Abstract
Accumulation of damaged proteins is causally related to many age-related diseases. The ubiquitin-proteasome pathway (UPP) plays a role in selective degradation of damaged proteins, whereas molecular chaperones, such as heat shock proteins, are involved in refolding denatured proteins. This work demonstrates for the first time that the UPP and molecular chaperones work in a competitive manner and that the fates of denatured proteins are determined by the relative activities of the UPP and molecular chaperones. Enhanced UPP activity suppresses the refolding of denatured proteins whereas elevated chaperone activity inhibits the degradation of denatured proteins. CHIP, a co-chaperone with E3 activity, plays a pivotal role in determining the fates of the damaged proteins. The delicate balance between UPP-mediated degradation and refolding of denatured proteins is governed by relative levels of CHIP and other molecular chaperones. Isopeptidases, the enzymes that reverse the actions of CHIP, also play an important role in determining the fate of denatured proteins.
Keywords: CHIP, heat shock proteins, isopeptidase, luciferase
A growing body of evidence indicates that accumulation of damaged or abnormal proteins is associated with various age-related diseases (1–3). Examples of such diseases include Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, cataract and age-related macular degeneration. Accumulation of damaged or abnormal proteins may arise from enhanced generation, or arise from deficiencies in repair or removal of these abnormal proteins. It is estimated that more than 30% of newly synthesized cellular proteins are degraded due to failure of proper folding (4). Even when proteins are normally folded into tertiary structures, there is spontaneous denaturation and generation of partially folded or unfolded proteins. In addition, environmental stress such as heat, oxidation and ultraviolet radiation, could generate denatured or damaged proteins. Unfolded or partially folded proteins are unstable, they tend to aggregate and precipitate and thus interfere with normal cellular functions (2, 5). To avoid disruption of cellular function by unfolded proteins, organisms evolved multiple levels of protein quality control systems that recognize proteins with abnormal structures and either refold them to normal conformation or target them for degradation.
It is well known that the ubiquitin-proteasome pathway (UPP) plays an important role in selective degradation of damaged or abnormal proteins. The UPP is a major cytosolic proteolytic pathway in eukaryotic cells (6). UPP-mediated protein degradation involves two discrete and successive steps: 1) tagging of the substrate by covalent attachment of multiple ubiquitin molecules and 2) degradation of the tagged protein by the 26S proteasome complex with release of free and reusable ubiquitin. Conjugation of ubiquitin to the protein substrate proceeds via a three-step relay reaction. The ubiquitin-activating enzyme (E1) activates ubiquitin in an ATP-dependent reaction to generate a high-energy E1-S-ubiquitin thiol ester. One of several E2s, ubiquitin-conjugating enzymes (Ubcs) transfers the activated ubiquitin moiety from E1, also via a high-energy thiol ester, to the substrate that is specifically bound to a member of the ubiquitin-protein ligase family, E3. The combination of E2s and E3s determines the substrate specificity. Proteins tagged by polyubiquitin chains are often recognized and degraded by the 26S proteasome. In some cases, the ubiquitin moiety is removed from the substrate by isopeptidases, a large family of deubiquitinating enzymes (7). Thus, isopeptidases provide a proofreading or rescuing mechanism in order to prevent the destruction of proteins that had been mistakenly ubiquitinated.
Molecular chaperones play a key role in folding newly synthesized proteins or refolding denatured proteins. In response to various types of stress, cells increase the expression of heat-shock proteins (Hsps), such as Hsp90, Hsp70, and small Hsps, such as Hsp40 and Hsp27 (8). It has been demonstrated that Hsp90 and Hsp70 are involved in the folding and maturation of various newly synthesized proteins, such as protein kinases, glucocorticoid receptor (9, 10), and the cystic fibrosis transmembrane regulator (CFTR) (11). Hsp70, coordinated with other molecular chaperones, can also refold denatured or partially unfolded proteins that are generated by various types of stresses (12, 13). The activity of Hsp90 and Hsp70 is modulated by accessory proteins called co-chaperones (14). CHIP (C-terminus of Hsp70-Interacting Protein) is one of the co-chaperones (15). In addition, CHIP is a member of the U-Box E3s of the UPP. The dual functions of CHIP provide a bridge between the chaperones and the UPP (16). CHIP interacts with Hsp70 and Hsp90 via three tandem tetratricopeptide repeat (TPR) motifs, whereas its carboxyl-terminal U-box domain associates with ubiquitin-conjugating enzymes. Therefore, CHIP could play a coordinating role in cellular protein quality control (17).
Several lines of evidence indicate that there are functional relationships between the UPP and molecular chaperones (18, 19). For example, inhibition of the UPP results in up-regulation of heat shock proteins (20) and that Hsp90 or Hsp70 are required for ubiquitination and degradation of some substrates (21, 22). It has also been shown that CHIP converts clients of Hsp90 to the UPP for degradation (23). However, several questions remain unanswered. Why are certain damaged proteins degraded by the UPP whereas others are repaired by chaperones? What is the functional relationship between the UPP and molecular chaperones? Do they work in a concerted fashion or in a competitive manner? This work was designed to address these fundamental questions. We demonstrated that 1) the UPP and molecular chaperones recognize the same features of denatured proteins; 2) Hsp90 is used by the UPP to recognize denatured proteins; 3) the UPP and molecular chaperones work in a competitive manner; 4) CHIP plays a pivotal role in determining the fate of denatured proteins by modulating the chaperone activity and the ubiquitin conjugating activity; 5) deubiquitinating enzymes are a determinant factor that governs the triage of denatured proteins.
MATERIALS AND METHODS
Na125I was purchased from PerkinElmer (Boston, MA). The rabbit reticulocyte was from PelFreeze (Rogers, AR). Nitrocellulose membrane and protein molecular mass standards were purchased from BioRad (Richmond, CA). MG-132 and lactacystin were purchased from BostonBiochem (Cambridge, MA). Heat-shock protein 90 from bovine brain (cat # H6774), heat shock protein 70 from bovine brain (cat # H9776) and recombinant human Hsp40 (cat # H7910) were purchased from Sigma (St. Louis, MO). Cell culture medium and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA). BioPorter was purchased from Gene Therapy Systems (San Diego, CA). Unless specified, all other reagents were purchased from Sigma and were the highest grade available.
Preparation of 125I-labeled luciferase
Luciferase was radioiodinated as described previously (24). In brief, luciferase was dissolved in 50 mM Tris-buffer (pH. 7.4) and mixed with Na125I. The iodination reaction was started by addition of chloramine T, and the vials were shaken for 2 min at room temperature. The reaction was then terminated by the addition of sodium metabisulfite and sodium iodide. Free 125I and small protein fragments were removed from the protein solution by a Sephadex-G25 column (Amersham, Piscataway, NJ USA).
Recombinant proteins
Recombinant Ubc4-1 (also called UbcH5c), and His(6)tagged-CHIP were produced in E. coli (Novagen Inc.). The Ubc4-1 plasmid was generously provided by Dr. Wing. The expression and purification of Ubc4 was performed as described previously (25). The CHIP was purified by affinity chromatography using a Ni-column.
Cell transfection
Cos7 cells were transfected with UBPY and Cezanne plasmid for 4 h using Lipofectamine according to the manufacturer’s instructions. Transfected cells were then incubated for 48 h in DMEM containing 10% fetal bovine serum and collected in 50 mM Tris-HCl (pH 7.6) containing 1 mM DTT. The cytosolic fraction was used as the source of UBPY and Cezanne. The UBPY plasmid was kindly provided by Dr. Di Fiore (26) whereas the Cezanne cDNAs were constructed in Dr. Evans’s laboratory (27).
Thermal denaturation and refolding of firefly luciferase
Firefly luciferase (20 µg/ml) was dissolved in a buffer containing 50 mM Tricine and 1 mM DTT, pH 7.5 and was thermally inactivated by incubating at 43°C for 10 min in the presence or absence of Hsp90 (0.7 µg/µl). After heating, the samples were quickly chilled in ice. Unheated firefly luciferase was used as a control. Renaturation was assessed in rabbit reticulocyte lysate (RRL) in the presence or absence of CHIP, an ubiquitin ligase. In brief, for refolding assays, the thermally inactivated luciferase was diluted 10-fold in the renaturation solution, which contained 2 mM ATP, 2 mM DTT, 20 mM phosphocreatine, 0.2 mg/ml creatine phosphokinase, 160 mM KCl, 5 mM Mg(OAc)2 and RRL. To demonstrate the effect of ubiquitin conjugation on the renaturation process, E1 was removed from RRL by immunoprecipitation with antibodies to E1 (28). To determine the role of deubiquitinating enzymes on renaturation, Cos-7 cells were transfected with an empty plasmid or with plasmids encoding UBPY or Cezanne for 48 h and the cell lysates were used to determine the renaturation capability. To determine the effect of proteasome on renaturation of denatured protein, the proteasome was removed from Cos-7 cell lysate by centrifuging at 100,000 × g for 5 h (29). Renaturation was monitored by restoration of luciferase activity, which was measured using a luminometer. Aliquots of 10 µl were removed from the renaturation reactions at the indicated times and mixed with 190 µl of 50 mM Tris-HCl (pH 7.6). 20 µl of the diluted sample was mixed with 100 µl luciferase assay buffer, which contains 25 mM Tricine-HC1, pH 7.8, 8 mM MgSO4, 0.1 mM EDTA, 33 µM DTT, 100 µM D-luciferin, 240 µM CoA, and 0.5 mM ATP. Each assay was done in duplicate and was repeated at least 3 times for each experimental condition.
Proteolysis assays
125I-labeled-luciferase was denatured in the same way as described above. Native and denatured 125I-labeled-luciferase was used as substrates for the degradation assay. ATP-dependent degradation was performed in 25 µl assays that contained 13 µl of RRL and 10 µl of buffer containing 50 mM Tris-HCl, pH 7.8, 5 mM MgCl2, 2 mM DTT, 2 mM ATP, 10 mM creatine phosphate, 4.5 µg/ml creatine phosphokinase, 2 µg ubiquitin and 0.5 µg 125I-labeled luciferase. Some of these assays were supplemented with 0.5 µg recombinant Ubc4, CHIP and/or Hsp70. The reaction was carried out at 37°C for 90 min and then stopped by the addition of 200 µl of 1% (w/v) bovine serum albumin, immediately followed by 50 µl of 100% (w/v) TCA. After standing on ice for 10 min, the samples were centrifuged 14,000 rpm at 4°C for 10 min. Aliquots of supernatants and the pellets were counted to determine the TCA-soluble radioactivity. The extent of degradation was determined as the percentage of 125I released as TCA-soluble fragments. Each assay was performed in triplicate.
Ubiquitin conjugation assays
Ubiquitin-protein conjugates were formed by incubation of native or thermally denatured luciferase with fraction II of RRL, in the presence or absence of Hsp90. Ubiquitination was performed in a total volume of 50 µl, which contains 50 mM Tris-HCl, 2 µg CHIP, 6 µg ubiquitin, 1 mM DTT, 5 mM MgCl2 and 2 mM ATP, and 30 µl fraction II. Native or thermally denatured, 125I-labeled luciferase was incubated in the ubiquitination solution at 30°C for 30 min. After terminating the reaction by the addition of 50 µl 2× Laemmli buffer (30), proteins in the reaction mixture were separated by SDS-PAGE and the dried gels were exposed to X-ray film. Ubiquitinated luciferase was detected on the autoradiogram as bands, which showed higher molecular weight than free luciferase.
RESULTS
Thermally denatured luciferase is preferentially ubiquitinated in a CHIP and Hsp90 dependent manner
To study the role of the UPP in protein quality control and its relationship to molecular chaperones, we chose thermally denatured firefly luciferase as a model substrate. To facilitate the detection of ubiquitination, luciferase was labeled with 125I prior to thermal denaturation. Denaturation of luciferase was monitored by the loss of luciferase activity. When incubated at 43°C for 10 min, 99% of the luciferase activity was lost. Although Hsp90 prevented the aggregation of heat-denatured luciferase, the presence of Hsp90 cannot prevent the loss of activity during the incubation period (data not shown). To prevent degradation of ubiquitinated luciferase by the proteasome, the ubiquitination assay was performed in a proteasome-free fraction II of rabbit reticulocyte lysate (RRL) supplemented with Ubc4. The ubiquitination of firefly luciferase was detected as bands that have higher masses than that of luciferase on the radiogram. As shown in Fig. 1A, in the presence of CHIP and Hsp90, thermally-denatured luciferase is preferentially ubiquitinated (compare lane 8 with lane 7). If CHIP or Hsp90 was omitted, ubiquitinated native or denatured luciferase was barely detectable (Fig. 1A, lanes 1–6). These data are consistent with a previous report that CHIP and Hsp90 are involved in ubiquitination of thermally denatured luciferase (31).
Figure 1.
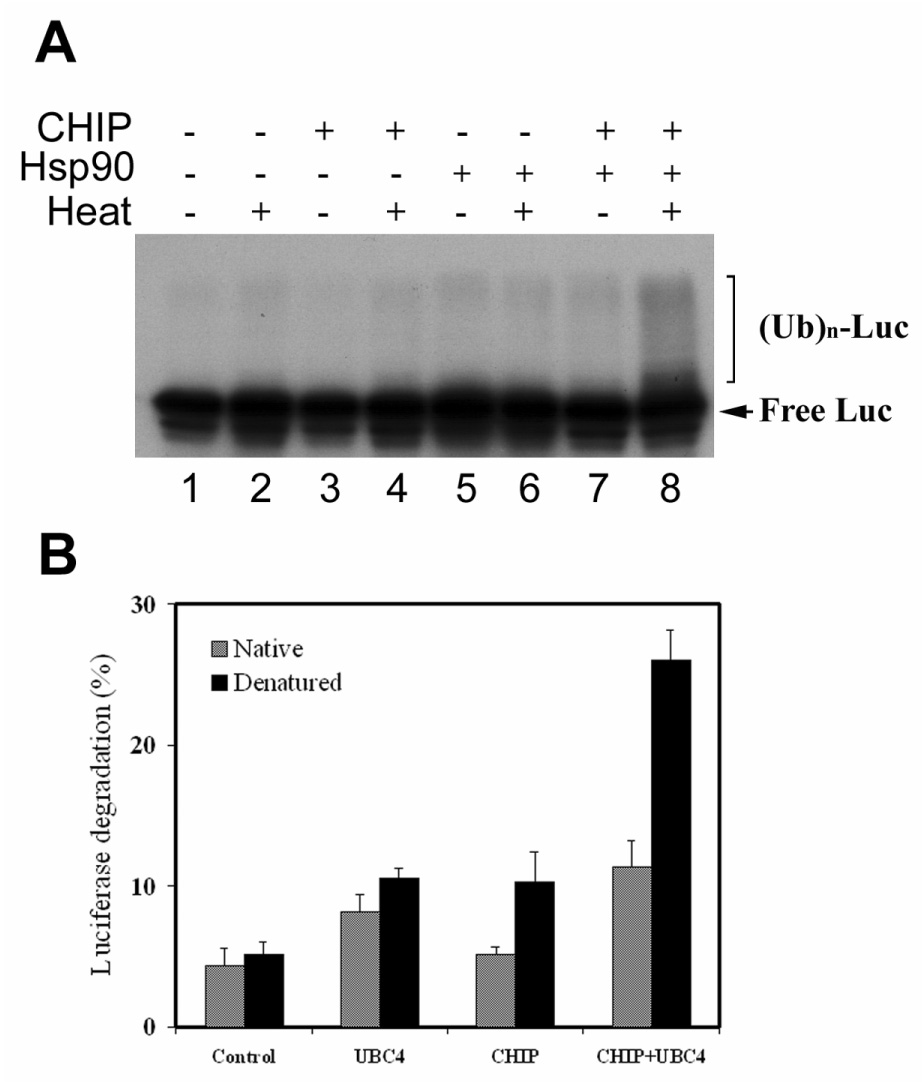
Denatured luciferase is preferentially ubiquitinated and degraded in a CHIP and Hsp90-dependent fashion. Firefly luciferase was first labelled with 125I and then thermally denatured in the presence or absence of Hsp90. Native or thermally denatured luciferase was subjected to ubiquitination assay or degradation assay as described in experimental procedures. A) the ubiquitination assay was performed at 30°C for 30 min in the presence or absence of CHIP in proteasome-free reticolucyte faction II, which was supplemented with ubiquitin and Ubc4. The ubiquitinated luciferase was indicated by the species with higher molecular weight than that of free luciferase. B) The degradation assay was performed in reticulocyte with or without supplementation of Ubc4 or CHIP. Percentage of degradation was calculated from acid-soluble radioactivity recovered in supernatants after 90 min of incubation at 37°C. Values are means ± SD of three independent determinations; each was done in duplicate.
Thermally denatured luciferase is preferentially degraded by the UPP in a CHIP-dependent fashion
The primary role of ubiquitination is to target the substrate to the 26S proteasome for degradation. To determine the fate of ubiquitinated luciferase, we assessed the susceptibility of the thermally denatured firefly luciferase to UPP-mediated degradation in RRL. Modest degradation of native and denatured luciferase was observed during the 90 min incubation with RRL. The rate of degradation of denatured luciferase was slightly higher (10%) than that of native luciferase (Fig. 1B). Addition of exogenous Ubc4 to the degradation reaction increased the degradation rates of both native and denatured luciferase, but the difference between the degradation rates of native and denatured luciferase remained the same (Fig. 1B). In contrast, addition of CHIP enhanced the degradation of denatured luciferase by 100%, but only enhanced the degradation of native luciferase by ∼10% (Fig. 1B). When CHIP and Ubc4 were added simultaneously, it increased the degradation of denatured luciferase by 400% and the degradation of native luciferase increased only ∼140%. Under these conditions, the rate of degradation of denatured luciferase was 2.3-fold of that of native one (Fig. 1B). These data clearly show that denatured luciferase was preferentially degraded by the UPP in a CHIP-dependent fashion. Although addition of Ubc4 boosted the degradation of luciferase, it did not provide selectivity between native and denatured luciferase in the absence of CHIP.
Hsp70 enhances renaturation, but reduces degradation of denatured luciferase
It has been shown that degradation of some proteins requires Hsp70 (21, 32, 33). To test if Hsp70 is involved in degradation of denatured luciferase, we determined the effect of exogenous Hsp70 on degradation of denatured luciferase in RRL. As shown in Fig. 2A, addition of Hsp70 to RRL inhibits degradation of denatured luciferase by 25%. Consistent with the data in Fig. 1B, addition of exogenous CHIP boosted the degradation of denatured luciferase (Fig. 2A). Even when CHIP was added, the inhibitory effect of Hsp70 on degradation of denatured luciferase was still detected (Fig. 2A). These data indicate that Hsp70 reduces, rather than stimulates, UPP-mediated degradation of denatured luciferase.
Figure 2.
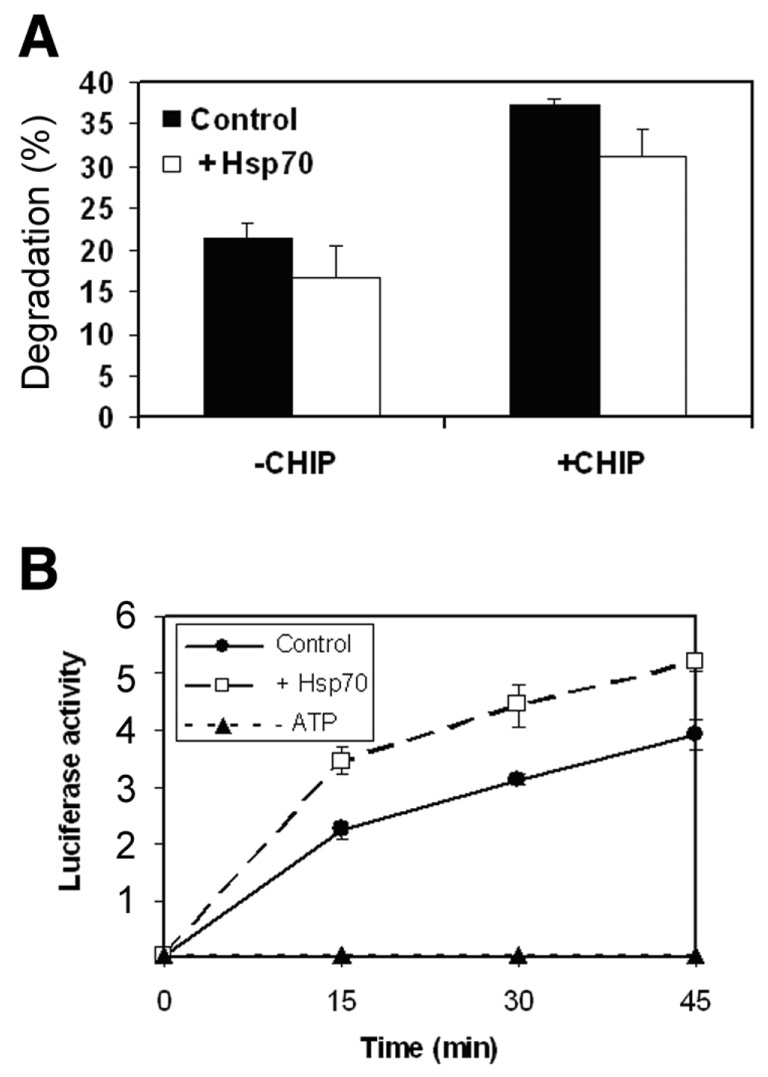
Hsp70 reduces UPP-mediated degradation of denatured luciferase and promotes renaturation. 125I-labeled firefly luciferase was denatured in the presence of Hsp90 as described in legend to Fig. 1. A) the degradation assay was performed in Ubc4 supplemented RRL. As indicated in the figure CHIP and Hsp70 were added to test their effects on degradation of denatured luciferase. The percentage of degradation was calculated from acid-soluble radioactivity recovered in supernatants after 90 min of incubation at 37°C. B) The renaturation assay was performed in RRL with or without addition of Hsp70. The renaturation was monitored by restoration of luciferase activity, which was expressed as a relative activity and the units were arbitrary. Values are means ± sd of three independent determinations.
It is well established that Hsp70 is a prominent chaperone, which works together with other chaperones to refold denatured proteins. To investigate the mechanism by which Hsp70 inhibits the UPP-mediated degradation of denatured luciferase, we determined the effects of Hsp70 on renaturation of luciferase in RRL. Consistent with previous reports (34), thermally denatured luciferase was readily refolded in RRL as indicated by the restoration of luciferase activity (Fig. 2B). The renaturation of denatured luciferase is ATP-dependent. If ATP was omitted from the renaturation reaction, there was no recovery of luciferase activity (Fig. 2B). Addition of exogenous Hsp70 increased the rate of renaturation by ∼30% (Fig. 2B). The inhibitory effects of Hsp70 on degradation and its stimulatory effect on renaturation indicate that molecular chaperones may compete with the UPP for denatured luciferase.
Enhancing ubiquitinating activity decreases renaturation of denatured luciferase
To further test the hypothesis that the UPP and molecular chaperones compete for denatured proteins, we measured the effect of ubiquitination on renaturation of denatured luciferase in RRL. First we tested if enhancing ubiquitination by addition of CHIP, the ubiquitin-ligase that promotes ubiquitination of denatured luciferase, affects the renaturation of denatured luciferase. As shown in Fig. 3A, addition of CHIP to RRL decreased the renaturation of luciferase in a dose dependent manner. Addition of Ubc4, the E2, which enhances the ubiquitination and degradation of denatured luciferase, further diminished the renaturation of luciferase (Fig. 3B). To verify that the effects of CHIP and Ubc4 are due to enhanced ubiquitinating activity, rather than directly inhibiting chaperone activities in RRL, we performed the experiment using a reconstituted system. Although Hsp70 or Hsp40 alone was capable of refolding denatured luciferase, their capability was limited (data not shown). However, the combination of Hsp70 and Hsp40 showed a robust refolding activity. Therefore, we chose the combination of Hsp70 and Hsp40 as the reconstituted protein folding system (Fig. 3C). Addition of CHIP alone or together with Ubc4 had no effect on renaturation in this reconstituted system (Fig. 3C). However, if the ubiquitinating activity was reconstituted with E1, Ubc4 and CHIP, the Hsp70/Hsp40-mediated renaturation was almost abolished (Fig. 3C). These data demonstrated that the effects of CHIP and Ubc4 on the renaturation of luciferase are due to the enhancement of ubiquitinating activity. These data also imply that the UPP competes with chaperones for denatured luciferase.
Figure 3.
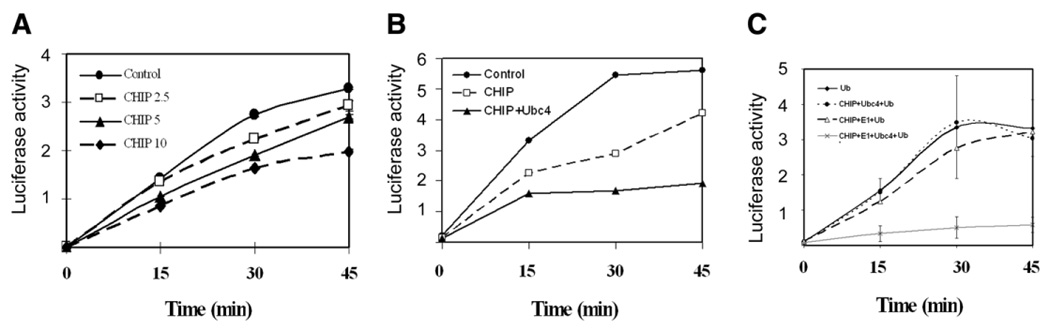
CHIP and Ubc4 inhibit renaturation of denatured luciferase. Renaturation of heat-denatured luciferase was performed in RRL (A and B) or using a reconstituted system (combination of Hsp70 and Hsp40) (C). A) 0, 2.5, 5 and 10 µg/ml of CHIP was added to the renaturation reactions. Luciferase activity was measured at the indicated time points. B) 5 µg/ml CHIP with or without 5 µg/ml Ubc4 was added to the renaturation reaction. C) The renaturation was mediated by the combination of Hsp70 and Hsp40. Effects of ubiquitination on renaturation were determined by addition of ubiquitin (Ub), E1, Ubc4 and CHIP as indicated in the figure. Luciferase activity was measured at time points indicated. Each point represents the mean of six measurements.
Depletion of E1 enhances renaturation of denatured luciferase
To further test the hypothesis that the ubiquitin conjugating system competes with chaperones for denatured luciferase, we determined the effect of depleting E1, the first enzyme in the ubiquitination reaction, on renaturation of luciferase. To do this, E1 was depleted from RRL by immunoprecipitation with antibodies specific to E1 (28). As shown in Fig. 4A, upper panel, ∼80% of E1 was removed from RRL by immunoprecipitation. The corresponding ubiquitin conjugating activity was also substantially diminished upon depletion of E1 (Fig. 4A, lower panel). Consistent with the hypothesis that the ubiquitin conjugating system competes with chaperones for denatured proteins, depletion of E1 enhanced the renaturation of luciferase (Fig. 4B).
Figure 4.
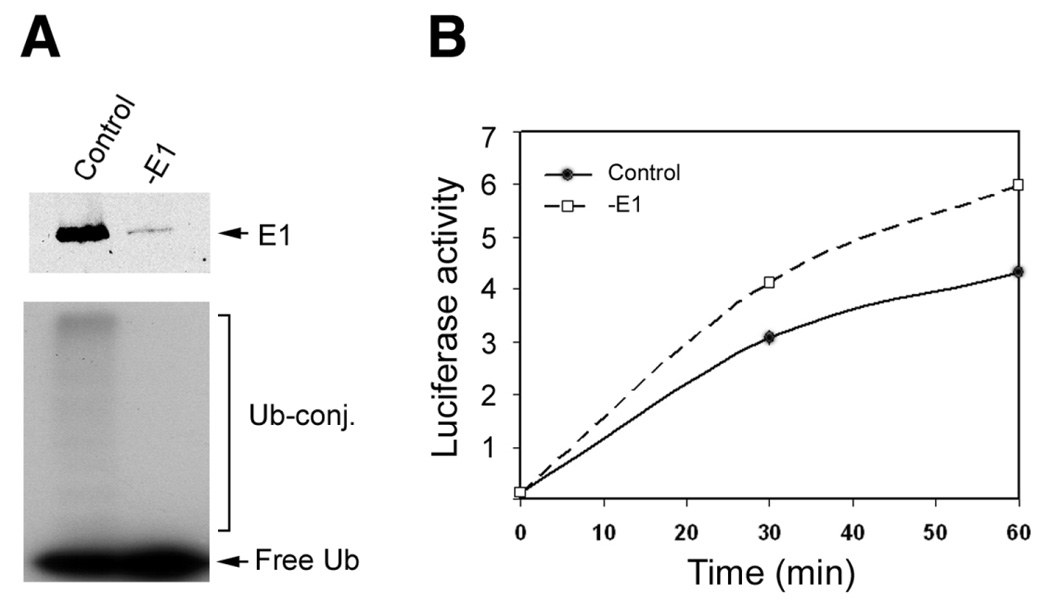
Depleting E1 enhances the renaturation of luciferase in reticulocyte lysate. E1 was depleted from RRL by immunoprecipitation with antibodies specific to E1. As a control, mock immunoprecipitation was performed using preimmune IgG. A) Shows the effectiveness of the immunoprecipitation; the upper panel shows the depletion of E1 and the lower panel shows the loss of ubiquitin conjugating activity. B) Shows the effects of depleting E1 on renaturation. Luciferase activity was measured at 0, 30, and 60 min of incubation. For each point a total of six replicates were measured.
Inhibition or depletion of proteasome activity enhances renaturation
To test if proteasome-mediated proteolysis plays a role in the competition between the UPP and chaperones for denatured proteins, we examined the effect of proteasome inhibition on the renaturation. We found that inhibition of proteasome activity by MG-132 or clasto-lactacystin β-lactone enhanced the renaturation of denatured luciferase (Fig. 5A). The effect of proteasome inhibitors is not due to direct enhancement of the chaperone activities, since addition of MG-132 had no effect on renaturation in the reconstituted renaturation reaction, in which the renaturation was mediated by the combination of Hsp70 and Hsp40 (Fig. 5B). The effect of proteasome on renaturation is not limited to the RRL, as depleting proteasome from lysate of Cos-7 cells also enhanced the renaturation activity substantially (Fig. 5C). These data further support the idea that the UPP competes with molecular chaperones for denatured proteins.
Figure 5.
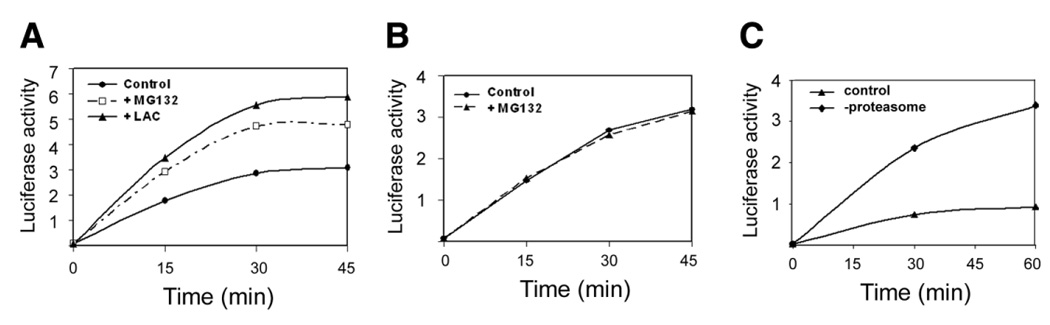
Inhibition or removal of proteasome enhances renaturation of denatured luciferase. A) Renaturation was performed in RRL in the presence or absence of proteasome inhibitors MG132 or clasto-lactacystin β-lactone (LAC). B) Renaturation was performed in a reconstituted renaturation reaction (Hsp70 and Hsp40) in the presence or absence of MG132. C) Renaturation was performed in Cos-7 cell lysate with or without depletion of the proteasome by centrifugation. Luciferase activity was measured at the indicated time points. Each point represents the mean of 12 measurements.
Over-expression of isopeptidases enhances renaturation
The data in Fig. 5 suggest that ubiquitinated proteins that are not degraded can be refolded in cell lysates. We have also shown, however, that ubiquitinated proteins cannot be refolded by chaperones in the reconstituted system (Fig. 3C). We hypothesized, therefore, that deubiquitination of cellular proteins may play an important role in the refolding process in cell lysates. To further test this hypothesis, we assessed the effect of isopeptidases on renaturation. To do this, Cos-7 cell were transfected with plasmids encoding isopeptidases. Cezanne is an isopeptidase that can remove ubiquitin from ubiquitin-protein conjugates (27). As shown in Fig. 6A, recombinant Cezanne was efficiently expressed in Cos-7 cells. Expression of Cezanne resulted in a significant decrease in levels of endogenous ubiquitin conjugates, indicating that it is capable of removing ubiquitin from the substrates (Fig. 6B). Fig. 6C showed that the supernatant of Cos-7 cells in which Cezanne was overexpressed had higher activity to refold denatured luciferase. To rule out the possibility that the effect of Cezanne was due to nonspecific interaction with the chaperones, we tested the effect of another isopeptidase. It has been demonstrated that UBPY is also capable of removing ubiquitin from ubiquitinated proteins (26). Expression of UBPY in Cos-7 cells resulted in a significant reduction in levels of endogenous ubiquitin conjugates, indicating enhanced isopeptidase activity (Fig. 6D). Consistent with the data obtained from overexpression of Cezanne, expression of UBPY also enhanced the renaturation of denatured luciferase (Fig. 6E). Taken together, these data further suggest that deubiquitination of the substrate is required for renaturation by molecular chaperones.
Figure 6.
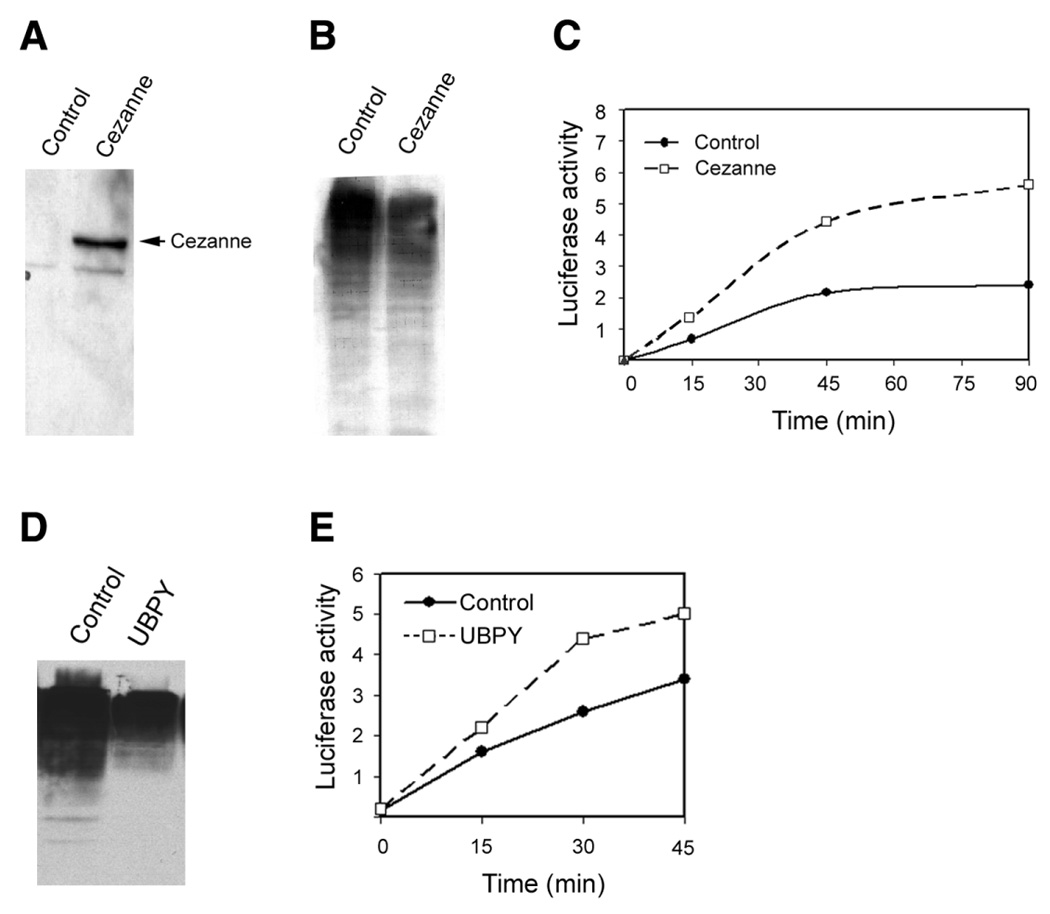
Expression of deubiquitinating enzyme promotes renaturation of denatured luciferase. Cos-7 cells were transfected with control plasmid or plasmid encoding the HA-tagged cezanne, a deubiquitinating enzyme, and chaperone activity was compared in the cell lysate. A) Shows the expression of cezanne, which were detected by Western blotting using antibody to HA. B) Shows the activity of cezanne as indicated by the decrease in levels of. endogenous ubiquitin conjugates. C) Shows the effect of cezanne on renaturation of denatured luciferase. D) Shows the activity of UBPY as indicated by the decrease in levels of endogenous ubiquitin conjugates. E) Shows the effect of UBPY on renaturation of denatured luciferase. Each point represents the mean of 12 measurements.
Intracellular over-expression of active CHIP, but not the catalytic inactive CHIP, diminishes the renaturation of imported luciferase
To verify that the results obtained from the cell-free assay pertain to intact cells, we determined the effects of overexpression of CHIP on the renaturation of luciferase in intact Cos-7 cells. To avoid heat-induced up-regulation of molecular chaperones, luciferase was first heat-denatured in a cell-free system and subsequently delivered into Cos-7 cells by liposome-based protein delivery reagents (BioPorter) and the renaturation of luciferase in the cells was determined by measuring the restoration of luciferase activity. The data in Fig. 7 showed that overexpression of active CHIP inhibited the renaturation, whereas expression of the TPR mutant form (K30A) or U-box mutant form (H260Q) had no effect on the renaturation. These data showed that both the Hsp90/Hsp70-interaction capability and ubiquitin ligase activity of CHIP is required for the inhibitory effect. To test if the CHIP-induced decrease in renaturation was related to enhancement of proteasome-mediated degradation of luciferase, we determined the effects of the proteasome inhibitor on the renaturation in Cos-7 cells. Treatment with lactacystin inhibited ∼90% of proteasome activity in the cells (data not shown), but it cannot reverse the inhibitory effects of CHIP (Fig. 7). These data further support the hypothesis that the UPP competes with molecular chaperones for denatured proteins and that ubiquitinated proteins need to be deubiquitinated prior to renaturation by molecular chaperones.
Figure 7.
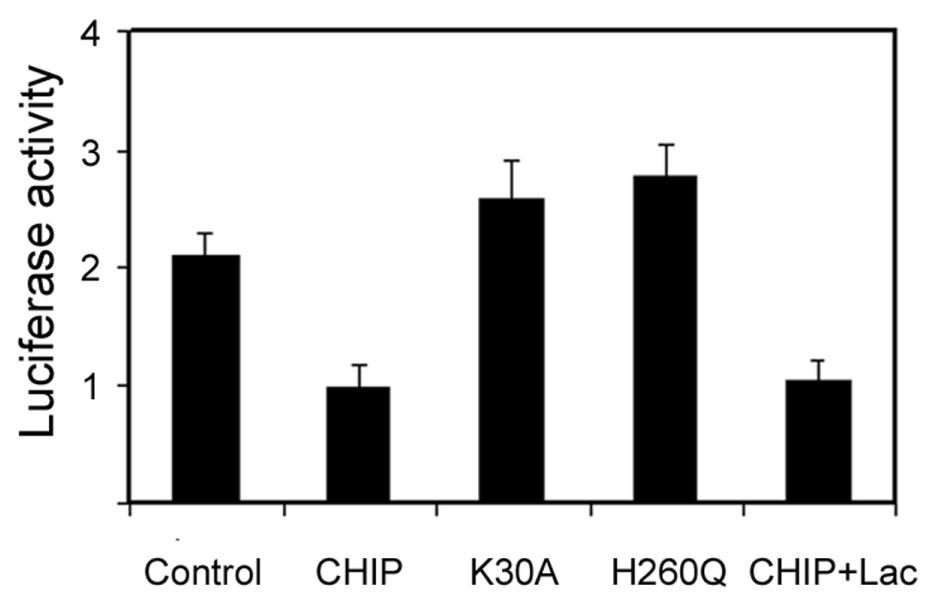
Expression of CHIP inhibits renaturation of denatured luciferase in Cos-7 cells. Cos-7 cells were transfected with control plasmid or with plasmids encoding wt CHIP or mutant CHIP. Thermally denatured luciferase was delivered into the cells by a liposome-based protein delivery reagent (BioPorter). Renaturation of the delivered luciferase was monitored by the recovery of luciferase activity. Each point represents the mean of six measurements.
DISCUSSION
Accumulation of damaged or abnormal proteins is cytotoxic and is causally related to various age-related diseases. Therefore effective removal or repair of damaged or abnormal proteins is essential for cellular function and cell survival. Whereas molecular chaperones, a large family of heat shock proteins, play a vital role in refolding denatured proteins, the UPP selectively degrades damaged or abnormal proteins. In this work we used thermally denatured luciferase as a model substrate to answer three fundamental questions related to cellular protein quality control systems. 1) What is the working relationship between the UPP and molecular chaperones? 2) How do cells coordinate the UPP and molecular chaperones in the protein quality control? 3) Do isopeptidases play a role in the process of protein quality control?
We demonstrated that thermally denatured luciferase is preferentially ubiquitinated and degraded by the UPP in an Hsp90 and CHIP dependent manner. These data suggest that the UPP recognizes the same features of denatured proteins that are recognized by molecular chaperones and that Hsp90 was used by the UPP in the recognition process. These results confirmed and extended a previous report that showed the selective ubiquitination of denatured luciferase in a CHIP and Hsp90 dependent fashion (31). The present work also demonstrated for the first time that the UPP and molecular chaperones work in a competitive manner in eliminating denatured proteins. The delicate balance between the UPP and chaperones appears to be modulated by levels of CHIP, a co-chaperone with ubiquitin ligase activity. In addition, isopeptidase activity is also a factor in determining the fate of denatured proteins.
The selective degradation of abnormal proteins has been known for ∼30 years (35, 36). The role of the UPP in selective degradation of abnormal proteins has been proposed since the discovery of this pathway (37–44). It has been demonstrated that the UPP is involved in degradation of various mutant proteins and damaged proteins, such as CFTR (45–48), SOD (49, 50) and oxidized proteins (43, 51). However, how the UPP distinguishes abnormal proteins from the native one remained to be an unsolved mystery. Although the 19S regulatory complex of the 26S proteasome interacted directly with denatured protein (52–55), most proteins degraded by the proteasome are first tagged by attachment of a polyubiquitin chain. It is believed that the ubiquitin conjugating enzymes (E2s) and ubiquitin ligases (E3s) are jointly responsible for substrate recognition and ubiquitination (6, 56). Thus these enzymes are the key in selecting proteins for degradation. Mutations in specific E2 proteins lead to defects in degradation of proteins with different classes of artificial hydrophobic degradation signals, suggesting that the ubiquitination system can target exposed hydrophobic regions in proteins (57). Consistent with previous reports (31, 33), this work indicates that molecular chaperones, particularly Hsp90, are used by the UPP to recognize denatured proteins. Exposure of hydrophobic regions that are normally buried in properly folded proteins provides a signal for the discrimination of denatured or unfolded proteins from native proteins by Hsp90. It is well established that Hsp90 and other molecular chaperones recognize exposed hydrophobic regions of denatured proteins (58). By interacting with Hsp90 and Ubc4/5, the co-chaperone CHIP catalyzes the ubiquitination of Hsp90-bound proteins. In this context, we may consider that the complex of Hsp90 and CHIP is the E3 that recognizes abnormal proteins for UPP-mediated degradation.
Both the UPP and molecular chaperones recognize proteins with abnormal structures and a common goal of these two pathways is to prevent the accumulation of abnormal proteins. The question is that why some denatured proteins are refolded by chaperones whereas others are degraded by the UPP. It appears that the cellular protein quality control systems have a triage mechanism. The first level of triage must be identification of the proteins that are damaged and require repair or removal. The quality control system must be able to distinguish between native (properly folded, assembled, and modified) proteins and everything else that might be considered non-native, including partially unfolded, misfolded, incorrectly modified, unassembled subunits of complexes, or proteins in wrong cellular compartments. Once damaged proteins have been identified, a second level of decision must be made: Is the protein repairable? In principle, chaperones should have the first opportunity to fix damaged proteins. The proteins that are damaged beyond repair must be degraded by the UPP.
How is the decision made to refold or to degrade a protein? The ability of both the UPP and chaperones to interact with damaged or misfolded proteins in similar ways allows these pathways for either repair or degradation of a given target protein to operate in a parallel or competitive manner. As illustrated in Fig. 8, we propose that the fate of the damaged protein depends on the kinetics of interaction between the damaged proteins and molecular chaperones or the chaperone component of the UPP (Hsp90). In general, proteins with exposed hydrophobic surfaces or other binding motifs interact with Hsp90. If the Hsp90-bound nonnative protein is efficiently refolded with the assistance of other chaperones, such Hsp70 and Hsp40, it is removed from the triage system. If the Hsp90-bound nonnative protein is not efficiently refolded by chaperones, the co-chaperone CHIP, which interacts with Hsp90 via its TPR domain and interacts with Ubc4/5 or other E2 s via its U-box domain, brings the Hsp90-bound proteins to the ubiquitination machinery. Ubiquitinated substrates are often targeted to the 26S proteasome for degradation. If the ubiquitinated proteins are deubiquitinated by isopeptidases, the denatured proteins would have a second chance to be refolded by chaperones. Therefore, the relative activities of ubiquitination and deubiquitination may control the fate of denatured proteins. It appears that CHIP plays a pivotal role in the triage process by switching chaperones into components of the UPP (17, 59).
Figure 8.
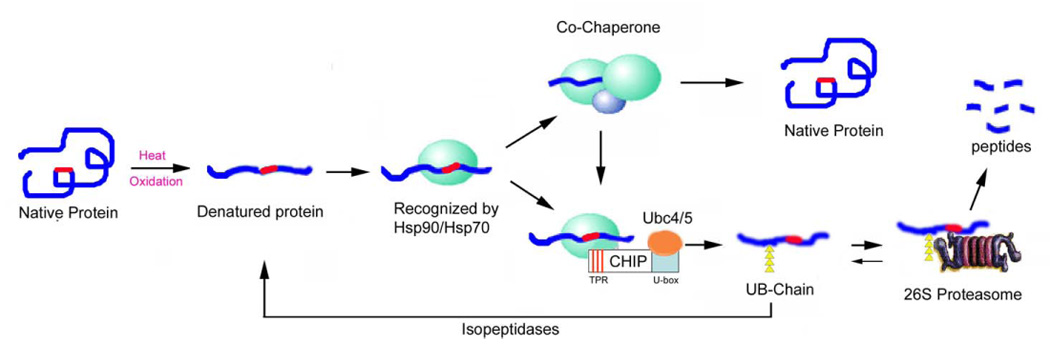
Triage model of the protein quality control system: the interaction between molecular chaperones and the UPP. This model predicts that most, if not all, proteins have intrinsic signals for interaction with molecular chaperones or the ubiquitination system. These signals (red) are hidden in properly folded native proteins and they are not recognized by the protein quality control systems. Upon environmental stress, such as heat or oxidation, proteins could be unfolded with exposure of the recognition signals, such as hydrophobic patches. The unfolded proteins are recognized by Hsp90 or Hsp70. With the help of other chaperones or co-factors, Hsp70 can refold the denatured proteins in an ATP-dependent manner. If the denatured proteins cannot be refolded rapidly, CHIP, a U-box E3, which interacts with Hsp90 and Hsp70 with its TPR domain, triggers the ubiquitination of Hsp90/hsp70-bound substrates. The ubiquitinated substrates will be recognized and degraded by the 26S proteasome. If the ubiquitinated proteins were deubiquitinated by isopeptidases, the denatured proteins would have a second chance to be refolded by molecular chaperones. The parallel/competitive functional relationship between the UPP and molecular chaperones assures the efficiency of the protein quality control systems to get rid of abnormal proteins.
A previous report indicated that over-expression of CHIP in fibroblasts promoted Hsp70-mediated renaturation of denatured luciferase (60). The apparent discrepancy of data presented here with the previous report may result from differences in the methods of denaturation. In the previous report, luciferase was first expressed in fibroblasts and was subsequently inactivated by heating the cells. Heat shock of the cells not only denatured luciferase, but it may also injure the ubiquitin conjugating system. If the ubiquitin conjugating system is inactivated by heat shock, over-expression of CHIP will not be able to enhance ubiquitination. Instead it may promote renaturation by modulating chaperone activities.
The parallel or competitive mode of action between the UPP and chaperones in the protein quality control process appears to not be energy efficient. It will end up with the undesirable destruction of some repairable proteins. This may explain why ∼30% of nascent proteins are degraded before they are folded properly (4). The cost of the undesired destruction can be justified for the security and efficiency of the protein quality control systems. If one system fails, the other system would be the backup. It has been shown that inhibition of the UPP results in up-regulation of heat shock proteins (20, 61–63). Vice versa, inhibition of chaperone activity also promotes the degradation of Hsp90 client proteins (64).
In healthy cells, the delicate balance between the UPP and the chaperones controls damaged proteins to a tolerable level. However, upon aging or severe stress, functions of both the UPP and the chaperones may be compromised. Dysfunction of the protein quality control mechanism may be causally related to the accumulation of damaged and aggregated proteins in the cells, which is associated with various age-related diseases. Therefore, means of protecting the function of the protein quality control system may be used to prevent a number of age-related diseases.
ACKNOWLEDGMENTS
The authors thank Dr. S. Wing and Dr. P. Di Fiore for their providing the plasmid of Ubc4-1 and UBPY, respectively. The authors also appreciate the critical comments and suggestions from Dr. M. Obin, Dr. E. Dudek and Dr. E. Whitcomb during preparation of this manuscript. This work is supported partially by NIH grants EY11717 (to FS), EY13250 (to AT), EY13078 (Tufts core), Fellowship from Foundation for Science and Technology, Portugal (PRAXIS XXI/BD/19532/99) (to CM), and USDA CRIS 1950- 51000-060-01A
REFERENCES
- 1.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 2.Julien JP. Amyotrophic lateral sclerosis. unfolding the toxicity of the misfolded. Cell. 2001;104:581–591. doi: 10.1016/s0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 3.Ross CA, Pickart CM. The ubiquitin-proteasome pathway in Parkinson's disease and other neurodegenerative diseases. Trends Cell Biol. 2004;14:703–711. doi: 10.1016/j.tcb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 5.Stathopulos PB, Rumfeldt JA, Scholz GA, Irani RA, Frey HE, Hallewell RA, Lepock JR, Meiering EM. Cu/Zn superoxide dismutase mutants associated with amyotrophic lateral sclerosis show enhanced formation of aggregates in vitro. Proc. Natl. Acad. Sci. USA. 2003;100:7021–7026. doi: 10.1073/pnas.1237797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson KD. Ubiquitin-dependent signaling: the role of ubiquitination in the response of cells to their environment. J. Nutr. 1999;129:1933–1936. doi: 10.1093/jn/129.11.1933. [DOI] [PubMed] [Google Scholar]
- 8.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 9.Morishima Y, Murphy PJ, Li DP, Sanchez ER, Pratt WB. Stepwise assembly of a glucocorticoid receptor.hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J. Biol. Chem. 2000;275:18054–18060. doi: 10.1074/jbc.M000434200. [DOI] [PubMed] [Google Scholar]
- 10.Rajapandi T, Greene LE, Eisenberg E. The molecular chaperones Hsp90 and Hsc70 are both necessary and sufficient to activate hormone binding by glucocorticoid receptor. J. Biol. Chem. 2000;275:22597–22604. doi: 10.1074/jbc.M002035200. [DOI] [PubMed] [Google Scholar]
- 11.Loo MA, Jensen TJ, Cui L, Hou Y, Chang XB, Riordan JR. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998;17:6879–6887. doi: 10.1093/emboj/17.23.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minami Y, Hohfeld J, Ohtsuka K, Hartl FU. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J. Biol. Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- 13.Cyr DM. Coupling chemical energy by the hsp70/tim44 complex to drive protein translocation into mitochondria. J. Bioenerg. Biomembr. 1997;29:29–34. doi: 10.1023/a:1022455621111. [DOI] [PubMed] [Google Scholar]
- 14.Luders J, Demand J, Schonfelder S, Frien M, Zimmermann R, Hohfeld J. Cofactor-induced modulation of the functional specificity of the molecular chaperone Hsc70. Biol. Chem. 1998;379:1217–1226. doi: 10.1515/bchm.1998.379.10.1217. [DOI] [PubMed] [Google Scholar]
- 15.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClellan AJ, Frydman J. Molecular chaperones and the art of recognizing a lost cause. Nat. Cell Biol. 2001;3:E51–E53. doi: 10.1038/35055162. [DOI] [PubMed] [Google Scholar]
- 17.Cyr DM, Hohfeld J, Patterson C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem. Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- 18.Wickner S, Maurizi MR, Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science. 1999;286:1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 20.Lee DH, Goldberg AL. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998;18:30–38. doi: 10.1128/mcb.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J. Biol. Chem. 1997;272:9002–9010. doi: 10.1074/jbc.272.14.9002. [DOI] [PubMed] [Google Scholar]
- 22.Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J. Biol. Chem. 2003;278:28490–28500. doi: 10.1074/jbc.M209682200. [DOI] [PubMed] [Google Scholar]
- 23.Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 24.Jahngen JH, Haas AL, Ciechanover A, Blondin J, Eisenhauer D, Taylor A. The eye lens has an active ubiquitin-protein conjugation system. J. Biol. Chem. 1986;261:13760–13767. [PubMed] [Google Scholar]
- 25.Wing SS, Jain P. Molecular cloning, expression and characterization of a ubiquitin conjugation enzyme (E2(17)kB) highly expressed in rat testis. Biochem. J. 1995;305:125–132. doi: 10.1042/bj3050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naviglio S, Mattecucci C, Matoskova B, Nagase T, Nomura N, Di Fiore PP, Draetta GF. UBPY: a growth-regulated human ubiquitin isopeptidase. EMBO J. 1998;17:3241–3250. doi: 10.1093/emboj/17.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans PC, Smith TS, Lai MJ, Williams MG, Burke DF, Heyninck K, Kreike MM, Beyaert R, Blundell TL, Kilshaw PJ. A novel type of deubiquitinating enzyme. J. Biol. Chem. 2003;278:23180–23186. doi: 10.1074/jbc.M301863200. [DOI] [PubMed] [Google Scholar]
- 28.Shang F, Deng G, Obin M, Wu CC, Gong X, Smith D, Laursen RA, Andley UP, Reddan JR, Taylor A. Ubiquitin-activating enzyme (E1) isoforms in lens epithelial cells: origin of translation, E2 specificity and cellular localization determined with novel site-specific antibodies. Exp. Eye Res. 2001;73:827–836. doi: 10.1006/exer.2001.1091. [DOI] [PubMed] [Google Scholar]
- 29.Gaczynska M, Goldberg AL, Tanaka K, Hendil KB, Rock KR. Proteasome subunits X and Y alter peptidase activities in opposite ways to the interferon-γ- induced subunits LMP2 and LMP7. J. Biol. Chem. 1996;271:17275–17280. doi: 10.1074/jbc.271.29.17275. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Murata S, Minami Y, Minami M, Chiba T, Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciechanover A, Laszlo A, Bercovich B, Stancovski I, Alkalay I, Ben-Neriah Y, Orian A. The ubiquitin-mediated proteolytic system: involvement of molecular chaperones, degradation of oncoproteins, and activation of transcriptional regulators. Cold Spring Harb. Symp. Quant. Biol. 1995;60:491–501. doi: 10.1101/sqb.1995.060.01.053. [DOI] [PubMed] [Google Scholar]
- 33.Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat. Cell Biol. 2001;3:100–105. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- 34.Yonehara M, Minami Y, Kawata Y, Nagai J, Yahara I. Heat-induced chaperone activity of HSP90. J. Biol. Chem. 1996;271:2641–2645. doi: 10.1074/jbc.271.5.2641. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg AL. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin) Proc. Natl. Acad. Sci. USA. 1972;69:422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etlinger JD, Goldberg AL. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc. Natl. Acad. Sci. USA. 1977;74:54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hershko A, Heller H, Eytan E, Reiss Y. The protein substrate binding site of the ubiquitin-protein ligase system. J. Biol. Chem. 1986;261:11992–11999. [PubMed] [Google Scholar]
- 38.Hershko A, Eytan E, Ciechanover A, Haas AL. Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. Relationship to the breakdown of abnormal proteins. J. Biol. Chem. 1982;257:13964–13970. [PubMed] [Google Scholar]
- 39.Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperature, starvation, and other stresses. Cell. 1987;48:1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- 40.Taylor A, Davies KJA. Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens. Free Radic. Biol. Med. 1987;3:371–377. doi: 10.1016/0891-5849(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 41.Shang F, Gong X, Taylor A. Activity of ubiquitin dependent pathway in response to oxidative stress: Ubiquitin activating enzyme (E1) is transiently upregulated. J. Biol. Chem. 1997;272:23086–23093. doi: 10.1074/jbc.272.37.23086. [DOI] [PubMed] [Google Scholar]
- 42.Shang F, Taylor A. Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem. J. 1995;307:297–303. doi: 10.1042/bj3070297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shang F, Nowell TR, Jr., Taylor A. Removal of oxidatively damaged proteins from lens cells by the ubiquitin-proteasome pathway. Exp. Eye Res. 2001;73:229–238. doi: 10.1006/exer.2001.1029. [DOI] [PubMed] [Google Scholar]
- 44.Shang F, Taylor A. Function of the ubiquitin proteolytic pathway in the eye. Exp. Eye Res. 2004;78:1–14. doi: 10.1016/j.exer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitinproteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 46.Benharouga M, Haardt M, Kartner N, Lukacs GL. COOH-terminal truncations promote proteasome-dependent degradation of mature cystic fibrosis transmembrane conductance regulator from post-Golgi compartments. J. Cell Biol. 2001;153:957–970. doi: 10.1083/jcb.153.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelman MS, Kannegaard ES, Kopito RR. A principal role for the proteasome in en0doplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2002;277:11709–11714. doi: 10.1074/jbc.M111958200. [DOI] [PubMed] [Google Scholar]
- 48.Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, et al. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J. Cell Biol. 2004;164:923–933. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyun DH, Lee M, Halliwell B, Jenner P. Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J. Neurochem. 2003;86:363–373. doi: 10.1046/j.1471-4159.2003.01841.x. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman EK, Wilcox HM, Scott RW, Siman R. Proteasome inhibition enhances the stability of mouse Cu/Zn superoxide dismutase with mutations linked to familial amyotrophic lateral sclerosis. J. Neurol. Sci. 1996;139:15–20. [PubMed] [Google Scholar]
- 51.Marques C, Pereira P, Taylor A, Liang JN, Reddy VN, Szweda LI, Shang F. Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. FASEB J. 2004;18:1424–1426. doi: 10.1096/fj.04-1743fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun BC, Glickman M, Kraft R, Dahlmann B, Kloetzel PM, Finley D, Schmidt M. The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat. Cell Biol. 1999;1:221–226. doi: 10.1038/12043. [DOI] [PubMed] [Google Scholar]
- 53.Liu CW, Millen L, Roman TB, Xiong H, Gilbert HF, Noiva R, DeMartino GN, Thomas PJ. Conformational remodeling of proteasomal substrates by PA700, the 19 S regulatory complex of the 26 S proteasome. J. Biol. Chem. 2002;277:26815–26820. doi: 10.1074/jbc.M201782200. [DOI] [PubMed] [Google Scholar]
- 54.Strickland E, Hakala K, Thomas PJ, DeMartino GN. Recognition of misfolding proteins by PA700, the regulatory subcomplex of the 26 S proteasome. J. Biol. Chem. 2000;275:5565–5572. doi: 10.1074/jbc.275.8.5565. [DOI] [PubMed] [Google Scholar]
- 55.Benaroudj N, Goldberg AL. PAN, the proteasome-activating nucleotidase from archaebacteria, is a protein-unfolding molecular chaperone. Nat. Cell Biol. 2000;2:833–839. doi: 10.1038/35041081. [DOI] [PubMed] [Google Scholar]
- 56.Hershko A, Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 57.Sadis S, Atienza C, Jr., Finley D. Synthetic signals for ubiquitin-dependent proteolysis. Mol. Cell. Biol. 1995;15:4086–4094. doi: 10.1128/mcb.15.8.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feldman DE, Frydman J. Protein folding in vivo: the importance of molecular chaperones. Curr. Opin. Struct. Biol. 2000;10:26–33. doi: 10.1016/s0959-440x(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 59.McDonough H, Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8:303–308. doi: 10.1379/1466-1268(2003)008<0303:calbtc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kampinga HH, Kanon B, Salomons FA, Kabakov AE, Patterson C. Overexpression of the cochaperone CHIP enhances Hsp70-dependent folding activity in mammalian cells. Mol. Cell. Biol. 2003;23:4948–4958. doi: 10.1128/MCB.23.14.4948-4958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hungness ES, Robb BW, Luo GJ, Pritts TA, Hershko DD, Hasselgren PO. Proteasome inhibitors activate the transcription factors C/EBP-beta and delta in human intestinal epithelial cells. Biochem. Biophys. Res. Commun. 2002;290:469–474. doi: 10.1006/bbrc.2001.6168. [DOI] [PubMed] [Google Scholar]
- 62.Mathew A, Mathur SK, Morimoto RI. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 1998;18:5091–5098. doi: 10.1128/mcb.18.9.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heatshock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J. Biol. Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- 64.Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol. Cancer Ther. 2004;3:551–566. [PubMed] [Google Scholar]


