Abstract
The study investigated the effects of smoking a nicotinized or denicotinized cigarette on craving, affect and posttraumatic stress disorder (PTSD) symptoms while recalling neutral, stressful and traumatic events in smokers with and without PTSD. Smokers completed laboratory sessions during which they were presented with audiotapes of personalized scripts followed by smoking a cigarette. The effect of the script and cigarette conditions on dependent variables was evaluated. There was a main effect of script type across groups for smoking craving, negative affect and PTSD symptoms, with increased symptoms in trauma and stressful conditions. Responses were significantly higher in PTSD smokers. Smoking either cigarette type resulted in decreased craving, negative affect and PTSD symptoms in both groups. A second script presentation following smoking elicited similar responses, suggesting the ameliorative effect of having smoked a cigarette was short-lived. These results support that context and non-pharmacologic effects of smoking are important variables in smoking craving and mood, particularly in smokers with PTSD.
Keywords: smoking, PTSD, context
1. Introduction
Posttraumatic stress disorder (PTSD) is a prevalent and often chronic psychiatric disorder in the United States population. Approximately 7% of trauma-exposed individuals develop lifetime PTSD (Kessler et al., 2005). It has been reported that sixty percent of help-seeking individuals with PTSD smoke cigarettes compared to 23% of the general U.S. population, and help-seeking persons with PTSD are more likely to be heavy smokers (Beckham et al. 1997; Beckham 1999). Analysis of individuals in a large health maintenance organization dataset suggested that PTSD status is associated with a fourfold increase in the odds ratio of smoking (Breslau et al. 2003).
A high prevalence of smoking in the PTSD population is consistent with smoking data in individuals with other psychiatric illnesses. Despite representing 22% of the adult U.S. population, individuals with lifetime psychiatric conditions consume 44% of all cigarettes sold in the United States (Lasser et al. 2000). Between 50% and 80% of those suffering from a mental illness smoke, whereas less than 40% of those who have never had mental illness smoke (Lasser et al. 2000). These rates are similar to another epidemiologic study (Grant et al. 2004). Taken together, these results indicate that smoking represents a much greater health risk to psychiatric populations than it does to the general population.
Although there is growing evidence regarding factors that influence smoking and nicotine self-administration, there are significant gaps in identifying under what conditions nicotine effects are particularly reinforcing, and for whom they are reinforcing (Perkins 1999). In self-medication models of substance abuse (Khantzian 1997), the substance is thought to assist the individual in their efforts to regulate mood. Virtually all smokers, at least in part, attribute their smoking to anxiolytic and sedative properties of smoking (Benowitz et al. 1990; Brandon and Baker, 1991; Juliano and Brandon 2002) and smokers reliably report they smoke more when they are more anxious, angry, stressed, or sad (Shiffman 1993).
In evaluating the aggregate of data pertaining to smoking, stress, and negative affect in the maintenance stage of smoking, Kassel and colleagues (Kassel et al. 2003) determined that between-subjects studies with non-clinical samples support a positive association between smoking status and negative mood states. However, the within-subject data obtained through having smokers monitor mood and smoking during daily activity (designed to evaluate whether negative affect acts as a cue for smoking) is less clear, with some studies suggesting an association (Delfino et al. 2001; Shapiro et al. 2002; Beckham et al. 2004) and others not (Shiffman et al. 2002; Shiffman et al. 2004).
Experimental data designed to evaluate whether smoking reduces stress have been limited by failure to control for nicotine delivery (Gilbert 1995; Kassel and Shiffman 1997; Parrott 1999). Although it is often assumed that smoking reduces anxiety through the pharmacologic effects of nicotine (Picciotto 1998; Balfour and Ridley 2000; Di Chiara 2000), in studies that controlled for nicotine delivery (Gilbert et al. 1989; Kassel and Unrod 2000; Juliano and Brandon 2002), results have suggested that context (e.g., stressful movie, distracting task) and the presence/amount of nicotine can interact to affect anxiety self-report. However, no previous studies have examined this issue experimentally in smokers with PTSD.
There is limited evidence that smoking is associated with negative affect and PTSD symptoms in veterans with PTSD. In a descriptive study (Beckham et al. 1995), PTSD smokers (compared to PTSD nonsmokers), reported higher levels of PTSD symptoms, depression and trait anxiety. In a follow-up study, withdrawal symptoms in combat veterans with PTSD were demonstrated to be higher after presentation of trauma-related words than neutral words (Beckham et al. 1996). These results suggested that trauma-related stimuli may serve as a compelling cue for smoking in individuals with trauma exposure or PTSD, but there were several important study limitations including use of a smoking urges measure that overlapped to some degree with PTSD symptoms. A recent ambulatory study reported that PTSD symptoms, positive and negative affect and craving all served as potent antecedents to ad lib smoking in PTSD smokers, but not in non-PTSD smokers (Beckham et al. 2004), but this has not been evaluated in a controlled laboratory setting.
The purpose of the current study was to improve upon the limitations of the previous PTSD and smoking studies, as well as to evaluate the possible effect of smoking a cigarette with or without nicotine upon negative affect and PTSD symptom reduction. Script-driven imagery (Orr et al. 1993) was used to evaluate the effect of trauma, general stressor and neutral scripts on craving, negative affect and PTSD symptoms. Male and female smokers with and without PTSD were included. In addition, a measure of craving that did not present overlap with PTSD symptoms, the Questionnaire of Smoking Urges (QSU (Tiffany and Drobes 1991)), was used to examine the effects of smoking after each script condition. Finally, a standardized method to deliver nicotine was utilized in order to evaluate the unique effect of nicotine (Levin et al. 1989).
We hypothesized the following: Across sessions, PTSD smokers will demonstrate the highest level of symptoms (craving, negative affect and PTSD symptoms) to the trauma script, second to the general stressor script, and third to the neutral script. Non-PTSD smokers will demonstrate similar response patterns to the general stressor and trauma scripts, but the magnitude of the responses will be significantly lower than in PTSD smokers. Although non-PTSD smokers will experience reductions in craving and negative affect after nicotine administration as compared to the denicotinized cigarette, PTSD smokers will experience a greater reduction in magnitude across dependent measures. Nicotine but not denicotinized cigarettes will result in decrease of symptoms (craving, negative affect, and PTSD symptoms). Finally, a second presentation of stressful or trauma scripts after smoking will result in a symptom increase similar to the first script presentation.
2. Method
2.1 Participants
Participants were 129 smokers (82 with PTSD and 47 without PTSD). Participants were recruited through two types of advertisements, one describing an assessment study on smoking and the other an assessment study on smoking and trauma. Smokers were paid $400 for their participation ($50 for the screening interview, $50 for the script development session and $50 for each of the six laboratory sessions). To qualify, participants had to be at least 18 years of age, be able to read, and smoke at least 10 cigarettes per day. Each participant completed the following: demographic information (age, race, socioeconomic status (Hollingshead and Redlich 1958), years of education), the Structured Clinical Interview for DSM-IV criteria (SCID) for Axis I disorders (First et al. 1994), and the Clinician Administered PTSD Scale (CAPS) (Blake et al. 1995), a smoking history questionnaire, and the Fagerström Test of Nicotine Dependence (FTND) (Heatherton et al. 1991). Any participant meeting criteria for current alcohol or other substance dependence/abuse, or a current psychotic disorder (including schizophrenia and bipolar with active manic symptoms) was excluded. Participants recruited for the comparison group were excluded if they met criteria for lifetime PTSD. Otherwise, participants were allowed to meet criteria for any other DSM-IV disorder assessed by the SCID. A subset of these participants examining ad lib smoking (Beckham et al. 2004) and smoking topography (McClernon et al. 2005) have been reported elsewhere.
Current diagnoses were determined by a one-month time frame for PTSD, major depressive episode, and anxiety disorder, and a six-month time frame for current substance abuse/dependence and psychotic disorder. Each rater was trained using SCID and CAPS standardized training (i.e., manual, videotapes, and co-rating training with a trained rater). Interrater reliability for diagnoses based on videotapes of patient interviews was kappa = .96.
Two hundred and five smokers completed the screening interview. Fifty-seven were excluded for the following: lifetime, but not current PTSD (n = 21); current drug or alcohol abuse or dependence or positive drug screen (n = 18); contraindicated medical condition (i.e., COPD or stroke; n = 2); psychiatric condition (n = 15 [nine for current psychosis or schizophrenia; five for bipolar with current manic symptoms]); and currently suicidal (n = 1). One hundred forty-eight met enrollment criteria. Eleven never returned. One hundred thirty-seven completed at least one of the six experimental laboratory sessions. One hundred twenty-nine completed all sessions. The exclusion, dropout, and data completion rates are similar to previous laboratory and ambulatory studies conducted with this population (Beckham et al. 2000; Beckham et al. 2002). After complete description of the study to the subjects, written informed consent was obtained.
Although the original study design proposed three groups of smokers (PTSD; trauma, no PTSD; and no trauma, no PTSD group), over the course of the four years data was collected, only five out of 137 smokers did not report a previous trauma exposure, consistent with other research showing high levels of trauma exposure in the general population (Vrana and Lauterbach 1994; Kessler et al. 1995), and also suggesting that trauma exposure may be overrepresented in smokers (Breslau et al. 2003). Consequently, only two groups were analyzed (PTSD versus non-PTSD). There was a range of trauma exposure histories represented in the sample. For the sample by group, the following were endorsed: combat (12% in non-PTSD; 41% in PTSD); childhood physical or sexual assault (14% in non-PTSD; 12% in PTSD); adult sexual assault (0% in non-PTSD; 5% in PTSD); accident (17% in non-PTSD; 7% in PTSD); domestic violence (12% in non-PTSD; 16% in PTSD); sudden death of a loved one (29% in non-PTSD; 9% in PTSD); adult violence (14% in non-PTSD; 9% in PTSD); and natural disaster (2% in non-PTSD; 1% in PTSD).
Current medications were recorded and classified into the following groups: lower dose anticholinergic; alpha-adrenergic blockade; beta-blockade; and other anti-hypertensive medications (diuretics, ACE inhibitors, and calcium channel blockers); specific serotonin reuptake inhibitor (SSRI); heterocyclic anti-depressant; lithium; MAOI anti-depressant; other antidepressant; benzodiazepene; neuroleptic; or sedative-hypnotic. This classification system is based on our previous work (Beckham et al. 2000). For simplifying the description in the current study, categories were further classified as either psychiatric or cardiovascular.
Statistical comparisons for demographic, diagnostic characteristics, medications and smoking variables between PTSD and non-PTSD groups are reported in Table 1. The Bonferroni multiple comparison correction procedure was applied to these comparisons (.05 divided by 35 comparisons = .001). Smokers with PTSD were significantly more likely to be prescribed psychiatric medications, including selective serotonin reuptake inhibitors and other antidepressant medication, and consistent with Kessler and colleagues (Kessler et al. 1995) were more likely to meet criteria for current major depressive disorder, lifetime major depressive disorder and lifetime specific phobia, and less likely to be employed.
Table 1.
Participant Characteristicsa
| Variable | PTSD
(n=82) M(SD) |
non-PTSD
(n=47) M(SD) |
Test statistic | p |
|---|---|---|---|---|
| Age | 45.77(10.76) | 44.28(12.74) | t(110) = -0.70 | NS |
| Education | 13.25(2.11) | 14.33(2.29) | t(110) = 2.67 | NS |
| SES | 51.95(14.27) | 43.89(14.81) | t(110) = -3.02 | NS |
| P Veteran | 62 | 45 | NS | |
| P Female | 45 | 47 | NS | |
| P Minority | 66 | 55 | NS | |
| African American | 94 | 96 | ||
| Other (including Asian, Native American) | 6 | 4 | ||
| P Married | 45 | 33 | NS | |
| P Employed | 31 | 63 | .0004 | |
| Fagerström Score | 6.88(1.91) | 5.49(2.54) | t(110) = -3.19 | NS |
| Cigarettes per day | 26.09(12.77) | 21.07(11.05) | t(110) = -2.23 | NS |
| Total years smoked | 28.30(12.04) | 25.61(13.97) | t(110) = -1.14 | NS |
| Pack Years | 39.77(29.96) | 29.58(28.56) | t(110) = -1.87 | NS |
| P Heavy Smokers | 45 | 28 | NS | |
| P Current MDD | 46 | 9 | <.0001 | |
| P Lifetime MDD | 80 | 43 | <.0001 | |
| P Lifetime Alcohol Dep | 50 | 26 | NS | |
| P Lifetime Alcohol Abuse | 55 | 36 | NS | |
| P Lifetime Drug Dep | 32 | 23 | NS | |
| P Lifetime Drug Abuse | 40 | 26 | NS | |
| P Current Panic Disorder | 10 | 0 | NS | |
| P Lifetime Panic Disorder | 16 | 6 | NS | |
| P Current OCD | 5 | 2 | NS | |
| P Lifetime OCD | 7 | 2 | NS | |
| P Current Specific/Social Phobia | 35 | 13 | NS | |
| P Lifetime Specific Phobia | 43 | 15 | .001 | |
| P Current GAD | 2 | 2 | NS | |
| P Lifetime GAD | 2 | 4 | NS | |
| P Current Bipolar | 2 | 0 | NS | |
| P Lifetime Bipolar | 4 | 4 | NS | |
| P Current Dysthymia | 12 | 6 | NS | |
| P Lifetime Dysthymia | 17 | 9 | NS | |
| P Psychiatric Medication | 55 | 11 | <.0001 | |
| P Cardiovascular Medication | 46 | 37 | NS |
For the Hollingshead SES measure, scores for middle class range from 28-43 and scores for middle lower class range from 44-60. For the Fagerström Questionnaire, ≥ 7 is high nicotine dependence and ≤ 6 is considered low-moderate nicotine dependence
2.2 Script development session
Script-driven imagery is a well-validated and reliable procedure across PTSD populations (Pitman et al. 1987; Pitman et al. 1989; Pitman et al. 1990; Orr et al. 1993; Shalev et al. 1993; Rauch et al. 1996). This procedure can provide a format for the development of individualized trauma-related material, and this has been shown to elicit stronger psychophysiological and mood responses than generic scripts (Pitman et al. 1987). Additionally, a general stressor script was developed that was unrelated to trauma exposure (Orr et al. 1993). Following the diagnostic session (visit 1), the script development session (visit 2) was conducted as outlined by the Pitman research group (Pitman et al. 1987; Pitman et al. 1989; Pitman et al. 1990; Orr et al. 1993; Shalev et al. 1993). Six individualized scripts portraying actual experiences from the participant’s past were composed, including the two most traumatic experiences the participant could recall, two neutral experiences and two of the most stressful non-trauma experiences. Participants were first asked to describe each experience in writing on a script preparation form, and then to select from a “menu” of subjective visceral and muscular reactions appearing at the bottom of the form those that he remembered accompanied the experience. The clinician then reviewed the participant’s written responses and asked him/her to clarify or expand on the details when necessary. The clinician then composed a 30-second script that portrayed each experience in the second person, present tense, incorporating five different visceral and muscular reactions, or as many as the participants selected, whichever was fewer. Two scripts of each type were used in each laboratory session in order to control for possible repetition effects. During this session smokers also smoked a cigarette of their own brand through a smoking apparatus (Levin et al. 1989). Smoking topography (number of puffs and puff interval) was calculated and these values were used to replicate each individual’s smoking topography using the smoking apparatus in all subsequent sessions.
2.3 Symptom Measures
In order to measure craving, anxiety, and PTSD symptoms, the 11-item Questionnaire on Smoking Urges (QSU (Tiffany and Drobes 1991)), the Positive Affect and Negative Affect Schedule (PANAS), and the Davidson PTSD Trauma Scale (DTS (Davidson et al. 1997)) were repeated four times in each session. From the Davidson Trauma Scale (DTS), a score for reexperiencing, avoidance and numbing, and hyperarousal symptoms were calculated for each measurement occasion. The PANAS is a 10-item positive affect and 10-item negative affect scale designed to measure positive and negative affect. The QSU, DTS and PANAS each have strong reliability and validity (QSU (Cox et al. 2001); DTS (Davidson et al. 1997)); PANAS (Watson et al. 1988; Watson et al. 1988)). The shortened version of the QSU has been shown to be quite sensitive to smoking urges within a session using imagery in vivo cue exposure (Elash et al. 1995).
2.4 Experimental Design and Procedures
The study employed a 2 Drug (puffs from a denicotinized cigarette; puffs from a participant’s usual brand cigarette) × 3 Script Condition (trauma-related, general stress, and neutral) × 4 Time (initial baseline, post first script presentation, post smoking, and post second script presentation) within-subjects design, with the first two variables manipulated across six separate sessions held at least one hour apart and presented in Latin square order to control for sequence effects. The Time variable was repeated within each session. PTSD group was a between-subjects variable.
For each of the six experimental sessions (visits 3-8, following the diagnostic and script development sessions), participants were instructed not to smoke one hour prior to the laboratory session and asked to consume their usual caffeine, and not to consume alcohol or illegal drugs the day of the session. At the beginning of the session, each participant “smoked” through a specialized device. In order to allow each participant to inhale the smoke in a normal fashion, with a draw resistance comparable to that of a cigarette, a device developed by Levin and colleagues was used (Levin et al. 1989; Pomerleau et al. 1989). This device controls for sensory cues associated with cigarette smoking while allowing for assessment of the pharmacological effects of nicotine delivered. The use of the smokers’ own brand was selected. Although use of the smokers’ own brand did not control for absolute levels of nicotine across participants, it did allow for greater representation of each smoker’s regular nicotine dose. After smoking, participants waited for 30 minutes before beginning the baseline measurement and individualized script presentation. This interval was chosen to produce a modest increase in the desire to smoke yet prevent the onset of overt symptoms and signs of nicotine withdrawal (Spohr et al. 1979). Following the 30-minute period of nicotine deprivation, participants completed initial baseline symptom measurement (time 0 min in Table 2 study time line) and were presented with an initial audiotaped script (neutral, stressful or trauma-related).
Table 2.
Experimental Procedure Timeline
| 0 min. | 10 | 20 | 25 | 35 | 40 | 50 | 55 |
|---|---|---|---|---|---|---|---|
| Initial baseline
Anxiety, Craving, and PTSD Measures |
Trauma script,
OR General stress OR Neutral script |
Post first script
Anxiety, Craving, and PTSD Measures |
Nicotine
Manipulation: Nicotinized OR Denicotinized Cigarette |
Post-smoking
Anxiety and Craving, and PTSD Measures |
Trauma
OR General stress OR Neutral Script |
Post
second script Anxiety Craving, and PTSD Measures |
|
| Continuous Cardiovascular measures--------------------------------------------------------------------------- | |||||||
The procedure for the initial audiotaped script presentation was as follows: a brief instructional tape was played followed by a 30-second relaxation baseline and then the script was presented for 30 seconds. After it ended, participants were instructed to imagine the situation for an additional 30 seconds before being signaled by the experimenter to open their eyes. (Complete instructions are available from the first author upon request). The participant then completed post first script symptom measures, and then smoked a nicotinized or denicotinized cigarette through a smoking apparatus1. Post smoking symptom measures were completed. A second script of the same type as the initial script (trauma-related, general stress, or neutral) was then presented, followed by a final symptom measurement. The two scripts of the same type were presented in the same order in across the two different cigarette condition sessions.
3. Results
3.1 Evaluation of Covariates
Age, gender, ethnicity, nicotine dependence, co-morbid psychiatric disorders (current and lifetime other anxiety disorders, major depression, bipolar disorder, and dysthymic disorder; lifetime substance abuse/dependence since current substance abuse/dependence was an exclusion) and cardiovascular and psychiatric medications were evaluated as potential covariates by including each independently as a covariate in an analysis of variance for each independent variable (e.g., script-provoked craving). When one of these potential covariates had a significant effect on the independent variable2, it was entered as a covariate in the reported analysis. This strategy is in accord with that recommended by experts in the PTSD field (Keane and Kaloupek 1997).
3.2 Effects of Script-Driven Imagery
Our first goal was to determine whether the script-driven imagery procedure would produce the hypothesized effect on smoking craving, PTSD symptoms, and affect in the two groups. Separate 2 Group (PTSD, non-PTSD) × 3 Script (neutral, stressful, trauma) repeated measures analyses of covariance (ANCOVAs) were conducted on the ratings taken immediately after the first imagery script, controlling for baseline values reported prior to the first script. Post hoc follow-ups of Script effects were performed using an improved Bonferroni procedure (Simes 1986) to correct for multiple comparisons. Results for craving (Figure 1), negative affect (Figure 2), and PTSD B reexperiencing symptoms (Figure 3) are presented in Figures 1-3.
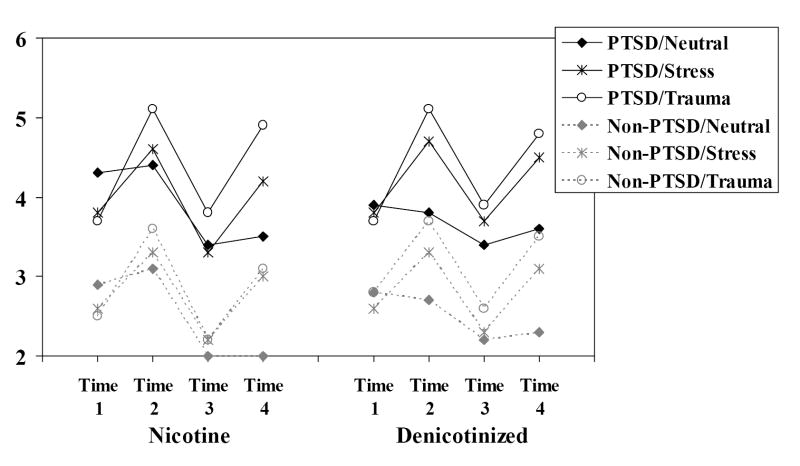
Figure 1.
Mean craving as a function of group, nicotine, and script conditions.
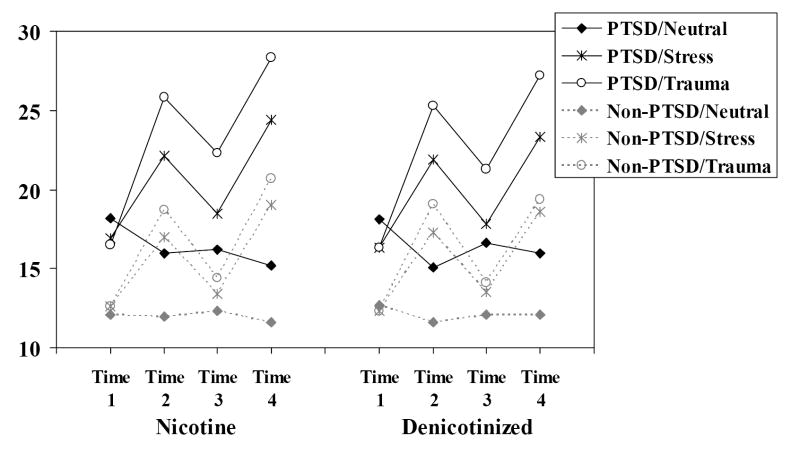
Figure 2.
Mean negative affect as a function of group, nicotine and script conditions.
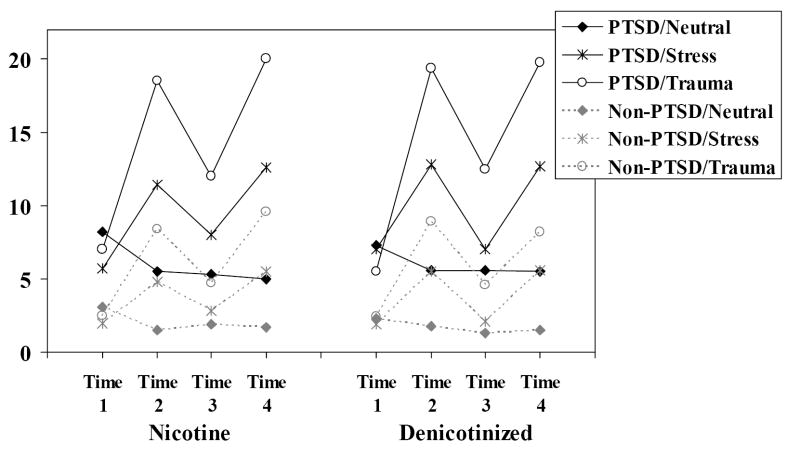
Figure 3.
Mean PTSD B reexperiencing symptoms as a function of group, nicotine and script conditions.
Trauma-related scripts produced greater cigarette craving and negative affect than did stress scripts, which in turn produced greater craving and negative affect than neutral scripts, (craving F[2,253] = 44.34; negative affect F[2,247] = 90.16, both p < .0001). Compared to neutral scripts, stress and trauma-related resulted in reduced positive affect, F(2,247) = 34.54, p < .0001. Further, individuals with PTSD showed a greater increase than did the no-PTSD group in negative affect for the stress and trauma scripts, Group × Script F(2,243) = 3.0, p < .05. There was also a Group × Script interaction for positive affect, F(2,247) = 3.0, p < .05; in this case both groups had similarly low positive affect for the stress and trauma cues but the PTSD group had lower positive affect during the neutral condition. Finally, across script types, the PTSD group reported more smoking urges, F(1,126) = 5.28, p < .03, and less positive affect, F(1,123) = 4.83, p < .03, than the no-PTSD group.
The pattern of data was the same for all three PTSD symptom cluster variables: the PTSD group reported higher symptoms overall, all Fs > 10.0, ps < .002; trauma-related scripts increased PTSD symptoms more than did stress-related scripts, which increased symptoms more than neutral scripts, all Fs >39.0, ps < .0001; and the PTSD group reported a greater increase in symptoms to the stress and (especially) the trauma-related cues than did the non-PTSD group, Group × Script, all Fs > 12.0, ps < .0001.
3.3 Effects of the Nicotine Manipulation
A second set of analyses was conducted to evaluate the effects of the nicotine manipulation (nicotinized versus denicotinized cigarette) on recovery from the effects of the emotional scripts. Figures 1-3 present the data for craving, negative affect and PTSD B (reexperiencing) symptoms. Note that Time 1 = prior to first script presentation; Time 2 = after first script presentation; Time 3 = after smoking a cigarette with or without nicotine; and Time 4 = after second script presentation. Each figure presents the data as a function of nicotine, condition, and time by group.
An analysis was conducted of the middle two time points on these graphs (from before to after the cigarette) in a Group × Script × Time (before cigarette, after cigarette; e.g., Time 2 vs. Time 3 × Cigarette condition (Nic/Denic) ANCOVA, using Time 1 data as a covariate (for other covariates in the analyses, see footnote 2). This allowed an examination, following the first script presentation, of recovery from the effects of the script as a function of smoking and nicotine (e.g., from 20 minutes to 35 minutes into the procedure in Table 2). There was a reduction in smoking craving from before to after the cigarette across groups, smoking conditions, and script conditions, Time F(1,127) = 141.29, p < .0001. There was an interaction between time and cigarette condition (F(1,127) = 10.53, p < .002), showing that smoking a nicotine cigarette reduced craving more than did smoking a denicotinized cigarette. There was also a Script × Time interaction, F(2,254) = 11.77, p < .0001, indicating that smoking reduced urges more following a trauma-related than a stress-related script, and more for a stress-related than a neutral script, which is not surprising given that urges were higher initially following the more negative scripts. This effect, however, did not interact significantly with the nicotine content of the cigarette3, F(2,254) = 2.40, p = .0924. The PTSD group continued to report greater craving overall (F(1,126) = 7.62, p < .01), though there were no differences in craving reduction between groups.
There was a decrease in negative affect after smoking overall, F(1,122) = 51.23, p < .0001, however, this was driven by substantial decreases in negative affect for the trauma and stressful conditions and a slight increase for the neutral script condition, Time × Script F(2,244) = 43.67, p < .001. The pattern was the same for PTSD or non-PTSD smokers and did not differ according to whether participants smoked nicotine or denicotinized cigarettes. In fact, the pattern of means suggested that smoking denicotinized cigarettes was slightly more successful in reducing negative affect after the stress and trauma scripts. Positive affect decreased slightly after smoking in the neutral script condition, but increased slightly after smoking for the stress and trauma script condition, F(1,122) = 17.25, p < .0001. Again, nicotine condition did not interact with this effect.
For PTSD reexperiencing symptoms, there was a significant reduction in symptoms in the trauma and stressful script conditions after smoking either cigarette type, Script × Time F(2,248) = 42.58, p < .0001, and the magnitude of reduction in both script conditions was significantly greater in the PTSD smokers compared to the non-PTSD smokers, Group × Script × Time F(2,248) = 3.08, p < .05. Further, across all three types of scripts and both groups, the denicotinized cigarette was marginally more successful at reducing PTSD reexperiencing symptoms, Cigarette condition × Time F(1,124) = 3.60, p < .07.
A similar pattern of results was observed for PTSD avoidance and numbing symptoms and hyperarousal symptoms. Significant reductions in symptoms occurred following either a nicotine or denicotinized cigarette in the trauma and stressful script conditions (Script × Time F(2,248) = 12.38, p < .0001 for avoidance/numbing and F(2,248) = 14.07, p < .0001 for hyperarousal) with the magnitude of this effect being greater in PTSD smokers (Group × Script × Time F(2,248) = 1.39, p < .26 for avoidance/numbing and F(2,248) = 3.51, p < .05 for hyperarousal). Nicotine content of the cigarette did not significantly influence these results.
Taken together, these results show that smoking reduces craving for cigarettes, and reduces the negative affect and PTSD symptoms associated with stress and trauma imagery (especially in people with PTSD). Additionally, nicotine in the smoked cigarette decreases craving for cigarettes and increases cardiovascular response, but does not help reduce PTSD symptoms or negative affect associated with processing negative images—in fact, there is some evidence that nicotine hinders reduction of traumatic reexperiencing symptoms after negative imagery.
3.4 Effects of Nicotine on Script Driven Imagery
A third set of analyses was conducted to evaluate the effects of nicotine on modulation of affect following re-introduction of traumatic imagery. This was accomplished in a series of ANCOVAs parallel to the first analysis: Group × Script × Cigarette condition (Nic/Denic), on Time 4 data (immediately after second script presentation) using Time 3 (e.g., the rating immediately prior to script presentation) as a covariate. For smoking urges, there was a main effect of Script showing that urges were lower following the neutral than stress condition, which were in turn lower than following the trauma condition, F(2,253) = 41.87, p < .0001. Similarly, the scripts had the same effects on positive and negative affect as were seen for the initial presentation (positive affect F(2,241) = 26.10, negative affect F(2,241) = 65.06, both p < .0001).
For PTSD symptoms, the results from the second script presentation paralleled results from the first script presentation: the PTSD group had higher symptoms overall; the trauma script prompted greater PTSD symptoms than the stress, which prompted greater symptoms than the neutral scripts; and the PTSD group in particular exhibited an increase in symptoms following the trauma script compared to the other scripts. This pattern of findings resulted in significant effects of Group, Script, and Script × Group, for all three PTSD symptom clusters (all effects F> 4.90, p < .02). There were no significant main effects or interactions involving nicotine on any of the variables.
4. Discussion
A number of relevant findings were observed: 1) trauma and stress related imagery intensified craving, negative affect, and PTSD symptoms in both PTSD and non-PTSD smokers; 2) contrary to our hypothesis, regardless of nicotine content, smoking reduced symptoms in both PTSD and non-PTSD smokers for smoking craving, negative affect, and PTSD symptoms in response to a trauma or stressful script; 3) there were between group differences in PTSD C symptoms (avoidance and numbing) and PTSD D (hyperarousal) symptoms such that trauma and stressful scripts increased these symptoms more in PTSD smokers than in non-PTSD smokers; and 4) PTSD symptom clusters were reduced in all smokers in the trauma script presentation for PTSD B (reexperiencing), PTSD C (avoidance and numbing) and PTSD D (hyperarousal) symptoms in all smoking conditions. Group differences in employment (e.g., psychiatric samples typically have lower employment) and co-morbid psychiatric disorders are consistent with previous epidemiological and experimental study reports (Beckham et al., 1998; Kessler et al., 2005).
Collectively, these results show that smoking craving, negative affect and PTSD symptoms vary by imagery context for both PTSD and non-PTSD smokers and when symptoms are increased, smoking a cigarette either with or without nicotine results in craving and symptom reduction. There were several group effects that are of interest in evaluating the effect of smoking in PTSD smokers. As expected, PTSD smokers experienced an increase in all three PTSD symptom clusters in response to a trauma script to a much greater extent than was found in non-PTSD smokers. In instances during which a significant response was observed following the first script presentation, a significant effect was again observed (compared to baseline) for the second script presentation. These results suggest that the palliative effects of cigarette smoking were temporary.
These data are consistent with a wealth of empirical data on cues and smoking behavior. Gilbert (Gilbert 1995) for instance, has asserted that the effect of smoking on mood state is a result of both situational demands and individual differences in personality and psychopathology (i.e., Situation × Trait Adaptive Response model - STAR). Our data are consistent with findings suggesting that the nonpharmacologic components of cigarette smoking (e.g., sensory cues such as inhaling smoke into the lungs) can, in part, mediate craving and drug-seeking behavior (Rose et al. 2003), and that compared to nicotinized cigarettes, denicotinized cigarettes can produce comparable reductions in craving (Gross et al. 1997; Pickworth et al. 1999; Rose et al. 2000).
This study results extend previous studies examining denicotinized and nicotinized cigarettes’ effect by also demonstrating that denicotinized cigarettes can reduce ratings of negative affect and PTSD symptoms. Expectations about the ability of smoking to reduce negative mood is an important variable: in normal smokers, Juliano and Brandon (Juliano and Brandon 2002) found that a nicotinized cigarette or instructions alone that the cigarette was nicotinized (when it was not) were each sufficient to reduce anxiety. In this study, participants were told they would be smoking nicotinized or denicotinized cigarettes, but not explicitly told which during which session. Data using nicotinized and denicotinized cigarettes in smokers without a psychiatric condition have reported discrepant findings, with a recent study reporting that smokers were able to easily discriminate between cigarette types across all visits (McClernon and Rose 2005) and another (Juliano and Brandon 2002) finding that only 12% of participants reported that they might have been deceived when given differing instructions and levels of nicotine in the experimental cigarette. Lack of measurement of expectations or evaluation of participants’ belief regarding the cigarette type in the current study limits the results: it would be useful in future studies of this type to vary and assess smoking-related expectancies as well as smokers’ ability to discriminate nicotine content.
The association between increased negative affect/PTSD symptoms and craving in the PTSD group is consistent with previous laboratory findings in which exposure to trauma cues increased urges to smoke (Beckham et al. 1996), daily diary monitoring data linking negative affect and PTSD symptoms to smoking behavior (Beckham et al. 2004) and a study showing that people suffer from smoking withdrawal symptoms consistent with their psychiatric symptomatology (Pomerleau et al. 2000). The findings of the present study suggest that, for both PTSD and non-PTSD smokers, smoking may represent a form of mood management, although the magnitude of the effect was stronger in the PTSD smokers.
A daily diary monitoring study examining essentially the same sample of smokers (Beckham et al. 2004) found no association between ad lib smoking and negative affect or PTSD symptoms in smokers without PTSD. Taken together with the current study, this indicates there may not be disorder-specific symptom-smoking associations, but that certain psychiatric subgroups may be more likely to use smoking as a coping response or simply deliberately increasing the yield of highly symptomatic smokers of any kind in ambulatory studies may allow a mood effect to be detected.
In contrast, the current study and a study using daily diary methods (Beckham et al. 2004), PTSD smokers reported significantly more negative affect and PTSD symptoms. Whalen and colleagues (Whalen et al. 2001) found that adolescent smokers spend a relatively high proportion of their day in negative mood states, and it also showed that psychiatric problems were highly associated with smoking among adolescents. However, mechanisms of detection of this association across naturalistic and experimental settings need further replication and investigation.
In summary, results of this study suggest that craving varies by imagery context in both PTSD and non-PTSD smokers, and that smoking a cigarette with or without nicotine can result in significant reductions in craving, negative affect and PTSD symptoms. The results provide support for both the role of nicotine and non-nicotine effects of smoking on craving, mood, and symptoms specific to PTSD. With the finding that craving, mood and PTSD symptoms are improved with denicotinized cigarettes, addressing the non-pharmacological effects of smoking in smoking cessation efforts is indicated. For example, use of more intensive interventions such as an integrated care model (Miles et al., 2005), a staged care intervention (Hall et al., 2006), or extinction methods using denicotinized cigarettes (McClernon, 2007), may be effective approaches in smoking cessation for smokers with PTSD. Taken together, the results suggest that addressing the behavioral effects of cigarette smoking in cessation treatment for this population will be vital to the success of treatment.
Acknowledgments
This work was supported by VA Merit Review MH-0011, and VA Merit Review MH-0012. NIDA K24DA016388, NIDA R21DA019704-01, NCI R01CA81595, VA Merit Review MH-0011, and VA Merit Review MH-0012. We thank those who kindly volunteered to participate in this study.
Footnotes
After completion of the preliminary analyses, it was discovered that there were no differences in the Drug (puffs from a denicotinized cigarette; puffs from a 1.0 mg nicotine research cigarette), thus raising the hypothesis that reductions in craving, negative affect and PTSD symptoms found after smoking were occurring merely because of the passage of time. An additional condition was included for eleven new participants included in the overall analyses, resulting in a 3 Drug (puff on denicotinized cigarette; smoke fresh smoke from a 1.0 mg nicotine research cigarette; passage of time equivalent to the average free smoke time for each participant) × 3 Script condition (trauma-related, general stress, and neutral) × 4 Time (initial baseline, post first script, post smoking baseline, and post second script).
Because of the large number of potential covariates tested on each of the independent variables, a criterion of p < .01 was used in deciding whether each should be used in the model. The variables ultimately used as covariates were as follows. First analysis set (Group × Script ANCOVA on the ratings immediately after the first imagery script): QSU— current substance abuse disorder; DTS total score and DTS re-experiencing symptoms score—current other anxiety disorder and lifetime other anxiety disorder; DTS avoidance symptoms score—current other anxiety disorder and currently taking psychiatric medication. Second analysis set (Group × Script × Nicotinized/ Denicotinized cigarette × Time [immediately after the first imagery script, after smoking for symptom ratings; during first script presentation, script 2 baseline immediately after smoking] ANCOVA): DTS total score and DTS reexperiencing symptoms score—current other anxiety disorder and lifetime other anxiety disorder; DTS avoidance symptoms score—lifetime other anxiety disorder. Third analysis set (Group × Script × Nicotinized/ Denicotinized cigarette × Time [for ratings: after smoking, immediately after the second imagery script; during second script presentation as change from second script baseline] ANCOVA): DTS total score and hyperarousal symptoms—current substance abuse disorder.
Exploratory analyses were conducted to evaluate the hypothesis that the mere passage of time (as opposed to the act of smoking) would result in a decrease of negative affect, PTSD symptoms and craving. A 2 Cigarette (cigarette versus no cigarette, passage of time) × 2 Time (Time 2 = after first script presentation; Time 3 = after smoking a denicotinized cigarette or the passage of time) ANOVA was conducted with eleven subjects (four PTSD, seven non-PTSD) who completed three additional laboratory sessions to measure the passage of time. There was a significant Smoking (cigarette versus no cigarette) × Time interaction such that administration of a denicotinized cigarette resulted in a significant decrease in craving: F (1,9)=11.97, p < .01; negative affect F(1,9) = 7.39, p < 05; compared to the passage of time, and a trend for a decrease in PTSD reexperiencing symptoms F(1,9) = 2.14, p = 0.177. Although this is only a partial examination of this hypothesis and must be further evaluated, it lends some support to the hypothesis that cigarette administration (compared to the passage of time) was responsible for the observed decrease in self-reported symptomatology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balfour D, Ridley DL. The effect of nicotine on neural pathways implicated in depression: A factor in nicotine addiction. Pharmacology Biochemistry & Behavior. 2000;66:79–85. doi: 10.1016/s0091-3057(00)00205-7. [DOI] [PubMed] [Google Scholar]
- Beckham JC. Smoking and anxiety in combat veterans with chronic posttraumatic stress disorder: A review. Journal of Psychoactive Drugs. 1999;31:103–110. doi: 10.1080/02791072.1999.10471731. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Feldman ME, Barefoot JC, Fairbank JA, Helms MJ, Haney TL, Hertzberg MA, Moore SD, Davidson JRT. Ambulatory cardiovascular activity in Vietnam combat veterans with and without posttraumatic stress disorder. Journal of Consulting & Clinical Psychology. 2000;68:269–276. doi: 10.1037//0022-006x.68.2.269. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Feldman ME, Vrana SR, Mozley SL, Erkanli A, Clancy CP, Rose JE. Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: A preliminary study. Experimental & Clinical Psychopharmacology. 2004 doi: 10.1037/1064-1297.13.3.219. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Moore SM, Feldman ME, Hertzberg MA, Kirby AC, Fairbank JA. Health status, somatization, and severity of posttraumatic stress disorder in Vietnam combat veterans with posttraumatic stress disorder. American Journal of Psychiatry. 1998;155:1565–1569. doi: 10.1176/ajp.155.11.1565. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, Davidson JRT, Fairbank JA. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addictive Behaviors. 1997;22:637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Lytle BL, Vrana SR, Hertzberg MA, Feldman ME, Shipley RH. Smoking withdrawal symptoms in response to a trauma-related stressor among Vietnam combat veterans with PTSD. Addictive Behaviors. 1996;21:93–101. doi: 10.1016/0306-4603(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Roodman AA, Shipley RH, Hertzberg MA, Cunha GH, Kudler HS, Levin ED, Rose JE, Fairbank JA. Smoking in Vietnam combat veterans with posttraumatic stress disorder. Journal of Traumatic Stress. 1995;8:461–472. doi: 10.1007/BF02102970. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Vrana SR, Barefoot JC, Feldman ME, Fairbank JA, Moore SM. Magnitude and duration of cardiovascular responses to anger in Vietnam veterans with and without posttraumatic stress disorder. Journal of Consulting & Clinical Psychology. 2002;70:228–234. doi: 10.1037//0022-006x.70.1.228. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P. Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott S, Russell MAH, Stolerman IP, editors. Nicotine Psychopharmacology: Molecular, cellular, and behavioral aspects. Oxford University Press; London: 1990. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered posttraumatic stress disorder scale. Journal of Traumatic Stress. 1995;8:75–80. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Baker TB. The smoking consequences questionnaire: The subjective expected utility of smoking in college students. Psychological Assessment. 1991;3:484–491. [Google Scholar]
- Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. In: Kirsch I, editor. How expectancies shape experience. American Psychological Association; Washington, D.C: 1999. pp. 263–299. [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:3–6. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Davidson JRT, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg MA, Mellman T, Beckham JC, Smith RD, et al. Assessment of a new self-rating scale for posttraumatic stress disorder: The Davidson Trauma Scale. Psychological Medicine. 1997;27:153–160. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Jamner LD, Whalen CK. Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine & Tobacco Research. 2001;3:235–248. doi: 10.1080/14622200110050466. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. European Journal of Pharmacology. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief imagery procedure: Self-report, psychophysiological and startle probe responses. Experimental & Clinical Psychopharmacology. 1995;3:151–156. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders. New York, NY: Biometrics Research Department; 1994. [Google Scholar]
- Gilbert DG. Smoking: Individual differences, psychopathology and emotion. Taylor and Francis; Washington, DC: 1995. [Google Scholar]
- Gilbert DG, Robinson JH, Chamberlin CL, Spielberger CD. Effects of smoking/nicotine on anxiety, heart rate, and lateralization of EEG during a stressful movie. Psychophysiology. 1989;26:311–320. doi: 10.1111/j.1469-8986.1989.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DF, Chou P, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Gross J, Lee J, Stitzer ML. Nicotine-containing versus denicotinized cigarettes: Effect on craving and withdrawal. Pharmacology Biochemistry & Behavior. 1997;57:159–165. doi: 10.1016/s0091-3057(96)00309-7. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich RL. Social class and mental illness. John Wiley; New York: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. Journal of Abnormal Psychology. 2002;111:88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Shiffman S. Attentional mediation of cigarette smoking’s effect on anxiety. Health Psychology. 1997;16:359–368. doi: 10.1037//0278-6133.16.4.359. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Unrod M. Smoking, anxiety, and attention: Support for the role of nicotine in attentionally mediated anxiolytics. Journal of Abnormal Psychology. 2000;109:161–166. doi: 10.1037//0021-843x.109.1.161. [DOI] [PubMed] [Google Scholar]
- Keane TM, Kaloupek DG. Comorbid psychiatric disorders in PTSD: Implications for research. Annals of the New York Academy of Sciences. 1997;821:24–34. doi: 10.1111/j.1749-6632.1997.tb48266.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rose JE, Behm F. Controlling puff volume without disrupting smoking topography. Behavior Research Methods, Instruments, & Computers. 1989;21:383–386. [Google Scholar]
- McClernon FJ. fMRI studies of craving and cue reactivity: Elucidating the role of bottom-up and top-down neural networks; Symposium presentation at the 13th Annual Meeting of the Society for Resaerch on Nicotine and Tobacco; Austin, Texas. 2007. [Google Scholar]
- McClernon FJ, Beckham JC, Mozley SL, Feldman ME, Vrana SR, Rose JE. The effects of trauma recall on smoking topography in posttraumatic stress disorder (PTSD) and non-PTSD trauma survivors. Addictive Behaviors. 2005;30:247–257. doi: 10.1016/j.addbeh.2004.05.013. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Rose JE. Mecamylamine moderates cue-induced emotional responses in smokers. Addictive Behaviors. 2005;30:741–753. doi: 10.1016/j.addbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Thompson CE, Yoshimoto D, Malte C, Straits-Troster K, Kanter E, Zhou AJ, Dougherty CM, Steele B. Improving the rates of quitting smoking for veterans with posttraumatic stress disorder. American Journal of Psychiatry. 2005;162:1311–1319. doi: 10.1176/appi.ajp.162.7.1311. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology. 1993;102:152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? American Psychologist. 1999;55:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Nicotine self-administration. Nicotine & Tobacco Research. 1999;1(suppl 1):133–137. doi: 10.1080/14622299050011951. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine & Tobacco Research. 2002;4:405–422. doi: 10.1080/1462220021000018425. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Common aspects of the action of nicotine and other drugs of abuse. Drug & Alcohol Dependence. 1998;51:165–172. doi: 10.1016/s0376-8716(98)00074-x. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effect of new denicotinized cigarettes. Nicotine & Tobacco Research. 1999;1:357–364. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, Altman B, de Jong JB, Herz LR. Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. Journal of Abnormal Psychology. 1990;99:49–54. doi: 10.1037//0021-843x.99.1.49. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, Clairborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Steketee GS. Psychophysiological investigations of posttraumatic stress disorder imagery. Psychopharmacology Bulletin. 1989;25:426–431. [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerleau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine & Tobacco Research. 2000;2:275–280. doi: 10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Rose JE. Methodological review: Controlled dosing of nicotine: A review of problems and progress. Annals of Behavioral Medicine. 1989;11:158–163. [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using Positron Emission Tomography and script-driven imagery. Archives of General Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacology Biochemistry & Behavior. 2003;76:243–250. doi: 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and non-nicotine components of cigarette smoking. Pharmacology Biochemistry & Behavior. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Pitman RK. Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with posttraumatic stress disorder. American Journal of Psychiatry. 1993;150:620–624. doi: 10.1176/ajp.150.4.620. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Jamner LD, Davydov DM, James P. Situations and moods associated with smoking in everyday life. Psychology of Addictive Behaviors. 2002;4:342–345. doi: 10.1037//0893-164x.16.4.342. [DOI] [PubMed] [Google Scholar]
- Sharon SM, Tsoph JY, Prochaska JJ, Eisendrath S, Rossi JS, Redding CA, Rosen AG, Meisner M, Humfleet GL, Gorecki JA. Treatment for cigarette smoking among depressed mental health outpatients: A randomized clinical trial. American Journal of Public Health. 2006;96:1808–1814. doi: 10.2105/AJPH.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Assessing smoking patterns and motives. Journal of Consulting & Clinical Psychology. 1993;61:732–742. doi: 10.1037//0022-006x.61.5.732. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Snys M. Immediate antecendents of cigarette smoking: An analysis from Ecological Momentary Assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: An analysis of unrestricted smoking patterns. Journal of Abnormal Psychology. 2004;113:166–171. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Spohr J, Hofman K, Steck W, Harenberg J, Walter E, Hengen N, Augustin J, Morel J, Koch A, Horsch A, et al. Evaluation of smoking-induced effects on sympathetic, hemodynamic and metabolic variables with respect to plasma nicotine and COHB levels. Arteriosclerosis. 1979;33:271–283. doi: 10.1016/0021-9150(79)90179-5. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Lauterbach D. Prevalence of traumatic events and posttraumatic psychological symptoms in a non-clinical sample of college students. Journal of Traumatic Stress. 1994;7:289–302. doi: 10.1007/BF02102949. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorder. Journal of Abnormal Psychology. 1988;97:346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affects: The PANAS scales. Journal of Personality & Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whalen CK, Jamner LD, Henker B, Delfino RJ. Smoking and moods in adolescents with depressive and aggressive dispositions: Evidence from surveys and electronic diaries. Health Psychology. 2001;20(2):99–111. [PubMed] [Google Scholar]