Abstract
Pain requires the integration of sensory, cognitive, and affective information. The use of placebo is a common methodological ploy in many fields, including pain. Neuroimaging studies of pain and placebo analgesia (PA) have yet to identify a mechanism of action. Because PA must result from higher-order processes, it is likely influenced by cognitive and affective dimension of the pain experience. A network of brain regions involved in these processes includes: the anterior and posterior insula (A-Ins, P-Ins), dorsal anterior cingulate cortex (DACC), dorsolateral prefrontal cortex (DLPFC), and the supplementary motor area (SMA). We used connectivity analyses to investigate the underlying mechanisms associated with placebo analgesia in a group of chronic pain patients. Structural equation models (SEM) of fMRI data evaluated the interregional connectivity of these regions across three conditions: 1) initial baseline (B1), 2) placebo (PA), and 3) placebo match (PM). SEM results of B1 data in the left hemisphere confirmed hypothesized regional relationships. However, interregional relationships were dynamic and the network models varied across hemisphere and conditions. Deviations from the B1 model in the PA and PM conditions correspond to our manipulation of expectation for pain. The dynamic changes in interregional influence across conditions are interpreted in the context of a self-reinforcing feedback loop involved in the induction and maintenance of PA. Although it is likely that placebo analgesia results partly from afferent inhibition of a nociceptive signal, the mechanisms likely involve the interaction of a cognitive-affective network with input from both hemispheres.
Introduction
Neuroimaging studies have identified large reductions in pain ratings and neural activation that accompany placebo analgesia (PA) during visceral stimulation and in proximity to when subjects rated pain (Price, Craggs, Verne, Perlstein, & Robinson, 2007; Wager, 2005a). Research suggests that placebo-induced analgesia is a complex phenomenon brought about by the interaction of multiple functional processes (e.g., sensory, cognitive, and affective) (Amanzio & Benedetti, 1999; Colloca & Benedetti, 2005).
The manner in which cortico-subcortical and cortico-cortical connections respond to and process nociceptive information may be modulated by previous experience and expectation. Work in neurophysiology, and more recently in neuroimaging, frequently identify several brain regions including the dorsolateral prefrontal cortex (DLPFC), dorsal anterior cingulate cortex (DACC), anterior insula (A-Ins), and posterior insula (P-Ins) as being involved with the functional processing of painful stimuli (Brooks, Zambreanu, Godinez, Craig, & Tracey, 2005; Kong et al., 2006; Kong et al., 2005). It follows then, that the interaction of these regions plays an important role in processing nociceptive information, ascribing valence and determining the level of pain experienced. Consequently, these regions may reflect a crucial cortico-cortical network for nociceptive processing whose role in pain modulation is not well defined.
Neuroimaging studies have identified distinct but overlapping networks involved in processing sensory and affective aspects of nociceptive stimuli (Kong et al., 2005). Questions about the manner in which these networks function with respect to context continue to emerge. It has been reasonably argued that nociceptive information may be differentially processed across pain-related affective networks under different conditions (Ohara, Crone, Weiss, & Lenz, 2006). Thus, information is needed to help clarify the implications of changes to the functional processing of nociceptive information brought about by placebo.
The present fMRI study analyzed the consistency with which a cognitive-affective network of brain regions processed pain-related information across several conditions using structural equation modeling (SEM). Based on research and theory from the fields of neurophysiology, psychology, and neuroimaging, we hypothesized a specific set of interactions for processing pain-related information (Kim, Zhu, Chang, Bentler, & Ernst, 2006; Solodkin, Hlustik, Chen, & Small, 2004). These interactions were first estimated in an initial baseline (B1) condition to validate the model and establish a baseline characterization of the model during nociception. Data from two additional conditions, placebo analgesia (PA) and one with decreased stimulation (to match the placebo response), referred to as placebo match, (PM) were then fitted to the established B1 model. The pain ratings of the latter two conditions were significantly lower than those of the B1 condition, indicating a substantial change in the processing related to the cognitive evaluation and affect. The inter-regional associations were carefully examined, compared, and contrasted across the three conditions in order to identify the corresponding manifest changes in the way the information was processed. This method of identifying the resultant changes in processing nociceptive information due to a placebo suggestion represents an important preliminary step in understanding the implications of how manipulating expectation, a cognitive process, may alter how sensory information is processed.
Materials and methods
The work presented here extends that of a previously published study. In that study, relative to a baseline condition, reductions in response pain ratings were accompanied by less activation in pain-related brain regions as a result of a placebo (Price et al., 2007). Repeated here is information related to subjects, experimental design, and procedures to give the reader sufficient background to evaluate the analysis in the present report.
Subjects
A painful condition affecting women to a greater extent than men, irritable bowel syndrome (IBS), has a huge impact on the individual, including significantly interfering with their quality of life, as well as on the economy, with estimates of annual costs of $8 billion in the US (Talley, Gabriel, Harmsen, Zinsmeister, & Evans, 1995). While prevalence rates vary by study, it is estimated that between 9% to 22% of individuals in the United States have IBS (as cited in Borum, 2001). The fact that IBS is so prevalent, and affects so many facets of life, makes it an ideal chronic pain population to study. As such, nine pre-menopausal women with IBS participated in the study (mean age = 27.7, SD = 9.6) years. Seven subjects were Caucasian, one was African-American and one was Hispanic. Seven of nine were married, six were employed and three were students. The diagnosis of IBS was made by an experienced gastroenterologist based on the ROME II criteria and exclusion of organic disease (Thompson, 1999). Six of the patients had diarrhea-predominant IBS, while three had constipation-predominant IBS. None of the patients had any symptoms other than those closely related to the IBS and none of the patients were on pain medications, serotonin uptake inhibitors, serotonin antagonists or tricyclic antidepressants at the time of the study. The study was approved by the University of Florida and Gainesville Veterans Administration Institutional Review Boards. All patients signed informed consent prior to the start of the study.
Experimental design
All patients fasted for 12 hours before each session. The B1 condition served as a control for the PA and PM conditions. Patients were always tested in the B1 condition first in order to determine level of hyperalgesia and identify the stimulus strength that evoked a pain rating between: 40–60, on a 100 point scale. Thus, the B1 stimulus intensity was individually tailored. Subsequent scanning sessions tested pain in the PA and PM conditions respectively. Sessions were spaced 3–10 days apart and on days when spontaneous ongoing abdominal pain was ≥ 30. These criteria maintained consistency in pain sensitivity across sessions. Stimulus strength was adjusted in the PM condition to evoke a pain rating that was similar to that of the PA condition (see Price et al., 2007). Each condition consisted of seven rectal-distensions, each lasting 20 seconds, and each separated by 20 seconds. Patients rated perceived pain intensity following each stimulus.
A saline jelly was applied to the rectal balloon prior to insertion in each condition. During the B1 condition patients were told “You have been given no treatment, that is, no lidocaine or any other active medication.” The PA instruction set was: “The agent you have been given has been shown to powerfully reduce pain in some people.” To help identify the psychological mechanisms associated with placebo, subjects were told the following during the PM session.
“We are interested in recreating the sensation you experienced when you got the pain killer in the last session, but without using any treatments such as lidocaine. We will be doing some test balloon inflations to determine the inflation that matches the sensations from the last session. We will ask you for a rating on the same 100-point scale we have used before to rate pain intensity. Now we will continue the test as in previous session, however, this time, you will be given no lidocaine or any other treatment. We will inflate the balloon to a lower pressure to achieve the same level of sensation that you felt in the drug session.”
Setting, drugs and materials
Scanning was at the University of Florida McKnight Brain Institute. Patients were placed in the left lateral decubitus position while the rectal balloon was inserted to within 4 cm from the anal sphincter. A lubricant (and placebo agent) of 200 mg saline jelly (Surgilube, E fougera and CO, Melville, NY 11747) was applied to the rectal balloon prior to insertion.
The rectal balloon (Zinetics Medical, Inc., Salt Lake City, UT) was controlled with a Visceral Stimulator® (Metronics, Minneapolis, MN) which was programmed to distend the balloon at a rapid rate (14.5 mL per second) to a precise and constant pressure plateau between 10 and 55 mg Hg, and to simultaneously record pressures, volumes, and compliance (Mertz, Naliboff, Munakata, Niazi, & Mayer, 1995; Whitehead et al., 1990).
Model of cognitive-affective network
The hypothesized network model is based upon psychophysiological and psychological research, and corresponds to an established anatomic model of signaling, shown in Figure 1-top (Price, 2002). In our previous work (Price et al., 2007) a Random Effects General Linear Model (RFX-GLM) of the whole-brain was used to identify areas of the brain where pain-stimulus onset was significantly convolved with the hemodynamic response function (HRF). As a precaution against Type I error, resultant statistical parameter maps (SPMs) were thresholded at p <.05, and had a spatial-extent of 50 contiguous voxels; the combination of which established an imagewise p-value of .00002 and an effective pixel-wise alpha of p < .0002 (Forman et al., 1995).
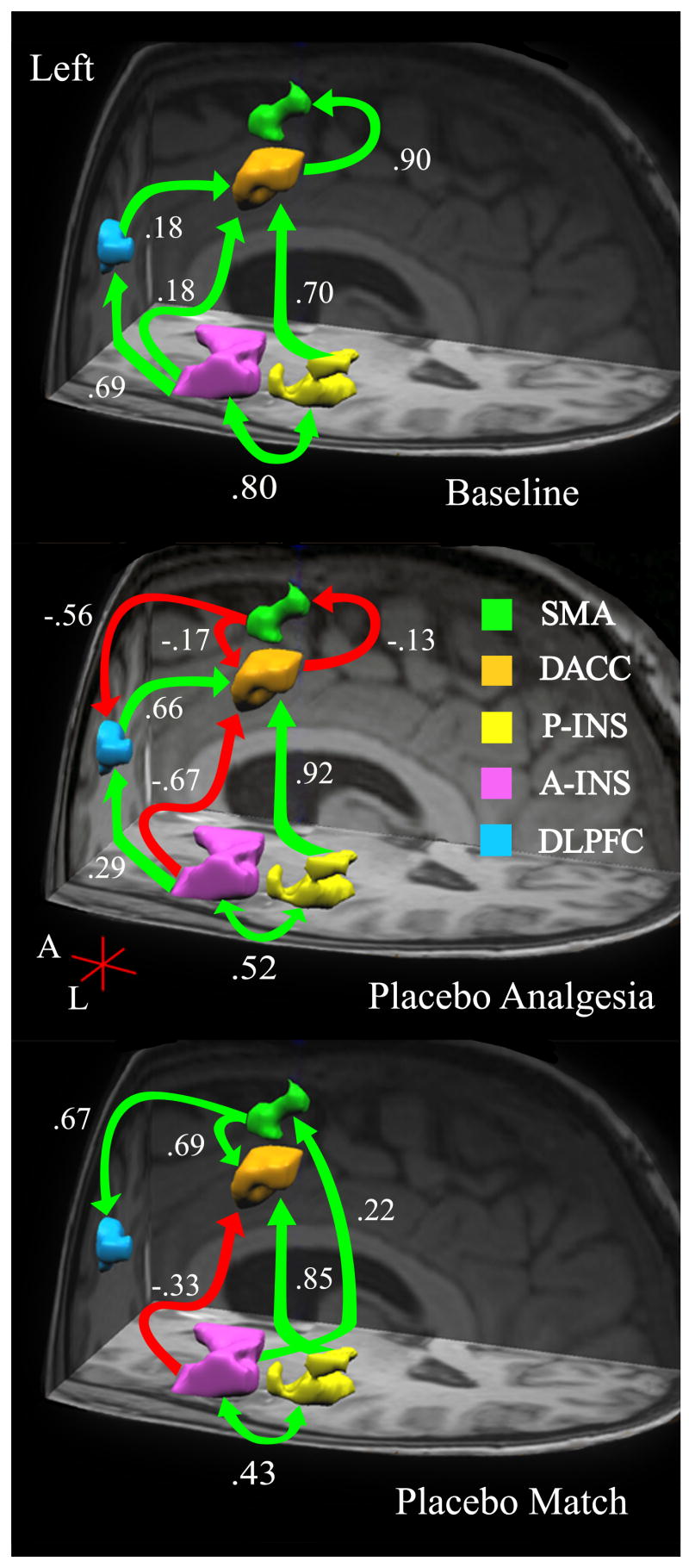
Figure 1.
Left hemisphere: Anatomical model of a cognitive – affective pain-related network. Based on a priori hypotheses, brain regions in the left hemisphere (Table 1) were identified a regions of interest (ROIs). The interregional relationships among ROIs are shown here for the left hemisphere across three conditions: Baseline (B1 - top), Placebo (PA – middle), and Placebo Match (PM – bottom). Arrows indicate estimated direction of influence. Green arrows indicate positive path coefficients and red arrows indicate negative coefficients.
In addition to estimating the main effect for balloon inflation, condition-related effects were estimated by examining BOLD signal changes in specific regions of interest (ROIs). The geographical boundaries of the ROIs were established by the t-contrast <inflate vs. rest> for the first experimental condition. Because the B1 condition was the most painful, this method of establishing ROIs increased the construct specificity and contrast sensitivity of our analyses. Similar to above, active voxel clusters were classified as a specific ROI under the following conditions: voxels survive second-level threshold of p <.05, maintained a minimum spatial-extent of 50 contiguous voxels, and the center-of-gravity of the cluster was in the targeted cortical area. Once the geographical extents of each ROI were determined, the data from the voxels within the ROI were used for the SEM analyses.
To properly model condition-related variability in the BOLD signal, data from all the voxels, in all conditions were used in the SEM analyses; not just those that would have been significant for a within-condition contrast via an ROI-GLM. This approach hedges against the possibility that some voxels would not survive the within-condition contrast (i.e., rest v inflation), and therefore lead to an unequal number of voxels contributing to the activity within an ROI across conditions (e.g., B1 v PA).
Because we were interested in investigating interactions related to cognitive affective processing, five regions in the left hemisphere (i.e., A-Ins, P-Ins, DLPFC, DACC, and SMA) were chosen (see Table 1). Preliminary models that included somatosensory (SI and SII) areas resulted in unwieldy complexity and poor fit. Data from the B1 condition were fitted to this network model initially for two reasons; to establish the validity of the model, and to develop a baseline characterization of pain processing among the regions in the model. Data from the other conditions were then used to estimate the established model. The best-fitting models for the subsequent two conditions are presented here. Deviations from the established B1 model appear to represent placebo-induced changes in processing identical stimuli in the B1 and PA conditions, and changes in the cognitive-affective responses to less painful stimuli (see Figures 1 and 2).
Table 1.
Regions of Interest (ROI) Center-of-Gravity coordinates
| Regions | Coordinates* | μL | ||
|---|---|---|---|---|
| X (±)† | Y | Z | ||
| Left dorsal ACC | −1 | 8 | 45 | 1743 |
| SMA | 0 | 6 | 61 | 513 |
| Left DLPFC | −32 | 38 | 35 | 1077 |
| Left anterior insula | −45 | 3 | 12 | 3885 |
| Left posterior insula | −46 | −22 | 12 | 1963 |
Coordinates are in the standard Talairach space
Left (−) and Right (+) hemisphere ROIs are mirror images.
Figure 2.
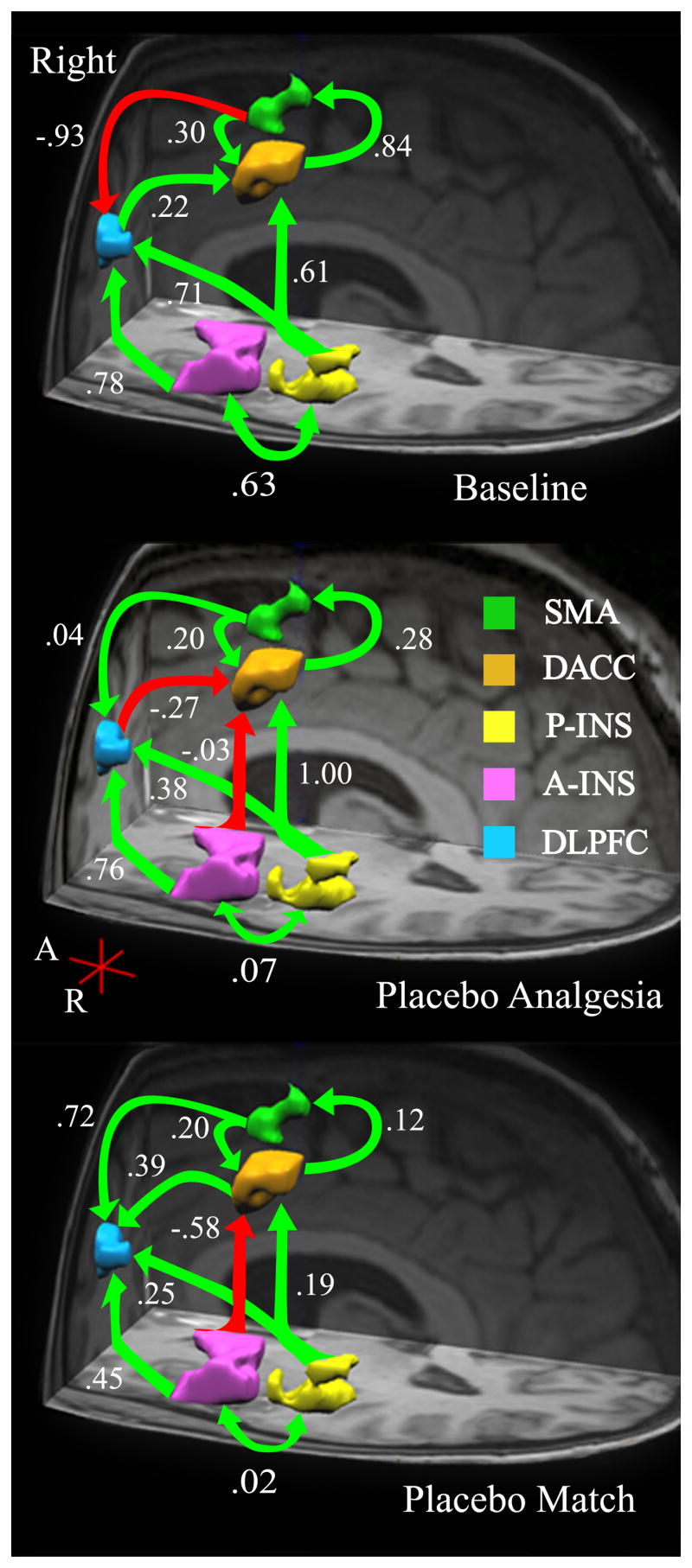
Right hemisphere: Anatomical model of a cognitive – affective pain-related network. Based on a priori hypotheses, brain regions in the right hemisphere (Table 1) were identified a regions of interest (ROIs). The interregional relationships among ROIs are shown here for the left hemisphere across three conditions: Baseline (B1 - top), Placebo (PA – middle), and Placebo Match (PM – bottom). Arrows indicate estimated direction of influence. Green arrows indicate positive path coefficients and red arrows indicate negative coefficients.
Functional MRI Data Reduction and Analyses
MRI data were acquired with a research-dedicated Siemens Allegra (3.0 Tesla) MR scanner using a standard head RF coil. High-resolution 3D anatomical images were acquired using a T1-weighted MP-RAGE protocol (128 1-mm axial slices; repetition time, TR = 2000ms; echo time, TE = 4.13ms; flip angle, FA = 8°, matrix = 256 × 256mm; field-of-view, FOV = 24cm). Functional images were acquired using a T2* gradient echo planar imaging (EPI) sequence which captured 33 axial slices of the whole brain parallel to the anterior commissure - posterior commissure (AC-PC) plane. Additional parameters were: (TR/TE = 2000 ms/30 ms), FA = 90°, FOV = 240 × 240 mm, 64 × 64 matrix; 3.75 mm isotropic voxels with 0.4 mm slice gap. Scan acquisition was time-locked to the stimulus onset. The first two volumes of each run were discarded at the scanner and two additional volumes were discarded during pre-processing to reduce saturation effects. All scanning conditions consisted of 7 functional runs, each with a “rest” period preceding a 20 second stimulus period.
fMRI data were processed using BrainVoyager (BVQX 1.6 - Brain Innovation, Maastricht, the Netherlands; http://www.brainvoyager.com). Image pre-processing consisted of re-sampling the functional data to 3mm3, rigid-body 3D motion correction using trilinear interpolation, slice-scan time correction with sinc interpolation, spatial smoothing with a 4-mm full-width at half maximum (FWHM) Gaussian kernel, voxel-wise linear detrending, and high-pass temporal filtering to remove nonlinear drifts below 3 Hz. Functional images were co-registered to a high resolution 3D anatomic volume and transformed into standard Talairach space (Talairach & Tournoux, 1988).
Following preprocessing, a Random Effects General Linear Model (RFX-GLM) was used to evaluate how well a convolved idealized hemodynamic response was predicted by the acquired BOLD signal (Smith, 2004). A linear contrast of the B1 and PA conditions established the presence of a placebo analgesic effect. A hypothesis driven approach was used to identify relevant clusters of voxels within each brain region believed to be critical for the cognitive - affective modulation of nociceptive input. Chosen clusters i) were thresholded at p <.05, ii) maintained a spatial-extent of at least 50-contiguous voxels, and iii) the Talairach coordinates for cluster’s center-of-gravity (mass) were in the expected brain region (see Table 1 for center-of-gravity coordinates).
For the network analyses, the data from each ROI was aggregated and extracted, resulting in a single data point for each volume of functional data. Per subject, this process was repeated in both hemispheres and all three conditions.
SEM Analysis of fMRI Data
Path analysis tests the plausibility of hypothetical relationships between/among variables. Statistically, the procedure estimates the coefficients corresponding to the structural equations that reflect the relationships being studied. An advantage of this technique is the ability to describe variable relationships in terms of i) direct, ii) indirect, and iii) the total effects a variable exerts on others. A direct effect is the unmediated influence of one variable on another. An indirect effect is the influence of variable on another that is mediated by at least one intervening variable. The sum of the direct and indirect effects yields the total effects. Importantly, the decomposition of model effects (i.e., path loadings) is always done with respect to a specific model. If the system of equations is altered by including or excluding variables and/or changing path specifications, the estimates of total, direct, and indirect effects may change (Bollen, 1989).
The consideration of the indirect effect leads to a more robust understanding of the relationship between variables. For example, the typical regression analysis provides an estimate of the direct effect of a variable. To ignore the indirect effects may obscure the true nature of the relationship between variables (Bollen, 1989).
LISREL methodology
Because our previous work showed that the placebo suggestion resulted in decreases in pain-related activity during stimulus presentation (Price, 2007), we believe the placebo effects may be the results of changes in the network nodes before and during the stimulation period. Thus, the entire time-series of BOLD measurements were extracted for each ROI.
Path Analysis
For all models the insula regions were classified as exogenous variables, while all the other ROI’s served as endogenous variables. The statistical model is consistent with an anatomical model of pain processing (Price, 2000) and our goal of modeling higher-order processing of pain-related information.
Model interpretation/Goodness of Fit
Goodness of Fit describes how well an identified model can reproduce the actual data. LISREL provides several fit indices for each model produced. For the present study, we used the Non-Normed Fit Index (NNFI), the Root Mean Square Error of Approximation (RMSEA), and the Standardized Root Mean Square Residual (SRMR).
Traditionally, the Normed Fit Index (NFI) is used to describe a null model (i.e., one in which the variable covariances are equal to zero). Obtained values of .90 – .95 are generally considered acceptable. Good fitting models are those with index values of .95 and above. The NFI is limited by the fact that no penalty is assessed for adding parameters to an established model. To correct this, the Non-Normed Fit Index (NNFI) was developed. The NNFI adjusts the NFI index by correcting for the χ2/df ratio in the model. Any obtained value greater than one is set to one, and the results are interpreted as with the NFI mentioned above.
The RMSEA fit index is based on the not centrality parameter. Good models have an RMSEA of .05 or less, while models with a value of .10 or greater are said to have poor fit. A confidence interval (CI) for this index is also computed. Ideally, the values for the 90% CI range from a low of zero to a high of about .1 or less.
The SRMR index is the standardized difference between the observed covariance and covariance being predicted. A value of zero indicates perfect fit. Values of .08 and below generally indicate a well fitting model.
Lisrel 8.7 was used to estimate the inter-regional connectivity among the selected ROIs for each condition. The cognitive – affective network models for the B1 condition were evaluated in terms of conceptual integrity, parsimony, and goodness-of-fit (Browne & Cudeck, 1993). Any subsequent changes to the initial (B1) models were needed for the identification of a model best fit data.
Results
Pain rating results
Compared to the Baseline condition, the pain ratings for the placebo condition were significantly lower and confirmed the presence of a placebo analgesic effect. Specifically, a repeated measures ANOVA for pain ratings and condition identified a significant main effect for condition (F(2,18) = 10.88, p < .001, partial η2 = .547; mean B1 = 52, PA = 36, and PM = 31), and trial (F(6,54) = p < .036, η2 = .214). As noted previously, there was an increase in the placebo analgesic effect following the first two stimuli. The increased placebo effect is likely the result of a self-reinforcing feedback loop. This feedback mechanism likely underpins the development and maintenance of the placebo effect and has been previously described elsewhere (Vase, Robinson, Verne, & Price, 2003, 2005; Verne, Himes et al., 2003).
Network Models
Structural equation modeling was used to determine whether pain-related bold signal would conform to an anatomical model of brain regions involved in processing the cognitive-affective components of pain. The stability of this hypothetical model was also tested in both hemispheres and across three conditions: Baseline (B1), Placebo Analgesia (PA), and Placebo-Match (PM).
Baseline Model
Our first task was to test how well the data from the left hemisphere (LH) of the Baseline condition fit an anatomical model of processing pain across 5 brain regions: the anterior insula (A-Ins), posterior insula (P-Ins), the dorsal aspect of the anterior cingulate cortex (DACC), and the supplementary motor area (SMA). Constraining the paths and direction of influence of this cognitive-affective network to be consistent with the model put forth by Price (2000) was successful. While all the hypothesized paths were present, the amount of influence from one region to another was either very modest (i.e., .18) or relatively strong (greater than or equal to .69). See Figure 1 (top).
After confirming our hypotheses with LH data, we examined whether this network structure fit the right hemisphere (RH) data. Imposing the LH network structure onto the right hemisphere did not yield an acceptable model. In fact, that structure failed to identify a model at all, as a solution would not converge. To identify an appropriate RH model, psychophysiological research, known anatomical connections, and theory were used to specify alternative models and were tested in an iterative process. This and all other models were chosen for two reasons. First, all of the estimated pathways conformed to anatomical and theoretical models of nociceptive information processing. Second, they accounted for the variance covariance structure in the data reasonably well, which was reflected in the Goodness of Fit indices.
Compared to LH, the final RH model contained many identical paths and their relative magnitudes of influence. However, notable change included the emergence of three new paths. The first went from the P-Ins to the DLPFC; the second was a reciprocal path from the SMA to the DACC; the third went from the SMA to the DLPFC. The first two pathways were positive in nature while the third was highly negative (i.e., −.93; See Figure 2 (top). Thus, while the B1 models of a cognitive-affective pain-related network in the LH and RH were similar, the additional RH paths indicate involvement in unique aspects of pain-related information processing.
Placebo Model
Placebos have been associated with decreased pain-related bold activation. Therefore, we next investigated whether these changes would lead to alterations in the B1 models of a cognitive-affective network for processing painful stimuli using data from the PA condition. The B1 network of the LH did not fit the PA data well. The final PA model for the LH was similar to the B1 network of the RH.
Compared to the B1 model, notable changes included a large shift in influence from the A-Ins to the DACC (β shift from .18 to −.71; See Figure 1 (middle). Additionally, a large decrease in influence of the A-Ins to the DLPFC (Δβ = .38) was also identified, and appeared to correspond with an increase of DLPFC → DACC influence (Δβ = .56). The dynamic changes in the A-Ins may have been partly responsible for changes to the integrity of the B1 network. Figures 1 and 2 (middle) also illustrates decreased influence of the DACC on the SMA (Δβ = 1.03). Similar to the B1 model of the RH, a reciprocal path from the SMA to the DACC emerged, as did an influential negative path from the SMA to the DLPFC (β = −.56). However, unlike the B1 model, the reciprocal SMA ↔ DACC paths were negative.
An additional path (A-Ins → DACC) was needed to identify the model that best fit the RH placebo data. Overall, only one path differentiated the LH and RH models of the pain-related cognitive-affective network, the P-Ins → DLPFC. That path aside, in the LH, the SMA ↔ DACC, and SMA to DLPFC relationships were negative, while in the RH they were positive (see Figure 2 - middle). These opposing relationships may have altered how the networks responded to painful stimuli (e.g., dampening the “fight or flight” response of the SMA), and thus enabled the placebo response.
Because the placebo suggestion decreased the anticipation of pain, and created an expectation of analgesia, the degree of similarity in the RH and LH models may reflect the convergence of these psychological processes at the time of stimulation, during which each stimulus was evaluated. Such a process would be consistent with the idea of an active self-reinforced feedback loop for the initiation and maintenance of a placebo effect.
Placebo-Match Model
Results from the PM condition revealed a striking departure from the B1 model of the LH (see Figures 1 and 2 - bottom). Specifically, the path from the A-Ins to the DLPFC, the DLPFC to the DACC, and the DACC to the SMA were no longer significant (i.e., present). Additionally, the LH model included a path from the A-Ins to the SMA (β = .22). Compared to the B1 model, the SMA to DACC and SMA to DLPFC paths both increased and became positive (β = .69 and .67 respectively).
The PM model of the RH also identified the loss of the DLPFC to DACC pathway, and a large reduction of influence from the P-Ins to the DACC (Δβ = .81). At the same time, the influence of the SMA on the DLPFC substantially increased (Δβ = .68), while the P-Ins to DLPFC pathway indicated and inverse relationship (see Figures 1 and 2 - bottom).
The reduced stimulus level used in the PM condition appears to be reflected in the changes to the network models. Thus, when compared to the placebo condition, the network changes identified in the PM data might reflect the psychological processes associated with the evaluation of non-painful visceral stimuli.
In summary, we successfully modeled a cognitive – affective network involved in processing pain-related information across several experimental conditions in both hemispheres. For each model, the parameter specification and estimated pathways yielded an acceptable fit for the data from each hemisphere and condition. See Table 2 for the Goodness of Fit Indices of each model. Tables 3 – 5 contain the direct, indirect, and total amount of influence exerted by each ROI for every model and condition.
Table 2.
Path analysis models and fit indices
| Goodness of Fit Indices | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Condition | Hemisphere | df | ?2 | ea | eaCI* | SRMR | NNFI | |
| 1 | Baseline | Left | 4 | 10.35 | .04 | .00 – .06 | .01 | 1.00 | |
| 2 | Right | 10 | 25.49 | .04 | .02 – .06 | .00 | 1.00 | ||
| 3 | Placebo | Left | 2 | 6.48 | .05 | .01 – .09 | .04 | 0.99 | |
| 4 | Right | 9 | 23.80 | .04 | .02 – .07 | .06 | 0.99 | ||
| 5 | Placebo Match | Left | 4 | 10.97 | .04 | .01 – .06 | .04 | 0.99 | |
| 6 | Right | 4 | 3.77 | .00 | .00 – .05 | .02 | 1.00 | ||
ea = Root Mean Square Error of Approximation (RMSEA, good models = .05 ).
eaCI = 90% confidence interval of the RMSEA (good models range from 0.0 to = 0.1)
SRMR = Standardized RMSEA, (i.e., the standardized difference between the observed covariance and covariance being predicted; good models = .08)
NNFI = Non-normed Fit Index (good models have values ˜ 1)
Table 3.
Regression Matrix of Estimated ROI Effects for the Baseline condition
| Baseline Model – ROI Relationships | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | ||||||||||
| Direct effects | |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | 0.80 | A-Ins | 0.63 | ||||||||
| P-Ins | 0.80 | P-Ins | 0.63 | ||||||||
| SMA | 0.90 | SMA | 0.84 | ||||||||
| DACC | 0.18 | 0.70 | 0.18 | DACC | 0.61 | 0.30 | 0.22 | ||||
| DLPFC | 0.69 | DLPFC | 0.78 | 0.71 | −0.93 | ||||||
| Indirect Effects | |||||||||||
|
| |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | A-Ins | ||||||||||
| P-Ins | P-Ins | ||||||||||
| SMA | 0.27 | 0.63 | 0.16 | SMA | 0.15 | 0.70 | 0.09 | 0.07 | 0.20 | ||
| DACC | 0.12 | DACC | 0.18 | 0.22 | −0.19 | 0.09 | 0.02 | ||||
| DLPFC | DLPFC | −0.14 | −0.65 | −0.08 | −0.85 | −0.19 | |||||
| Total Effects | |||||||||||
|
| |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | 0.80 | A-Ins | 0.63 | ||||||||
| P-Ins | 0.80 | P-Ins | 0.63 | ||||||||
| SMA | 0.27 | 0.63 | 0.90 | 0.16 | SMA | 0.15 | 0.70 | 0.92 | 0.20 | ||
| DACC | 0.30 | 0.70 | 0.18 | DACC | 0.18 | 0.83 | 0.09 | 0.24 | |||
| DLPFC | 0.69 | DLPFC | 0.63 | 0.06 | −0.85 | −0.19 | |||||
Note: For Tables 3–5 the numbers are standardized unidirectional loadings, except for the A-Ins and P-Ins, which is a correlation. Direction of influence is column → row. Figures 1 and 2 show direct effects. The mediated influence of one structure on another is an indirect effect (e.g., there is a relationship between the P-Ins → SAM, mediated by the DACC, and is − .70*.90 = .63). Total effects are the sum of direct and indirect effects, which may include reciprocal paths.
Table 5.
Regression Matrix of Estimated ROI Effects for the Placebo-Match condition
| Placebo–Match Model – ROI Relationships | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | ||||||||||
| Direct Effects | |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | 0.43 | A-Ins | 0.02 | ||||||||
| P-Ins | 0.43 | P-Ins | 0.02 | ||||||||
| SMA | 0.22 | 0.85 | SMA | 0.12 | |||||||
| DACC | −0.33 | 0.69 | DACC | −0.58 | 0.19 | 0.20 | |||||
| DLPFC | 0.67 | DLPFC | 0.45 | 0.25 | 0.72 | 0.39 | |||||
| Indirect Effects | |||||||||||
|
| |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | A-Ins | ||||||||||
| P-Ins | P-Ins | ||||||||||
| SMA | SMA | −0.07 | 0.02 | 0.02 | |||||||
| DACC | 0.15 | DACC | −0.01 | 0.01 | 0.01 | 0.02 | |||||
| DLPFC | 0.14 | DLPFC | −0.28 | 0.09 | 0.10 | 0.10 | |||||
| Total Effects | |||||||||||
|
| |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | 0.43 | A-Ins | 0.02 | ||||||||
| P-Ins | 0.43 | P-Ins | 0.02 | ||||||||
| SMA | 0.22 | SMA | −0.07 | 0.02 | 0.02 | 0.12 | |||||
| DACC | −0.18 | 0.85 | 0.69 | DACC | −0.59 | 0.20 | 0.21 | 0.02 | |||
| DLPFC | 0.14 | 0.67 | DLPFC | 0.17 | 0.35 | 0.82 | 0.49 | ||||
Discussion
Placebo manipulation decreases pain ratings and brain activation
In our previous work, examination of the BOLD signal revealed that the placebo response was active and identifiable within a few seconds of stimulus onset. This supported our hypothesis that reductions in pain-related brain activation resulted from the placebo manipulation rather than a response or report bias as others have suggested (Hrobjartsson & Gotzsche, 2001; Wager et al., 2004).
The observed placebo analgesic effect of that study was larger than in previous fMRI experiments, and was induced in IBS patients using a clinically relevant pain paradigm (Price et al., 2007). Contrary to other views (Hrobjartsson & Gotzsche, 2001, 2004, 2006), our previous work revealed large placebo effect sizes in IBS patients (Price et al., 2007; Vase et al., 2003, 2005; Verne, Robinson, Vase, & Price, 2003) and validated the significance and clinical relevance of this placebo manipulation for reducing pain. Those results highlighted a need for improved understanding of the underlying mechanisms associated with placebo analgesia, which were directly addressed in the present study.
Initial baseline model
Using a clinically relevant pain protocol for IBS and SEM, we have modeled the regional connectivity among five brain regions involved in the cognitive/affective components of pain processing. The proposed additional processing paths and interactions were based on theory, anatomical, neurological, and neurophysiological evidence (Price, 2000). This approach differs from other network studies where connections are identified via statistical analyses (Seminowicz & Davis, 2007). To our knowledge, this is the first confirmation of a cognitive-affective network involved in nociceptive information processing using SEM. The validation of the B-1 model in the left hemisphere provided justification for further examination of these processing paths in the right hemisphere. The integrity of these pathways and the existence of additional paths were investigated in the placebo and the placebo match conditions.
Compared to the left hemisphere model, all but 1 of the primary pathways (A-Ins → DACC) were intact in the right hemisphere. Further, three new paths were identified in the right hemisphere posterior insula → DLPFC, SMA → DACC, and SMA → DLPFC. Taken together, these models in the B-1 condition may reflect the mechanism by which a painful event is evaluated and placed within an emotional context to be used for later recall.
Placebo analgesia model
The changes from the B1 model for the PA condition in the left hemisphere indicate that the DACC, DLPFC, and SMA are dynamically involved in moderating a painful and/or analgesic experience, which is consistent with previous reports (Buchel et al., 2002; Kong et al., 2006; Kong et al., 2005; Petrovic & Ingvar, 2002). Specifically activity in the anterior insula became inversely related to that of the dorsal ACC; which may be related to a reduction in the anticipation of pain, lower input to the DACC (in the right hemisphere), and correspondingly lower output to the SMA.
Increased influence of DLPFC on DACC may also be related to a maintenance function. This would involve the recall and maintenance of the previous (B1) session to evaluate and compare the current set of stimuli. If the comparisons continue to confirm the expectation of an analgesic effect, this function could serve as the foundation of a self-reinforcing feedback loop underpinning the placebo effect. This mechanism has the potential to help explain increase in the placebo effect over time. The feedback loop would decrease the anticipation of pain and could be related to the decreased influence from the A-Ins to the DACC given the evidence for involvement of A-Ins in pain anticipation (Ardila, 1999; Brooks et al., 2005; Phillips et al., 2003). This explanation seems consistent with reports of the involvement of the DLPFC for context maintenance and working memory (Cohen et al., 1997), and expectation of pain relief (Lorenz, Minoshima, & Casey, 2003).
The congruence of influence-related changes in the left and right hemisphere models suggest a dynamic interhemispheric interplay. The interhemispheric convergence of cognitive and affective processes for a stimulus-by-stimulus evaluation of painful stimuli could increase the demand on working memory; likely reflected by the deviations from the B1 models.
Taken together, these models are consistent with our previous imaging work on the neural mechanisms underlying placebo analgesia (Price et al., 2007). That study showed that placebo manipulation led not only to a significant reduction in pain ratings, but also identified a corresponding decrease in neural activity across pain-related brain regions during the stimulus presentation. The dynamic changes in how the ROIs related to each other in the B1 and PA models presented here appear to reveal the potential of psychological constructs such as expectation for altering how one experiences pain.
Placebo match model
In the left hemisphere, the relationships among the P-Ins, A-Ins, and the DACC in the PM model are similar to those in the PA model. However, unlike the PA model, the path from DLPFC to the DACC is absent from both hemispheres during the PM condition. This is likely related to the decrease demand on the feedback mechanism as a result of the instruction set for this condition. The PM models may reflect the instruction efficacy to reduce anxiety and lower the expectation of pain.
Concluding remarks
Multiple studies support the view that afferent inhibition contributes to placebo analgesia and is associated with decreased pain-related neural activity (Fields & Price, 1997; Mayer & Price, 1976; Wager, 2005b). Our previous work has shown the effectiveness of this paradigm for reducing pain ratings and decreasing pain-related brain activation (Price et al., 2007). The models presented here provide additional insight into the coordination of multiple brain regions during the development and maintenance of a verbally induced placebo response. These findings represent an important next step in the understanding of pain perception because these models provide an indication of how cognitive and affective processes converge to alter the experience of pain.
This study investigated the underlying neural mechanisms of placebo analgesia. The combined use of chronic pain patients and a clinically relevant pain paradigm increased the ecological validity of our results. This is important because placebo effects are generally smaller in studies using healthy volunteers and experimentally induced pain (Kong et al., 2006). The apparent changes in how these brain regions interact following a placebo suggestion provides new insight into the neural mechanisms related to placebo analgesia. Taken as a whole, our models identify an important principle in the representation of pain. Specifically, the functional interactions within this cognitive-affective network change strength and even direction during conditions of pain and analgesia. Future studies are needed to relate these interactions to specific aspects of cognition and emotion.
Table 4.
Regression Matrix of Estimated ROI Effects for the Placebo condition
| Placebo Model – ROI Relationships | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left hemisphere | Right hemisphere | ||||||||||
| Direct Effects | |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | 0.52 | A-Ins | 0.07 | ||||||||
| P-Ins | 0.52 | P-Ins | 0.07 | ||||||||
| SMA | −0.13 | SMA | 0.28 | ||||||||
| DACC | −0.67 | 0.92 | −0.17 | 0.66 | DACC | −0.03 | 1.00 | 0.20 | −0.27 | ||
| DLPFC | 0.29 | −0.56 | DLPFC | 0.76 | 0.38 | 0.04 | |||||
| Indirect Effects | |||||||||||
|
| |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | A-Ins | ||||||||||
| P-Ins | P-Ins | ||||||||||
| SMA | 0.07 | −0.13 | 0.08 | −0.01 | −0.10 | SMA | −0.07 | 0.27 | 0.06 | 0.02 | −0.08 |
| DACC | 0.15 | 0.07 | −0.41 | 0.08 | 0.05 | DACC | −0.22 | −0.05 | 0.06 | −0.02 | |
| DLPFC | −0.04 | 0.07 | −0.04 | 0.08 | 0.05 | DLPFC | 0.01 | 0.01 | |||
| Total Effects | |||||||||||
|
| |||||||||||
| A-Ins | P-Ins | SMA | DACC | DLPFC | A-Ins | P-Ins | SMA | DACC | DLPFC | ||
| A-Ins | 0.52 | A-Ins | 0.07 | ||||||||
| P-Ins | 0.52 | P-Ins | 0.07 | ||||||||
| SMA | 0.07 | −0.13 | 0.08 | −0.14 | −0.10 | SMA | −0.07 | 0.27 | 0.06 | 0.30 | −0.08 |
| DACC | −0.52 | 0.99 | −0.58 | 0.08 | 0.71 | DACC | −0.25 | 0.95 | 0.20 | 0.06 | −0.29 |
| DLPFC | 0.25 | 0.07 | −0.6 | 0.08 | 0.05 | DLPFC | 0.76 | 0.39 | 0.04 | 0.01 | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A. The role of insula in language: an unsettled question. Aphasiology. 1999;13(1):79–87. [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Borum ML. Irritable bowel syndrome. Prim Care. 2001;28(3):523–538. vi. doi: 10.1016/s0095-4543(05)70051-8. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27(1):201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. [Google Scholar]
- Buchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22(3):970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nat Rev Neurosci. 2005;6(7):545–552. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- Fields HL, Price DD. The placebo effect: An interdisciplinary exploration. In: Harrington A, editor. Toward a neurobiology of placebo analgesia. Cambridge, MA: Harvard University Press; 1997. pp. 93–116. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344(21):1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Intern Med. 2004;256(2):91–100. doi: 10.1111/j.1365-2796.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Unsubstantiated claims of large effects of placebo on pain: serious errors in meta-analysis of placebo analgesia mechanism studies. J Clin Epidemiol. 2006;59(4):336–338. doi: 10.1016/j.jclinepi.2005.05.011. discussion 339–341. [DOI] [PubMed] [Google Scholar]
- Kim J, Zhu W, Chang L, Bentler PM, Ernst T. Unified structural equation modeling approach for the analysis of multisubject, multivariate functional MRI data. Hum Brain Mapp. 2006 doi: 10.1002/hbm.20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2005 doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(Pt 5):1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Price DD. Central nervous system mechanisms of analgesia. Pain. 1976;2(4):379–404. doi: 10.1016/0304-3959(76)90080-4. [DOI] [PubMed] [Google Scholar]
- Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109(1):40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates ‘pain networks’ defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123(3):244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95(1–2):1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Gregory LJ, Cullen S, Coen S, Ng V, Andrew C, et al. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126(Pt 3):669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288(5472):1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv. 2002;2(6):392–403. 339. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- Price DD, Craggs JG, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127(1–2):63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol. 2007;97(5):3651–3659. doi: 10.1152/jn.01210.2006. [DOI] [PubMed] [Google Scholar]
- Smith SM. Overview of fMRI analysis. Br J Radiol. 2004;77(Spec No 2):S167–175. doi: 10.1259/bjr/33553595. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14(11):1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. In: Co-Planar stereotaxic atlas of the human brain. Rayport M, translator. New York: Thieme Publishers Inc; 1988. [Google Scholar]
- Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109(6):1736–1741. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- Thompson WG. Irritable bowel syndrome: a management strategy. Baillieres Best Pract Res Clin Gastroenterol. 1999;13(3):453–460. doi: 10.1053/bega.1999.0039. [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105(1–2):17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115(3):338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103(1–2):99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105(1–2):223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Wager TD. Expectations and anxiety as mediators of placebo effects in pain. Pain. 2005a;115(3):225–226. doi: 10.1016/j.pain.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Wager TD. The Neural Bases of Placebo Effects in Pain. Current Directions in Psychological Science. 2005b;14(4):175–179. [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Cheskin LJ, Heller BR, Robinson JC, Crowell MD, Benjamin C, et al. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology. 1990;98(6):1485–1489. doi: 10.1016/0016-5085(90)91079-l. [DOI] [PubMed] [Google Scholar]