho small GTPases by S1P in over- and underexpressing glioma cell lines. A) Control or S1P2-overexpressing U-118 MG cells were treated without and with 100 nM S1P for 10 minutes. Activated Rac was isolated from cell lysates and subjected to Western analysis as described in Materials and Methods. Results of two independent experiments are shown. Total rac from whole cell lysates was blotted as a loading control. B) Control or S1P receptor-overexpressing U-118 MG cells were treated without and with 100 nM S1P for 10 minutes. Activated Rho was isolated from cell lysates and subjected to Western analysis as described in Materials and Methods. Two independent experiments provided similar results. Positive control samples for activated Rac or Rho were generated by adding the non-hydrolyzable GTP analog GTPγS to separate lysates.
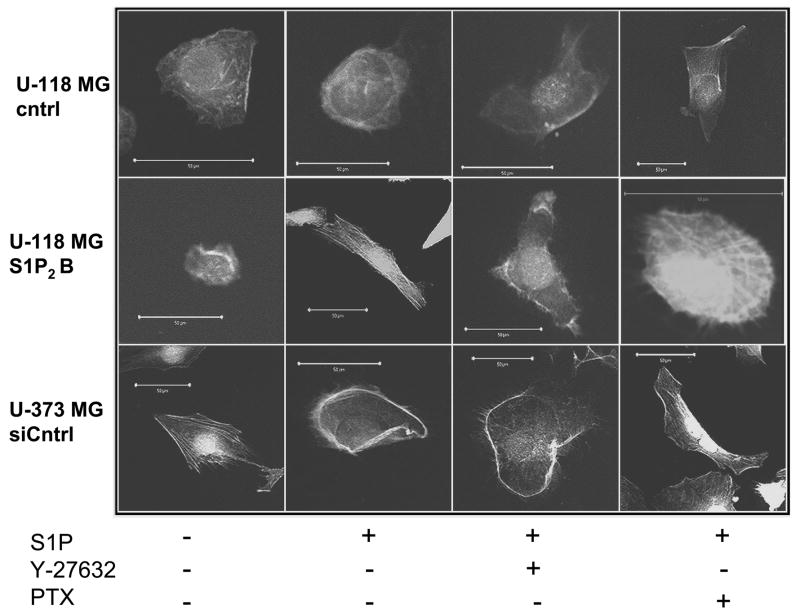
Rac and Rho small GTPases affect cell migration through formation of actin cytoskeletal structures. Thus, Rac activation is known to lead to formation of membrane ruffles or lamellipodia, while Rho activation causes formation of actin stress fibers [20]. Therefore, to further examine the regulation of these pathways by S1P receptors in glioma cells we treated U-373 cells and U-118-control and S1P2-overexpressing cells with or without S1P and stained with fluorescently-labeled phalloidin, which binds specifically to polymerized actin (Fig. 7). Confocal microscopy revealed that S1P stimulation led to lamellipodia formation in U-373-siCntrl cells but had no effect in U-118-control cells. This is consistent with our data showing that S1P stimulates migration of U-373-siCntrl but not U-118-control cells (Fig. 4). S1P2 overexpression in U-118 cells allowed S1P to stimulate formation of actin stress fibers, consistent with Rho activation seen in these cells (Fig. 6). Furthermore, S1P-induced stress fiber formation in these cells was blocked by the Rho kinase inhibitor Y-27632 but not by Pertussis toxin. S1P-induced lamellipodia formation in U-373-siCntrl cells was unaffected by Y-27632. In contrast, Pertussis toxin treatment of U-373-siCntrl cells changed the effect of S1P to induction of stress fibers.
Fig. 7.
Effect of S1P on the actin cytoskeleton. U-118-control or S1P2-overexpressing cells or U-373-siControl cells were pretreated with or without 10 μM Y-27632 for 30 minutes or with 200 ng/ml PTX for 3 hours and then treated with or withour 100 nM S1P for 3 hours. Cells were stained with TRITC-labeled phalloidin and visualized by confocal microscopy as described in Materials and Methods. Photographs of representative cells are shown. Scale bars indicate 50 μm.
We next examined whether Rho kinase signaling plays a role in the S1P-mediated decrease in migration and/or increase in invasion in S1P2-overexpressing U-118 cells. Y-27632 completely prevented the decrease in migration seen in S1P2-overexpressing U-118 cells (Fig. 8). Interestingly, it also partially prevented S1P-induced increased migration in both S1P1 and S1P3-overexpressing U-118 cells. S1P-induced invasion of U-118-S1P1 and S1P2-overexpressing cells appeared to decrease slightly although these changes were not statistically significant.
S1P-stimulated adhesion of glioma cells
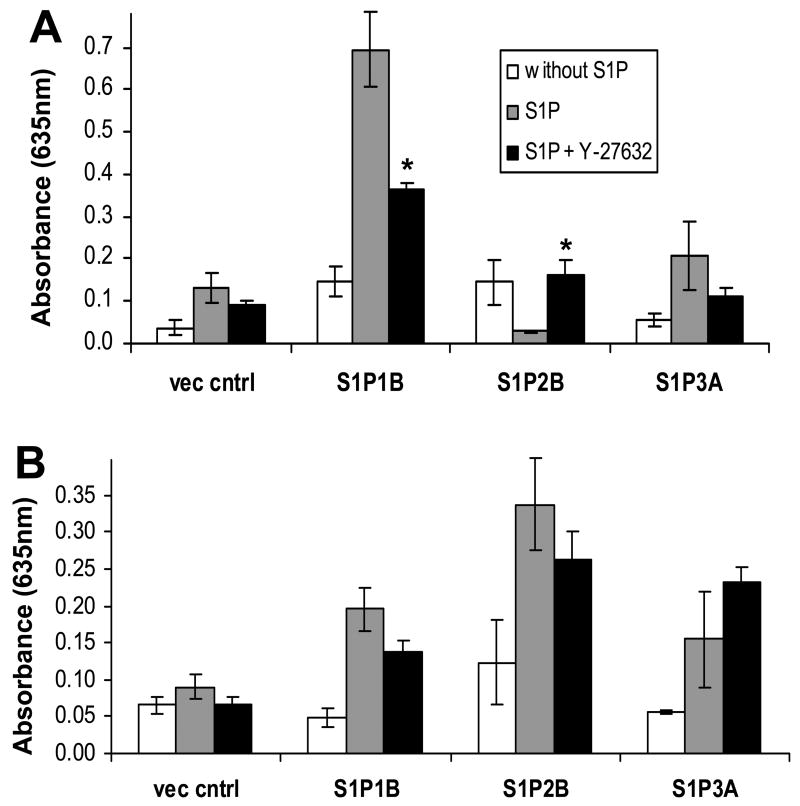
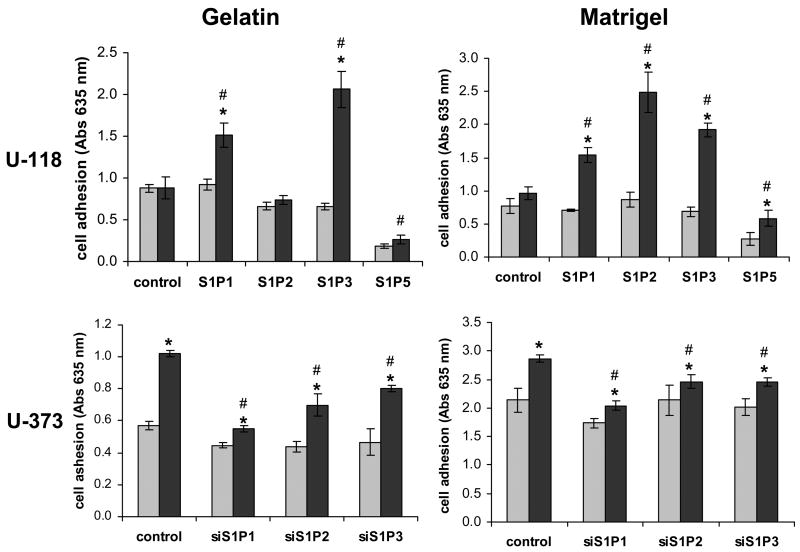
Cellular adhesion is an important aspect of cell motility and invasion. The difference between our migration and invasion assays is whether transwell inserts are coated lightly with gelatin or with a thicker layer of Matrigel, respectively. Thus, we next measured cellular adhesion to wells coated with gelatin, as used in the migration assay, or Matrigel, as in the invasion experiments. S1P pre-treatment had no effect on adhesion of U-118 MG control cells to either substrate. However, overexpression of either S1P1 or S1P3 led to significant increases in S1P-stimulated adhesion to both coatings (Fig. 9A,B). Interestingly, S1P stimulation of S1P2 overexpression in U-118 MG cells significantly increasing adhesion to Matrigel while not influencing adhesion to gelatin. This suggests that the difference in the effect of S1P2 on cell motility vs. invasiveness is at least partially due to the promotion of adhesion of S1P2-expressing cells to the Matrigel substrate.
Fig. 9.
Regulation of adhesion by S1P receptors. Serum-starved cells were treated with 100 nM S1P (black bars) or without S1P (gray bars) and adhesion to wells coated with either gelatin or Matrigel was measured as described in Materials and Methods (A). Data are means ± standard deviations of triplicate determinations. Two independent experiments provided similar results. The * represent a statistically significant difference (p< 0.05) between S1P-treated and untreated samples. The # represent a statistically significant difference (p< 0.05) between the S1P-treated control and the S1P-treated receptor overexpresser or underexpresser cell line.
Knockdown of S1P1, S1P2 or S1P3 in U-373 MG cells decreased S1P-stimulated adhesion to both gelatin and Matrigel. Notably, S1P1 knock down decreased S1P-stimulated gelatin adhesion more potently than S1P2 or S1P3 knock down. Knock down of any of the three receptors similarly decreased Matrigel adhesion (Fig. 9C,D). Taken together, these data suggest that all three receptors contribute to adhesion of glioma cells. The strong effect of S1P2 on adhesion to Matrigel suggests that this may be an important component of the enhanced invasiveness seen in response to this receptor.
Role of CCN1 in S1P-stimulated invasion
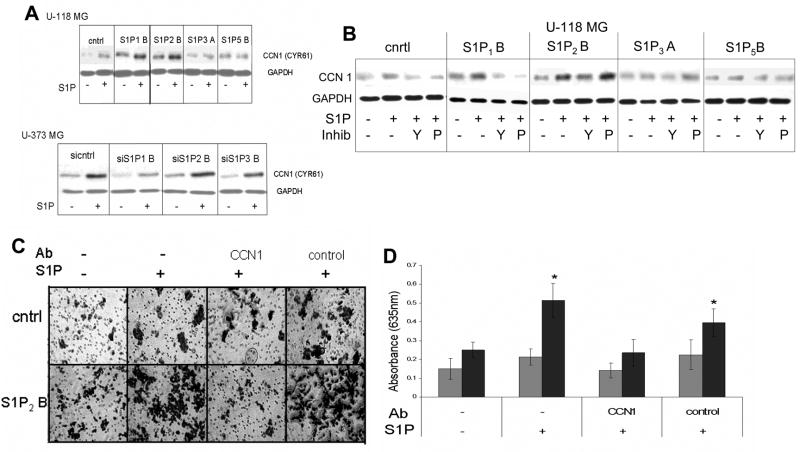
We next wished to further examine molecular mechanisms mediating S1P2-induced enhancement of invasion. We began by examining the regulation of expression of a protein known as CCN1/Cyr61. CCN1 is a heparin-binding, matricellular protein that has been shown to increase cellular motility, invasiveness, and adhesion, as well as angiogenesis [21]. In addition, CCN1 is commonly overexpressed in GBMs, and its overexpression correlates with short survival time of GBM patients [22]. S1P has been shown to induce expression of CCN1 in smooth muscle cells [23]. We therefore, examined CCN1 protein levels in S1P receptor over- and underexpressing cell lines (Fig. 10A). Expression of CCN1 was enhanced by S1P treatment in U-118 MG-control cells, indicating that even the low levels of S1P receptors present in these cells were able to induce moderate CCN1 expression. However, overexpression of S1P1 or S1P2, enhanced both basal levels and S1P-stimulation of CCN1 expression. In U-373 MG cells knock down of S1P1 expression decreased S1P induction of CCN1, while S1P2 and S1P3 knock down were without effect.
Fig. 10.
Role of CCN1/Cyr61 in S1P2-stimulated glioma cell invasion. (A) U-373 MG and U-118 MG cells were stimulated with or without S1P for 3 hours. (B) Cells were pretreated with or without 10 μM Y-27632 for 30 minutes (Y) or with 200 ng/ml PTX (P) for 3 hours and then treated with or withour 100 nM S1P for 3 hours. For both panels A and B cell lysates were subjected to western blot analysis for CCN1. Blots were stripped of antibodies and reprobed for GAPDH as a loading control. (C) S1P-stimulated invasion of U-118 MG control and S1P2-overexpressing cells was analyzed using the transwell assay in the absence and presence of antibodies to CCN1 or an irrelevant control antibody (anti-pAKT). (D) Results from the experiment shown in Panel C were quantitated as described in Materials and Methods. Gray bars represent U-118 MG control cells. Black bars represent S1P2-overexpressing cells. The * indicates a statistically significant difference (p< 0.05) between S1P-treated and untreated cells of the same cell line.
To evaluate pathways involved in CCN1 regulation we pretreated U-118 MG cells with or without either pertussis toxin or Y-27632, and determined the effect of S1P on CCN1 expression (Fig 10B). Inhibition of Rho kinase partially decreased the effect of S1P on CCN1 expression in U-118-S1P2 cells, while PTX had no effect. Interestingly, either inhibitor blocked the increase in CCN1 expression in U-118-S1P1 cells. As S1P1 has been shown to signal exclusively through Gi proteins, the effectiveness of the Rho kinase inhibitor in S1P1-overexpressing cells may indicate that S1P1 cooperates with the small amount of endogenous S1P2 in these cells to augment the induction of CCN1.
To determine whether CCN1 plays a role in the enhancement of U-118 MG cell invasion by S1P2 overexpression, antibodies specific to CCN1 or irrelevant, control antibodies were added to the U-118 MG control or S1P2-overexpressing cells prior to performing an invasion assay. Addition of CCN1 antibody decreased S1P-stimulated invasion of S1P2-overexpressing cells while control antibodies were without effect (Fig. 10C,D). A slight apparent increase in invasion, which did not reach statistical significance, was noted in S1P-treated U-118 MG control cells. This response was also prevented by CCN1, but not control, antibodies. Thus, the sensitivity of the strong S1P induction of invasiveness in S1P2 overexpressing cells and the weak induction in control cells, correlate with the strong and weak induction of CCN1 expression in the same cell lines respectively. Taken together these data indicate that CCN1 partially mediates the S1P2-induced invasion of U-118 MG glioma cells.
Discussion
The results of this study show that the pleiotropic effects of S1P on glioma cells are mediated by a combination of S1P receptors, i.e. rather than individual receptor subtypes stimulating cell proliferation or invasion, these responses are contributed to by several receptor subtypes. Indeed, all three of the S1P receptors commonly expressed in glioma tissue contributed positively to S1P regulation of cell proliferation, as evidenced by experiments utilizing both overexpression and knock down of endogenous receptor expression. In addition, all three of these receptors, including S1P2, which negatively affects cell motility, positively influenced glioma cell invasiveness. It is interesting to note that, although high expression of SphK1 correlates strongly with decreased GBM patient survival, expression levels of S1P receptors do not [9]. This may be partially due to the fact that no one receptor subtype is alone responsible for enhancing malignant behavior of glioma cells. Therefore, high expression levels of individual receptors are not necessary. Moreover, because the receptors share in stimulating malignant behavior, it is possible that one receptor subtype mediates such effects in some tumors while another is more important in other tumors. Therefore, when examining expression of receptor subtypes in a number of cases no one receptor can be found that correlates with clinical data. Thus, even though this correlation is not found, it is unlikely that the receptors are unnecessary. Rather, while the expression level of SphK appears to be the important aspect that triggers contributions of this pathway to glioma malignancy, it is still likely that the downstream effects of S1P produced by SphK are mediated at least in part through these receptors. However, these data would also suggest that targeting individual S1P receptors therapeutically might not be a viable strategy, but rather a therapeutic agent that targets S1P1, S1P2 and S1P3 would be necessary. The alternative, of course, would be to therapeutically target SphK or common mediators downstream of S1P receptors. With regard to the latter possibility, CCN1 represents a potentially useful target, especially given the correlation of CCN1 expression with GBM patient survival [22], and its known contributions to various aspects of malignant behavior [21].
Nevertheless, differences do exist between the responses of glioma cells to the various receptors. Overexpression or knock down of S1P1 had a more potent effect on glioma cell proliferation than either S1P2 or S1P3, suggesting that S1P1 is the most important S1P receptor for this response These results are in good agreement with our previously published work, which showed that glioma cell lines that did not express significant levels of S1P1 were mitogenically unresponsive to S1P [7]. In agreement, S1P1 overexpression in U-118 MG cells led to a more potent activation of ERK, a pathway crucial for S1P-stimulation of glioma cell growth. However, S1P2, at least in U-373 MG cells, and S1P3 also contribute to ERK activation, consistent with their positive effects on glioma cell proliferation. Moreover, knockdown of S1P2 or S1P3 expression decreases S1P-stimulated proliferation of U-373 MG cells, even though S1P1 expression remains high. Thus, S1P1 is not sufficient to mediate a maximal mitogenic response to S1P in the absence of S1P2 and S1P3, and these three receptors serve overlapping, but not entirely redundant, functions in S1P-induced mitogenesis. Notably, S1P-stimulation of glioma cell proliferation is only partially sensitive to pertussis toxin [7]. As S1P1 couples exclusively to the pertussis toxin-sensitive Gi while S1P2 and S1P3 couple to Gi, Gq, and G12/13 [24], it is likely that S1P2 and S1P3 contribute to glioma cell proliferation through Gq, and G12/13 signaling.
The S1P5 receptor subtype caused quite a different response when overexpressed in glioma cells. Both with and without S1P stimulation, cell proliferation and DNA synthesis were significantly decreased by S1P5 overexpression. This was supported by decreased levels of activated ERK in the absence of S1P and even lower levels of activated ERK upon S1P stimulation. The anti-mitogenic effect of S1P5 in glioma cells likely explains the lack of high levels of expression of this receptor in GBM tissue compared to S1P1–3, as significant expression of S1P5 may be incompatible with tumor growth. In normal brain tissue S1P5 is commonly expressed in oligodendrocytes where it enhances both survival and process retraction depending on the status of cellular differentiation [11]. Thus, it would be interesting to determine if S1P5 plays a significant role in oligodendroglioma as opposed to the more astrocytic GBM. It is also interesting to note that S1P5 overexpression decreased glioma cell adhesion, a response that may be related to the process retraction seen in immature oligodendrocyte precursors [11].
The most intriguing results of this study were observed with S1P2 signaling with regard to motility and invasiveness of glioma cells. As previously reported [19, 25], S1P2 stimulation led to decreased motility of glioma cells. In contrast, S1P stimulation of this receptor subtype caused an increased invasiveness of glioma cells through Matrigel. Invasion is a far more complicated process than motility, as invading cells must adhere to extracellular matrix, degrade matrix components, and migrate across the space created. This suggests that S1P2 may alter interaction of glioma cells with extracellular matrix components. In agreement, S1P2 enhanced adhesion of glioma cells to Matrigel but not gelatin. Matrigel is a basement membrane-like material secreted by a mouse sarcoma cell line which is commonly used in tumor cell invasion experiments. Its major components include laminin, collagen IV and heparan sulfate proteoglycans. Proteoglycans are major constituents of normal brain extracellular matrix [26, 27]. Although laminin and collagen are not, both are secreted by glioma cells, and glioma cells are thought to use secreted matrix components to support their migration along white matter tracts [27, 28]. Thus, adhesion of glioma cells to these matrix components is likely an important aspect of glioma invasion.
Effects of S1P on cell migration are known to be regulated by signaling through the small GTPases Rac and Rho and the impact of these pathways on the actin cytoskeleton. In particular, S1P1 stimulates migration through Rac activation and lamellipodia formation, while S1P2 blocks migration through Rho activation, which in many cell types leads to inhibition of Rac [16–18]. In U-118 MG cells S1P2 does not inhibit Rac activation, however it does activate Rho ([19] and Fig. 6). Rho signaling through Rho kinase leads to stress fiber formation, and this pathway is necessary for S1P2-induced decreased motility.
To further examine molecular mechanisms of S1P-regulated glioma cell adhesion and invasion we assessed the role of CCN1/Cyr61. This secreted matricellular protein enhances adhesion through binding to both heparan sulfate proteoglycans and cellular integrins [21]. CCN1 expression was enhanced by both S1P1 and S1P2 in U-118 MG cells. Moreover, CCN1 is necessary for S1P2-stimulated invasion of U-118 MG cells, as this response was blocked with a neutralizing antibody directed against CCN1. However, only S1P1 knockdown effectively blocked S1P-stimulation of CCN1 expression in U-373 MG cells. Thus, S1P receptor regulation of this protein may occur through different receptor subtypes in different cells. In addition, it is likely that other proteins besides CCN1, possibly including additional proteins that modulate cell interaction with or degradation of the extracellular matrix, also contribute to this effect of S1P2, particularly in U-373 MG cells.
CCN1 may also play roles in other aspects of S1P-regulated glioma cell biology. CCN1 binding to integrins stimulates cellular signaling pathways that have been shown to participate in cell proliferation [29]. Furthermore, CCN1 is known to be pro-angiogenic [30], and thus S1P may affect tumor angiogenesis through CCN1 induction. Thus, further studies will explore in more detail the roles played by CCN1 in S1P regulation of glioma cell behavior, as well other mechanisms involved in S1P regulation of glioma cell interaction with extracellular matrix, proliferation and invasion.
Acknowledgments
This work was supported by Grant # R01 NS41517 from the National Institute of Neurological Disorders and Stroke (NINDS) to JRVB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicholas Young, Integrated Biomedical Science Graduate Program, The Ohio State University, Columbus, OH.
James R. Van Brocklyn, Department of Pathology, The Ohio State University, Columbus, OH
References
- 1.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 2.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Young N, Van Brocklyn JR. Signal transduction of sphingosine-1-phosphate G protein-coupled receptors. TheScientificWorldJOURNAL. 2006;6:946–66. doi: 10.1100/tsw.2006.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- 5.Lefranc F, Sadeghi N, Camby I, Metens T, Dewitte O, Kiss R. Present and potential future issues in glioblastoma treatment. Expert Rev Anticancer Ther. 2006;6:719–732. doi: 10.1586/14737140.6.5.719. [DOI] [PubMed] [Google Scholar]
- 6.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6:1087–1104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- 7.Van Brocklyn JR, Letterle CA, Snyder PJ, Prior TW. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: Role of ERK MAP kinase and phosphatidylinositol 3-kinase β. Cancer Lett. 2002;181:195–204. doi: 10.1016/s0304-3835(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 8.Van Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi: 10.1016/s0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 9.Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme. Roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 10.Gräler MH, Bernhardt G, Lipp M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 1998;53:164–169. doi: 10.1006/geno.1998.5491. [DOI] [PubMed] [Google Scholar]
- 11.Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K, Zalc B, Lubetzki C. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Del Duca D, Werbowetski T, Del Maestro RF. Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neuro-oncol. 2004;67:295–303. doi: 10.1023/b:neon.0000024220.07063.70. [DOI] [PubMed] [Google Scholar]
- 15.Malek RL, Toman RE, Edsall LC, Wong S, Chiu J, Letterle CA, Van Brocklyn JR, Milstein S, Spiegel S, Lee NH. Nrg-1 belongs to the endothelial differentiation gene family of G protein-coupled sphingosine-1-phosphate receptors. J Biol Chem. 2001;276:5692–5699. doi: 10.1074/jbc.M003964200. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Kitayama J, Takuwa N, Arikawa K, Inoki I, Takehara K, Nagawa H, Takuwa Y. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and hematogenous metastasis of melanoma cells. Biochem J. 2003;374:715–722. doi: 10.1042/BJ20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arikawa K, Takuwa N, Yamaguchi H, Sugimoto N, Kitayama J, Nagawa H, Takehara K, Takuwa Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor requirement of inhibition of cellular Rac activity. J Biol Chem. 2003;278:32841–32851. doi: 10.1074/jbc.M305024200. [DOI] [PubMed] [Google Scholar]
- 19.Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–3795. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- 20.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 21.Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- 22.Xie D, Yin D, Wang HJ, Liu GT, Elashoff R, Black K, Koeffler HP. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10:2072–2081. doi: 10.1158/1078-0432.ccr-0659-03. [DOI] [PubMed] [Google Scholar]
- 23.Han JS, Macarak E, Rosenbloom J, Chung KC, Chaqour B. Regulation of Cyr61/CCN1 gene expression through RhoA GTPase and p38MAPK signaling pathways. Role of CREB and AP-1 transcription factors. Eur J Biochem. 2003;270:3408–3421. doi: 10.1046/j.1432-1033.2003.03723.x. [DOI] [PubMed] [Google Scholar]
- 24.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gi, Gq, and G12 families of heterotrimeric G proteins. J Biol Chem. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 25.Malchinkhuu E, Sato K, Horiuchi Y, Mogi C, Ohwada S, Ishiuchi S, Saito N, Kurose H, Tomura H, Okajima F. Role of p38 mitogen-activated kinase and c-Jun terminal kinase in migration response to lysophosphatidic acid and sphingosine-1-phosphate in glioma cells. Oncogene. 2005;24:6676–6688. doi: 10.1038/sj.onc.1208805. [DOI] [PubMed] [Google Scholar]
- 26.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Zamecnik J. The extracellular space and matrix of gliomas. Acta Neuropathol (Berl) 2005;110:435–442. doi: 10.1007/s00401-005-1078-5. [DOI] [PubMed] [Google Scholar]
- 28.Pilkington CJ. The role of the extracellular matrix in neoplastic glial invasion of the nervous system. Braz J Med Biol Res. 1996;29:1159–1172. [PubMed] [Google Scholar]
- 29.Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]