Abstract
Background
Recently, several new human coronaviruses have been identified.
Objectives
To define the seroepidemiology of group I human coronaviruses.
Study design
A recombinant protein enzyme linked immunosorbent assay (ELISA) based on portions of the nucleocapsid protein of group I human coronaviruses was developed and was used to screen serum from 243 children and young adults.
Results
For HCoV-229E, the percentages of seropositive individuals were 57.1% for infants <2 months old; 38.9% for infants 2–3 months old; 4.7% for infants 4–5 months old; 42.9–50.0% for infants 6–12 months old; 34.8–62.5% for individuals 1–20 years old. For HCoV-NL63, the percentages of seropositive individuals were 45.2% for infants <2 months old; 11.1% for infants 2–3 months old; 4.7% for infants 4–5 months old; 28.6–40.0% for infants 6–12 months old; 25.0–70.3% for individuals 1–20 years old.
Conclusions
Infection with these viruses is common in childhood though the prevalence of these viruses may vary from year to year.
Keywords: Human coronavirus, Seroepidemiology, Nucleocapsid
1. Introduction
Coronaviruses are a complex group of single-stranded, positive-sense RNA viruses that infect mammals and birds. The first human coronaviruses were discovered in the 1960s (Hamre and Procknow, 1966, Hambre and Beem, 1972, McIntosh et al., 1967, Tyrrell and Bynoe, 1965). Until the identification of the SARS coronavirus in 2003 (Drosten et al., 2003, Ksiazek et al., 2003, Peiris et al., 2003), HCoV-229E (group I) and HCoV-OC43 (group II) were the only known human coronaviruses though data from the 1960s suggested that other coronaviruses may be circulating in the human population (Kahn and McIntosh, 2005).
Recently, several new or emerging human coronaviruses have been discovered. The SARS coronavirus that caused a worldwide epidemic in 2002–2004 either represents a novel group of coronaviruses or is a group II coronavirus (Drosten et al., 2003). In 2004, van der Hoek et al. (2004) reported the discovery of a new group I coronavirus designated HCoV-NL63. Using molecular probes that target conserved regions of the coronavirus genome, we found evidence of HCoV-NL63 (designated the “New Haven coronavirus” in our initial description) in respiratory secretions obtained from children with respiratory tract disease (Esper et al., 2005). Several reports have demonstrated that HCoV-NL63 is a common virus and is associated with both upper and lower respiratory tract disease (Bastien et al., 2005, Chiu et al., 2005, Choi et al., 2006, Ebihara et al., 2005, Esper et al., 2005, Vabret et al., 2005, van der Hoek et al., 2005).
Coronaviruses contain the largest RNA viral genomes (∼29 kb) and encode at least four virion proteins (for review, see Holmes and Lai, 1996). The spike (S) is a large protein (>175 kDa) that forms oligomers on the viral surface. The nucleocapsid protein binds to viral RNA and is contained within the virion. The nucleocapsid elicits a humoral immune response and contains several linear epitopes (Wege et al., 1993).
It appears that HCoV-NL63, like the other known group I human coronavirus, HCoV-229E, is a common pathogen. The epidemiology of HCoV-NL63 is incomplete and there is relatively little data examining the seroprevalence of the virus. To address this, we developed a serological assay, based on recombinant nucleocapsid protein and screened serum from children and young adults. Here we report the results of a seroprevalence study of the group I human coronaviruses in children.
2. Materials and methods
2.1. Viruses and cells
HCoV-229E was obtained from the American Type Culture Collection (Manassas, VA). HCoV-NL63 strain New Haven (191) was identified and described previously (Esper et al., 2005).
2.2. RNA extraction and reverse transcriptase-PCR amplification of regions of the N gene
Viral RNA extraction and RT-PCR were performed as described previously (Esper et al., 2005). Primers for PCR amplification and cloning were as follows: HCoV-NL63 strain New Haven 191 N (GenBank accession number EF081296) amino acid 59 forward primer 5′-gcgcGGATCCTCAAGAGCGTTGGCGTATGC-3′; 234 forward primer 5′-gcgcGGATCCACCTCGTTGGAAGCGTGTT-3′; amino acid 271 reverse primer 5′-gcgcCTCGAGGGCATCAACACCATTCTG-3′; amino acid 377 reverse primer 5′-cgcgCTCGAGATGCAAAACCTCGTTGAC-3′. For HCoV-229E N (GenBank accession number NC_002645), the primers were as follows: amino acid 229 forward primer 5′-gcgcGGATCCTCTTGCTCGTTCTCAGAGTTCTG-3′; 389 reverse primer 5′-gcgcCTCGAGGTTTACTTCATCAATTATGTC-3′. The cloning restriction endonuclease site within each primer is underlined. G/C clamps are in lower case.
2.3. Cloning, expression and purification of recombinant N protein
The N gene amplicon for each virus was cloned separately into the expression vector pET22b (Novagen, San Diego, CA) in frame with a His tag at the 3′ end of the open reading frame. Expression of recombinant protein was performed following the protocols provided by the manufacturer. Purification of His tag protein was performed using the QIAexpress Ni-NTA Fast Start Kit (Qiagen) according to the manufacturer's specifications. EBV-Z protein (with a carboxy-terminal His tag) was provided by George Miller, Yale University, New Haven, CT.
2.4. Western blot analysis
Western blot analysis was performed as previously described (Leung et al., 2005). Briefly, purified protein (1.4 μm) was separated by SDS-polyacrylamide (10%) gel electrophoresis, transferred to a membrane filter and incubated with either human sera (1:400) or goat anti-HCoV-229E (1:800) (kindly provided by K.V. Holmes, University of Colorado Health Sciences Center, Denver, CO). Bound secondary antibody (horse radish peroxidase (HRP))-conjugated antibody (either anti-human or anti-goat, Jackson ImmunoResearch Laboratories, West Grove, PA) was detected using ECL Western blotting system (Amersham Pharmacia Biotech Inc., Piscataway, New Jersey).
2.5. Serum
Human serum specimens from individuals <20 years old were collected from the Clinical Chemistry Laboratory, Yale-New Haven Hospital (with the exception of serum #1 which was collected from an adult donor). Serum samples were collected from June 2003 through March 2004 and stored at −20 °C.
2.6. ELISA
ELISA assays with purified recombinant protein or HCoV-229E infected cell lysates were performed using a modification of a protocol described previously (Leung et al., 2005). Briefly, 50 ng of purified protein (in phosphate buffered saline [PBS]) was used to coat the wells of a 96-well plate. Serial dilutions of serum were added to the wells and bound antibody was detected with species appropriate HRP-conjugated anti-human antibody (1:20,000) (Jackson ImmunoResearch Laboratories). Optical densities were read at 450 nm (DynaTech MR5000). The optimal conditions were determined by checker board titrations using serum that tested positive for HCoV-229E (by Western blot and infected cell lysates-based ELISA). The criteria for a positive ELISA were defined as an OD450 > 0.2 (above background) at a dilution of 1:80 or greater for recombinant protein ELISA and OD450 > 0.25 for HCoV-229E infected whole cell lysates ELISA.
3. Results
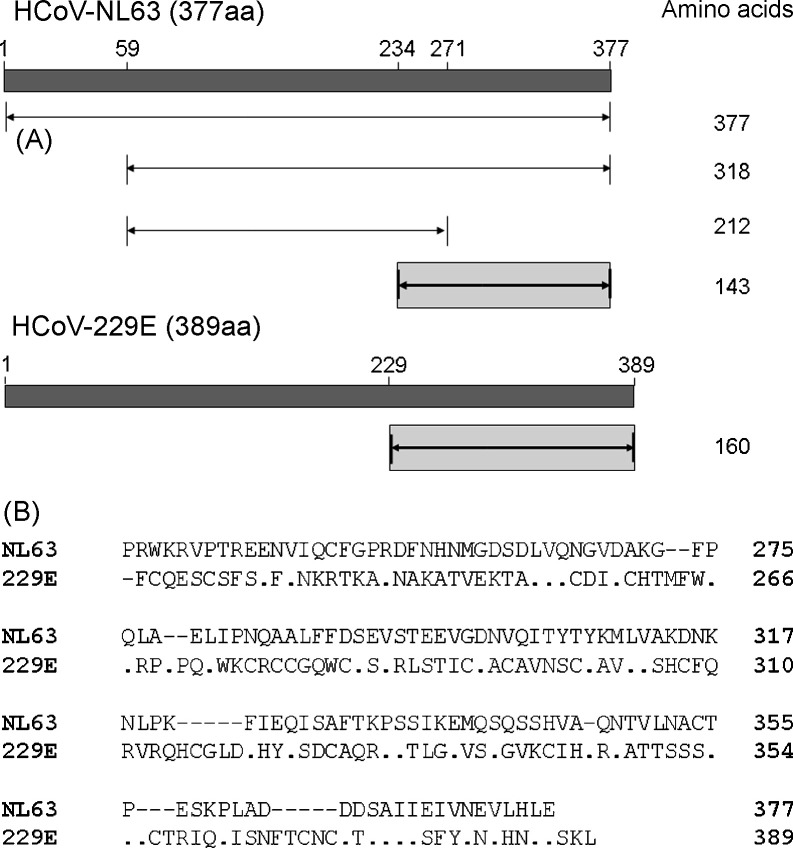
Comparison of the 1134 base nucleocapsid genes of HCoV-NL63 strain Amsterdam1, the first non-HCoV-229E group I human coronavirus characterized, and HCoV-NL63 strain New Haven 191 revealed as many as 10 polymorphisms. Of these, 5 resulted in amino acid substitutions (data not shown). Because the full length nucleocapsid gene of HCoV-NL63 could not be expressed efficiently (data not shown), we constructed clones which contained only portions of the nucleocapsid gene (Fig. 1A). Overall, amino acids 59–377, 59–271 and 234–377 of HCoV-NL63 and amino acids 229–389 of HCoV-229E were cloned and expressed. Comparison of the predicted amino acid sequence of the carboxy-terminus of the nucleocapsid proteins of HCoV-NL63 strain New Haven 191 (amino acids 234–377) and HCoV-229E (amino acids 229–389) revealed minimal homology (Fig. 1B) suggesting that these recombinant proteins may be useful in serological assays.
Fig. 1.
Map and amino acid sequence comparisons of the nucleocapsid genes of group I human coronaviruses. (A) Map of the full length and expressed regions of the nucleocapsid genes of HCoV-NL63 (strain New Haven 191) and HCoV-229E. The N protein of HCoV-NL63 and HCoV-229E are 377 and 389 amino acids, respectively. The region encoding amino acid 59–377, 59–271 and 234–377 of HCoV-NL63 and the region encoding amino acid 229–389 of HCoV-229E were cloned and expressed in bacteria. Recombinant proteins representing amino acid 234–377 of the HCoV-NL63 nucleocapsid and amino acids 229–389 of the HCoV-229E nucleocapsid were used in the seroepidemiological investigation (see text). (B) Sequence alignment of the predicted amino acid sequence of the nucleocapsid proteins of HCoV-NL63 (strain New Haven 191, amino acid 234–377) and HCoV-229E (229E) (amino acid 229–389). Sequence alignments were performed with Lasergene software. Amino acid residues in HCoV-229E that match HCoV-NL63 are represented with a dot (“.”). A dash (–) represent gaps in the alignment. Amino acid residue numbers are listed on the right of the figure.
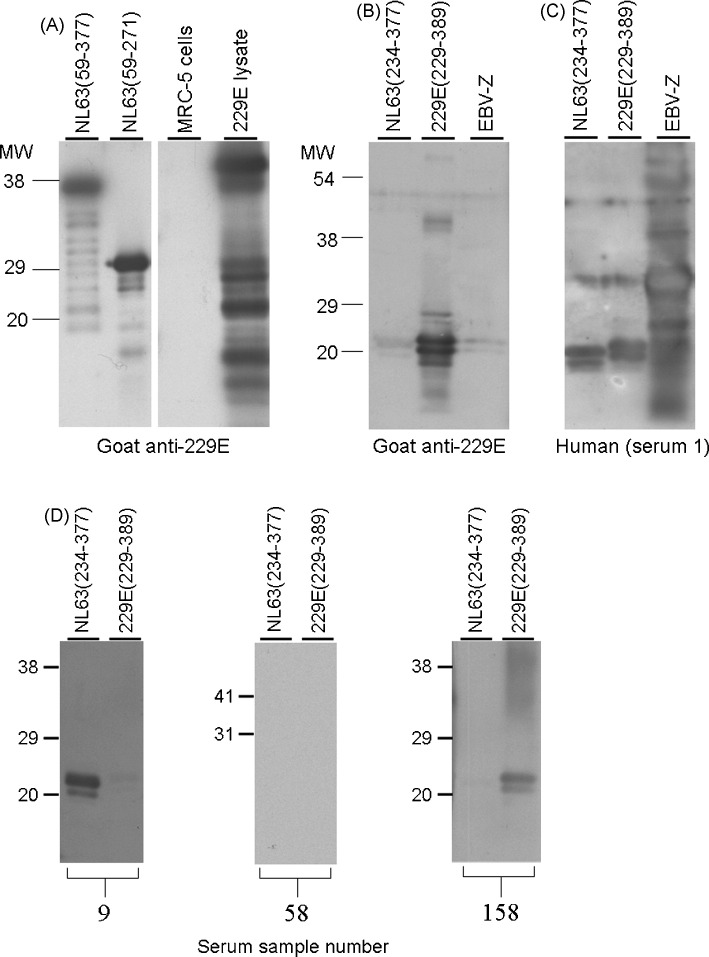
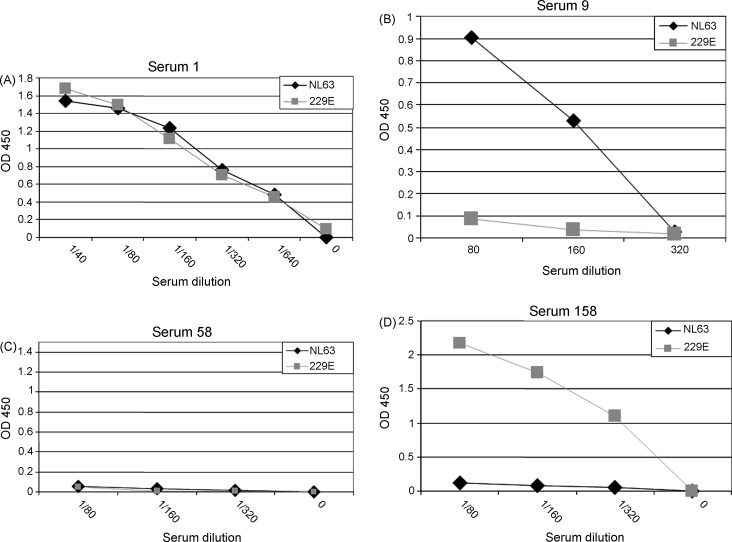
To establish that these recombinant proteins could be used to detect virus-specific antibodies, Western blot analyses were performed. As shown in Fig. 2A, recombinant protein representing amino acids 59–377 and 59–271 of HCoV-NL63 were bound by a goat anti-HCoV-229E anti-serum and therefore would not be suitable for use an antigen in an ELISA to detect HCoV-NL63-specific antibody. However, the goat anti-HCoV-229E antibody failed to detect purified recombinant protein corresponding to amino acid 234–377 of HCoV-NL63 (Fig. 2B). Individual serum #1 (Fig. 2C) bound to both HCoV-NL63 and HCoV-229E recombinant proteins suggesting that this person had been exposed to both viruses. Representative Western blots for a HCoV-NL63+, HCoV-229E− serum, HCoV-NL63−, HCoV-229E− serum and a HCoV-NL63−, HCoV229E+ serum (Fig. 2D) are also shown in the figure. The ELISAs for the serum represented in the Western blots in Fig. 2D are shown in Fig. 3 .
Fig. 2.
Goat anti-HCoV-229E antiserum is specific for recombinant HCoV-229E N protein and cross-reacts with recombinant HCoV-NL63 (59–377) N protein and recombinant HCoV-NL63 (59–271) N protein but not recombinant HCoV-NL63 (234–377) N protein. Purified recombinant proteins representing amino acid 59–377, 59–271 and 234–377 of HCoV-NL63 and amino acid 229–389 of HCoV-229E were probed by Western blots with goat anti-HCoV-229E (blots A and B) and serum from a human donor (blot C). Lysates of uninfected MRC-5 cells and HCoV-229E-infected MRC-5 cells were controls for the goat anti-HCoV-229E antiserum (blot A). Recombinant EBV Z protein (Zebra) (with a carboxy-terminal His tag) produced using the same bacterial expression system was used a control (blots B and C). Position of molecular weight markers are shown on the left. (Panel D) Recombinant proteins corresponding to amino acid 234–377 of HCoV-NL63/NH(191) (NL63/NH) and amino acid 229–389 of HCoV-229E (229E) were used as antigen for Western blot analysis of human serum. Serum from three different individuals was used (ELISAs using these serum are shown in Fig. 3). Positions of molecular weight markers are shown on the left.
Fig. 3.
Detection of HCoV-NL63 and HCoV-229E specific antibodies by ELISA. An ELISA assay was developed that used either HCoV-NL63 N protein (amino acid 234–377) or HCoV-229E N protein (amino acid 229–389) as antigen. Serial dilutions of sera were incubated with bound recombinant protein. Human antibodies specific for these recombinant proteins were detected with a chromogenic reaction following the addition and incubation with an enzyme (HRP)-linked anti-human antibody. Antibody levels were quantified by optical densities. (A) HCoV-NL63+, HCoV-229E+; (B) HCoV-NL63+, HCoV-229E−; (C) HCoV-NL63−, HCoV-229E−; (D) HCoV-NL63−, HCoV-229E+.
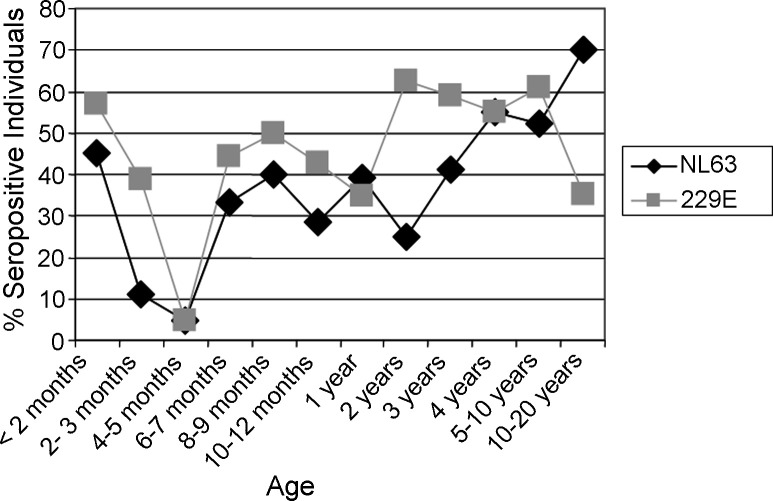
The recombinant protein-based ELISA was used to screen serum from individuals <20 years of age. Overall, 243 sera were screened (Fig. 4 and Table 1 ). The percentage of infants <2 months of age who are seropositive for HCoV-NL63 and HCoV-229E are 45.2% and 57.1%, respectively. The percentage of seropositive individuals drops to 4.7% for both viruses in infants 4–5 months of age (p value ≤ 0.001 for both viruses, Fisher exact test). For 2-year-old children, the percentage of seropositive children for HCoV-229E was 62.5% while the percentage of children seropositive for HCoV-NL63 was 25%. This difference was statistically significant (p = 0.0366, Fisher exact test). In the 4-year-old age group and the 5–10-year-old age group, >50% of children assayed tested positive for antibodies for both group I human coronaviruses. However, the percentage of 10–20-year olds who are seropositive for HCoV-229E was 35.1%.
Fig. 4.
Seroepidemiology of group I human coronaviruses based on an ELISA using recombinant N proteins. Serum from 243 individuals <20 years were screened by ELISA for HCoV-NL63 and HCoV-229E specific antibody. The percentage of seropositive individuals for each age group is displayed in the graph.
Table 1.
Screening of sera for group I human coronavirus specific antibodies
| Age group | Total number of individuals screened | HCoV-229E positive (%) | HCoV-NL63 positive (%) | Serum specimens collected per month |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| June 2003 | July 2003 | August 2003 | September 2003 | October 2003 | November 2003 | December 2003 | January 2004 | February 2004 | March 2004 | ||||
| <2 months | 42 | 24 (57.1) | 19 (45.2) | 2 | 5 | 2 | 9 | 9 | 0 | 4 | 10 | 1 | 0 |
| 2–3 months | 18 | 7 (38.9) | 2 (11.1) | 1 | 4 | 3 | 3 | 1 | 0 | 1 | 3 | 2 | 0 |
| 4–5 months | 21 | 1 (4.8) | 1 (4.8) | 3 | 0 | 0 | 6 | 1 | 0 | 3 | 3 | 5 | 0 |
| 6–7 months | 9 | 4 (44.4) | 3 (33.3) | 0 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 4 | 0 |
| 8–9 months | 10 | 5 (50.0) | 4 (40.0) | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 4 | 0 |
| 10–12 months | 7 | 3 (42.9) | 2 (28.6) | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 |
| 1 year | 23 | 8 (34.8) | 9 (39.1) | 0 | 1 | 2 | 2 | 6 | 0 | 5 | 1 | 6 | 0 |
| 2 years | 16 | 10 (62.5) | 4 (25.0) | 0 | 3 | 1 | 2 | 1 | 0 | 1 | 2 | 6 | 0 |
| 3 years | 17 | 10 (58.8) | 7 (41.2) | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 8 | 3 |
| 4 years | 20 | 11 (55.0) | 11 (55.0) | 0 | 8 | 0 | 0 | 1 | 0 | 0 | 1 | 10 | 0 |
| 5–10 years | 23 | 14 (60.9) | 12 (52.2) | 1 | 4 | 2 | 7 | 2 | 0 | 1 | 3 | 3 | 0 |
| 10–20 years | 37 | 13 (35.1) | 26 (70.3) | 0 | 4 | 3 | 10 | 10 | 0 | 2 | 6 | 2 | 0 |
| Total | 243 | 110 | 100 | 8 | 37 | 16 | 42 | 33 | 0 | 19 | 31 | 54 | 3 |
A negative result in the ELISA would indicate that either the individual has not been infected with the specific virus or that that individual has been infected but did not develop antibodies to the domain of the nucleocapsid represented in the bacterial-expressed protein. To address this issue, we randomly chose serum that tested negative for both HCoV-229E (229–389) and HCoV-NL63 and assayed these sera for HCoV-229E-specific antibodies using an HCoV-229E infected cell lysate based-ELISA (assuming that the HCoV-229E-infected cell lysate could be used as a “gold-standard”). Of the 18 serum selected that tested negative for HCoV-229E based on the recombinant protein ELISA, 16 tested negative in the HCoV-229E ELISA (negative predictive value = 89.9%). The two sera that tested positive for HCoV-229E by the infected cell lysate ELISA had low recombinant protein ELISA titers (OD450 of 0.315 and 0.286 at a 1:80 dilution). (A similar set of experiments with HCoV-NL63 could not be performed because the virus could not be propagated to sufficient titers.)
4. Discussion
In this study, we demonstrated that recombinant proteins representing a portion of the nucleocapsid protein of group I coronaviruses can be used to discriminate between antibodies specific for the two viruses. We chose to use recombinant proteins rather than whole virus for this assay for several reasons. First, use of whole virus or infected cell lysates as antigens would require efficient replication of these viruses in vitro. HCoV-NL63 replicates poorly in cell culture and this would limit the ability to screen a large number of serum specimens. Second, whole virus or infected cell lysates may detect cross-reactivity antibody, complicating the use of these materials for seroepidemiology. Indeed, portions of the nucleocapsid of HCoV-NL63 were detected by a goat anti-HCoV-229E suggesting that cross-reactive antibody to the two group I human coronaviruses may be elicited after infection with these viruses. Lastly, recombinant protein representing the nucleocapsid of the SARS coronavirus and HKU1, a newly identified human group II coronavirus (Huang et al., 2004, Shi et al., 2003, Timani et al., 2004, Woo et al., 2004, Woo et al., 2005b) has been used in antibody detection assays.
Since we could not rely on an infected cell lysates-based ELISAs as a “gold standard,” because of a concern for cross-reactive antibody, we could not test the sensitivity for the recombinant protein-based ELISA. Nonetheless, we demonstrated that the recombinant proteins could be used to specifically detect antibodies for HCoV-229E and HCoV-NL63. The high negative predictive value (89%) indicates that our assay is reliable though may result in an (albeit small) underestimation of the true percentage of negatives. However, this negative predictive value was determined for HCoV-229E; we could unfortunately not confirm this for HCoV-NL63, because we could not propagate the HCoV-NL63 virus to sufficient titers.
It appears that infection with known group I coronaviruses is common in childhood. Although maternally acquired N-directed antibodies to both HCoV-229E and HCoV-NL63 appeared to wane by 4–5 months of life, >30% of children have serological evidence of infection with either of the two group I human coronaviruses by the age of 12 months. The most likely explanation for this is the exposure and infection with these viruses in the first year of life. Our cross-sectional study revealed that the percentage of seropositive individuals 2 years of age was much greater for HCoV-229E as compared to HCoV-NL63 suggesting that HCoV-229E was more prevalent in the 1–2 years prior to the acquisition of the serum for these children. Our findings are similar to that of Hofmann et al. who found, with the use of pseudotype virus expressing the S protein, that most sera from adults had HCoV-NL63-neutralizing antibodies (Hofmann et al., 2005). Indeed, the youngest children who had detectable HCoV-NL63-specific antibodies were ∼1.5 years old and by age 8 years, the sera from most of the children screened had HCoV-NL63-specific antibodies, which are consistent with our findings. Furthermore, HCoV-229E specific antibodies were infrequently detected in the adult sera screened by Hofmann et al. and by our group suggesting that the circulation of the two group I coronaviruses differ.
Previous studies of non-SARS human coronaviruses, which focused on HCoV-229E and HCoV-OC43, demonstrated that the seroprevalence varies greatly and depends on the age of the population and the method used to detect coronavirus-specific antibodies (Hambre and Beem, 1972, Hasony and Macnaughton, 1982, Kaye et al., 1971, McIntosh et al., 1970, Monto and Lim, 1974, Pohl-Koppe et al., 1995). However, these studies used whole virus as antigen and, because of the apparent cross-reactivity of HCoV-229E antibodies demonstrated in our study, it is likely that the assays used in these studies detected group-specific antibody rather than type-specific antibody. With the discovery of HCoV-NL63 the results of these publications should now be viewed differently: in the studies in which HCoV-229E antibody was measured, the data likely represents the seroepidemiology of group I coronaviruses. (The same is likely true for group II coronaviruses with the discovery of HCoV-HKU1, Woo et al., 2005a.)
In conclusion, our data indicates that infection with these group I coronaviruses is common in childhood. Application of the recombinant protein-based ELISA will lead to the further understanding of the epidemiology of these viruses.
Conflict of interest
None.
Acknowledgements
The work presented in this manuscript was supported by the National Institutes of Health, grant R21AI067762-01 (JSK). This work was supported in part by National Institutes of Health grant T32 AI07210-20 (FE). We are indebted to George Miller, M.D., John F. Enders Professor of Pediatric Infectious Diseases for his continued support, intellectual and scientific input and exchange and critical review of the data. We thank the staff of the Clinical Laboratories, Yale-New Haven Hospital for their assistance in this study. This work was approved by the Yale University Human Investigation Committee.
References
- Bastien N., Robinson J.L., Tse A., Lee B.E., Hart L., Li Y. Human coronavirus NL-63 infections in children: a 1-year study. J Clin Microbiol. 2005;43(9):4567–4573. doi: 10.1128/JCM.43.9.4567-4573.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S.S., Chan K.H., Chu K.W., Kwan S.W., Guan Y., Poon L.L. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40(12):1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.H., Lee H.J., Kim S.J., Eun B.W., Kim N.H., Lee J.A. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43(5):585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Ebihara T., Endo R., Ma X., Ishiguro N., Kikuta H. Detection of human coronavirus NL63 in young children with bronchiolitis. J Med Virol. 2005;75(3):463–465. doi: 10.1002/jmv.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191(4):492–498. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambre D., Beem M. Virologic studies of acute respiratory disease in young adults. V. Coronavirus 229E infections during six years of surveillance. Am J Epidemiol. 1972;96(2):94–106. doi: 10.1093/oxfordjournals.aje.a121445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Hasony H.J., Macnaughton M.R. Prevalence of human coronavirus antibody in the population of southern Iraq. J Med Virol. 1982;9(3):209–216. doi: 10.1002/jmv.1890090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci USA. 2005;102(22):7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K., Lai M.M.C. Coronaviridae and their replication. In: Fields B., Knipe D., Howley P., editors. Virology. 3rd ed. Lippincott-Raven; Philadelphia: 1996. pp. 1075–1094. [Google Scholar]
- Huang L.R., Chiu C.M., Yeh S.H., Huang W.H., Hsueh P.R., Yang W.Z. Evaluation of antibody responses against SARS coronaviral nucleocapsid or spike proteins by immunoblotting or ELISA. J Med Virol. 2004;73(3):338–346. doi: 10.1002/jmv.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J.S., McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11 Suppl):S223–S227. doi: 10.1097/01.inf.0000188166.17324.60. discussion S226. [DOI] [PubMed] [Google Scholar]
- Kaye H.S., Marsh H.B., Dowdle W.R. Seroepidemiologic survey of coronavirus (strain OC 43) related infections in a children's population. Am J Epidemiol. 1971;94(1):43–49. doi: 10.1093/oxfordjournals.aje.a121293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Leung J., Esper F., Weibel C., Kahn J.S. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol. 2005;43(3):1213–1219. doi: 10.1128/JCM.43.3.1213-1219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 1967;57(4):933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Kapikian A.Z., Turner H.C., Hartley J.W., Parrott R.H., Chanock R.M. Seroepidemiologic studies of coronavirus infection in adults and children. Am J Epidemiol. 1970;91(6):585–592. doi: 10.1093/oxfordjournals.aje.a121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A.S., Lim S.K. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J Infect Dis. 1974;129(3):271–276. doi: 10.1093/infdis/129.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl-Koppe A., Raabe T., Siddell S.G., ter Meulen V. Detection of human coronavirus 229E-specific antibodies using recombinant fusion proteins. J Virol Methods. 1995;55(2):175–183. doi: 10.1016/0166-0934(95)00041-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yi Y., Li P., Kuang T., Li L., Dong M. Diagnosis of severe acute respiratory syndrome (SARS) by detection of SARS coronavirus nucleocapsid antibodies in an antigen-capturing enzyme-linked immunosorbent assay. J Clin Microbiol. 2003;41(12):5781–5782. doi: 10.1128/JCM.41.12.5781-5782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timani K.A., Ye L., Ye L., Zhu Y., Wu Z., Gong Z. Cloning, sequencing, expression, and purification of SARS-associated coronavirus nucleocapsid protein for serodiagnosis of SARS. J Clin Virol. 2004;30(4):309–312. doi: 10.1016/j.jcv.2004.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D.A., Bynoe M.L. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965;5448:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret A., Mourez T., Dina J., van der Hoek L., Gouarin S., Petitjean J. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11(8):1225–1229. doi: 10.3201/eid1108.050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C. Identification of a new human coronavirus. Nat Med. 2004;10(4):368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Sure K., Ihorst G., Stang A., Pyrc K., Jebbink M.F. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005;2(8):e240. doi: 10.1371/journal.pmed.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Schliephake A., Korner H., Flory E., Wege H. An immunodominant CD4+ T cell site on the nucleocapsid protein of murine coronavirus contributes to protection against encephalomyelitis. J Gen Virol. 1993;74(Pt 7):1287–1294. doi: 10.1099/0022-1317-74-7-1287. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Tsoi H.W., Huang Y., Poon R.W., Chu C.M. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192(11):1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Wong B.H., Tsoi H.W., Fung A.M., Chan K.H. Detection of specific antibodies to severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein for serodiagnosis of SARS coronavirus pneumonia. J Clin Microbiol. 2004;42(5):2306–2309. doi: 10.1128/JCM.42.5.2306-2309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]