Abstract
Working memory (WM) tasks engage a network of brain regions that includes primary, unimodal, and multimodal associative cortices. Little is known, however, about whether task practice influences these types of regions differently. In this experiment, we used event-related fMRI to examine practice-related activation changes in different region types over the course of a scanning session while participants performed a delayed-recognition task. The task contained separate WM processing stages (encoding, maintenance, retrieval) and different materials (object, spatial), which allowed us to investigate the influence of practice on different component processes. We observed significant monotonic decreases, and not increases, in fMRI signal primarily in unimodal and multimodal regions. These decreases occurred during WM encoding and retrieval, but not during maintenance. Finally, regions specific to the type of memoranda (e.g. spatial or object) showed a lesser degree of sensitivity to practice as compared to regions activated by both types of memoranda, suggesting that these regions may be specialized more for carrying out processing within a particular modality than for experience-related flexibility. Overall, these findings indicate that task practice does not have a uniform effect on stages of WM processing, the type of WM memoranda being processed or on different types of brain regions. Instead, regions engaged during WM encoding and retrieval may have greater capacity for functional plasticity than WM maintenance. Additionally, the degree of specialization within brain regions may determine processing efficiency. Unimodal and multimodal regions that participate in both object and spatial processing may be specialized for flexible experience-related change, while those supporting primary sensorimotor processing may operate at optimal efficiency and are less susceptible to practice.
Keywords: event-related fMRI, learning, practice, plasticity
1. Introduction
The ability to adapt flexibly to new experiences with practice is a critical feature of human learning. While the cognitive processes that change as a task becomes practiced have been well explored (Anderson 1982, Logan 1988, Schneider & Shiffrin 1977), there is a limited understanding of how the underlying neural architecture may be specialized for practice-related change.
A number of possible mechanisms have been proposed to underlie neural plasticity related to practice, including changes to dentrite, synapse, and glial structure; and metabolic alterations (Kolb & Whishaw 1998, Sanes & Donoghue 2000). It is unknown, however, the extent to which these mechanisms may operate differently for brain regions that are more or less susceptible to practice-related plasticity. Mesulam (1998) has explicated a model of varying functional properties of brain regions in which high-level cognitive processes such as working memory (WM) engages a network of primary, unimodal, and multimodal cortical regions that corresponds to a continuum of increasingly abstract levels of processing. Each type of region in the network is thought to have a set of specialized adaptations, although multimodal regions (e.g. posterior parietal and prefrontal cortex) in particular are thought to be critical for the flexible, adaptive skills required for WM (Mesulam 1998).
In this experiment, we set out to examine practice-related functional plasticity across at network of brain regions supporting attentional and executive control processes thought to be required early in learning (Kelly & Garavan 2005, Schneider & Chein 2003). We also sought to investigate changes that occur during processing of different types of information (e.g. verbal, visual, and spatial), which differentially recruit different nodes of these attentional/executive networks (D’Esposito et al 1998, Postle & D’Esposito 1999, Wager & Smith 2003). While practice-related neuroplasticity has been reported within these networks, there is little agreement in the literature about whether some processes and brain regions are disproportionately influenced. We predict that functional changes in primary, unimodal, and multimodal brain regions would vary along a continuum such that primary regions would show the least amount of practice-related change and multimodal regions would show the most. To test this prediction we conducted an exploratory analysis of practice-related neural changes using a well-studied WM task (Smith et al 1995) that required the temporary retention of different types of information (object and spatial). Based on previous findings that WM encoding was influenced by practice to a greater extent than other WM processes (Landau et al 2004), we also used an event-related design to examine separate WM components (target/encoding; delay/maintenance; probe/retrieval).
2. Results
Behavioral
Participants performed a delayed recognition test with object and spatial tasks (Figure 1) for a total of 10 fMRI scanning runs. Object and spatial trials were performed in separate scanning runs (see Experimental procedure), and the object and spatial runs were ordered in a pseudorandom interleaved fashion such that practice-related changes could be examined across series of five runs for each task individually (e.g. object: 1, 4, 6, 7, 9; spatial: 2, 3, 5, 8, 10) and collapsed across tasks (e.g. collapsing across object and spatial trials for each of the five runs).
Figure 1.

Schematic depiction of the two tasks in the delayed recognition task. Both object and spatial trials began with a 2-sec encoding period where two stimuli were presented in different locations on the screen, followed by a 12-sec delay. During the probe period, one stimulus appeared and participants had to decide whether the stimulus matched either one of the shapes (for object trials) or one of the positions (for spatial trials) of the stimuli during encoding. Participants pressed a right button to indicate a match and a left button to indicate a non-match. The correct response is “match” for both object and spatial trials shown here.
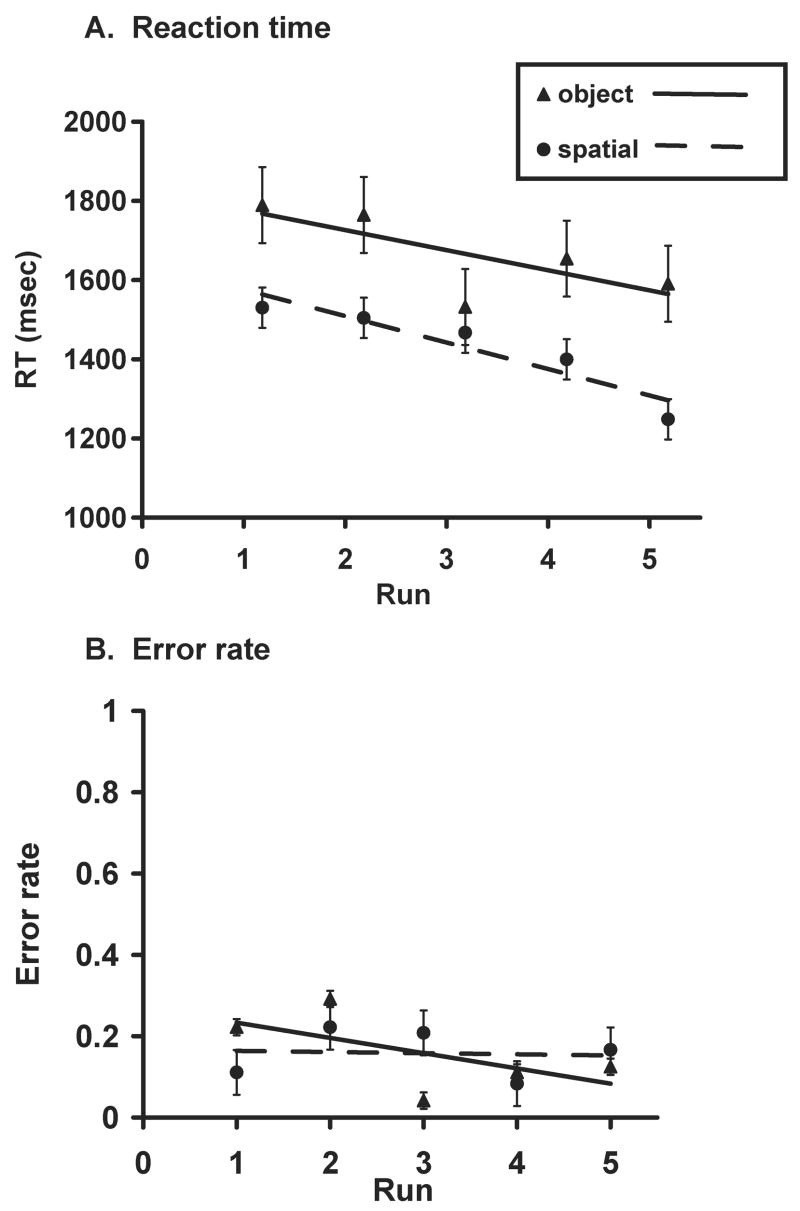
Figure 2 shows the change in RT and error rate over the course of the scanning session for object and spatial runs. An ANOVA with factors Practice (runs 1–5) and Task (object, spatial) showed a significant linear decrease in RTs across runs [F(1,8) = 7.86, p<0.03], collapsing across object and spatial trials. Decreases in RT for object and spatial trials separately were not significant. The mean RT, collapsed across runs, was 1666 ms for object trials and 1430 ms for spatial trials. The mean error rate was 0.15 for both object and spatial trials. Object trials had a higher mean RT than spatial trials [F(1,8) = 15.12, p<0.01], but there was no difference in accuracy between tasks [F(1,8) = 0.29, p>0.60] or across runs [F(1,8) = 1.16, p<0.30].
Figure 2.
Changes in (a) RT and (b) error rate with practice are shown separately for each set of five object and spatial runs.
Although the dissociation of object and spatial tasks per se was not the focus of this experiment, we did examine the RT and accuracy data to verify that participants attended selectively to object and spatial features. With respect to the influence of foils on accuracy and RT (see Experimental procedure), our results replicate the double dissociation shown by Smith et al. (1995). Briefly, within-subjects ANOVAs with factors Task (object, spatial) and Foil Difficulty (similar/dissimilar or near/far) revealed significant interactions for both object trials [F(1,8)=9.41, p<0.02] and spatial trials [F(1,8)=21.40, p<0.005]. RT was higher for similar than dissimilar foils for object trials, and higher for near than far foils for spatial trials. On object trials, RT was also higher for far foils. Similar to the RT data, ANOVAs performed on arcsine-transformed error rate scores revealed interactions of task and foil difficulty for object and spatial trials. Error rates for object trials were disproportionately higher for similar compared with dissimilar foils [F(1,8)=7.93, p<0.05], while error rates for spatial trials were disproportionately higher for far compared with near foils [F(1,8)=5.97, p<0.05].
Mapwise analysis: Identification of task-active regions
In order to test hypotheses about the relationship between practice and region type, we first identified a map of regions active across all trials relative to fixation for each trial period (target, delay, probe). As shown in Figure 3 and listed in Table 1, we found regions active across all three task periods (target, delay, probe) in bilateral occipital, parietal and frontal cortex. Regions of overlap across all three task periods included right middle prefrontal/premotor regions, and bilateral superior parietal regions.
Figure 3.

Regions significant for the main effect of task are shown for the target, delay, and probe periods. Regions are listed in Table 1 and were used to test for effects of practice over the course of the session.
Table 1. Functionally-defined regions of interest.
Locations of activation comprising statistical parametric maps of the main effect of task for each trial period (target, delay, probe). Regions are listed according to location, laterality, Brodmann Area (BA), type of region (primary, unimodal, or multimodal), cluster size of ROI, and any significant practice effect resulting from the ANOVA (see Experimental procedure). Significant effects of practice are all linear decreases.
| BRAIN REGION | R/L | subregion type | BA | #vox | practice |
|---|---|---|---|---|---|
| Target | |||||
| Postcentral gyrus | L | primary | 2,4 | 259 | |
| Postcentral gyrus | R | primary | 2,3,4 | 377 | |
| Precentral gyrus | L | unimodal | 6 | 1143 | 0.001 |
| Precentral gyrus | R | unimodal | 6 | 1263 | 0.003 |
| Mid/inferior occipital gyri | L | unimodal | 18,19 | 1447 | |
| Mid/inferior occipital gyri | R | unimodal | 18,19 | 1525 | |
| Superior frontal/Anterior cingulate gyri | L | multimodal -medial PFC | 8,24,32 | 527 | |
| Superior frontal/Anterior cingulate gyri | R | multimodal -medial PFC | 8,24,32 | 683 | 0.005 |
| Superior/inf parietal lobules | L | multimodal-parietal | 7,40 | 1402 | |
| Superior/inf parietal lobules | R | multimodal-parietal | 7,40 | 833 | 0.038 |
| Inferior temporal gyrus | L | multimodal-temporal | 37 | 71 | |
| Inferior temporal gyrus | R | multimodal-temporal | 37 | 228 | 0.032 |
| Middle/inferior frontal gyri | L | multimodal-lateral PFC | 9,44,45,46,47 | 410 | 0.007 |
| Middle/inferior frontal gyri | R | multimodal-lateral PFC | 9,44,45,46,47 | 863 | 0.008 |
|
| |||||
| Delay | |||||
|
| |||||
| Postcentral gyrus | L | primary | 2,3 | 273 | |
| Postcentral gyrus | R | primary | 2,4 | 87 | |
| Precentral gyrus | L | unimodal | 6 | 680 | |
| Precentral gyrus | R | unimodal | 6 | 646 | |
| Mid/inferior occipital gyri | L | unimodal | 19 | 85 | |
| Mid/inferior occipital gyri | R | unimodal | 19 | 213 | |
| Superior frontal/Anterior cingulate gyri | L | multimodal -medial PFC | 8,32 | 314 | |
| Superior frontal/Anterior cingulate gyri | R | multimodal -medial PFC | 8,32 | 112 | |
| Superior/inf parietal lobules | L | multimodal-parietal | 7,40 | 834 | |
| Superior/inf parietal lobules | R | multimodal-parietal | 7,40 | 560 | |
| Middle/inferior frontal gyri | L | multimodal-lateral PFC | 44,45,46,47 | 599 | |
| Middle/inferior frontal gyri | R | multimodal-lateral PFC | 44 | 110 | |
|
| |||||
| Probe | |||||
|
| |||||
| Postcentral gyrus | L | primary | 2,3 | 251 | 0.013 |
| Postcentral gyrus | R | primary | 2,3,4 | 691 | |
| Precentral gyrus | L | unimodal | 6 | 48 | |
| Precentral gyrus | R | unimodal | 6 | 31 | 0.002 |
| Mid/inferior occipital gyri | L | unimodal | 18,19 | 135 | |
| Mid/inferior occipital gyri | R | unimodal | 19 | 47 | 0.024 |
| Superior Frontal/Anterior Cingulate gyri | L | multimodal -medial PFC | 8,32 | 232 | |
| Superior frontal/Anterior cingulate gyri | R | multimodal -medial PFC | 8,32 | 231 | 0.045 |
| Superior/inf parietal lobules | L | multimodal-parietal | 40 | 522 | 0.045 |
| Superior/inf parietal lobules | R | multimodal-parietal | 7,40 | 1133 | 0.023 |
| Inferior temporal gyrus | L | multimodal-temporal | 37 | 88 | |
| Middle/inferior frontal gyri | L | multimodal-lateral PFC | 9,44 | 45 | |
| Middle/inferior frontal gyri | R | multimodal-lateral PFC | 44,45,46 | 361 | |
L, left; R, right; BA, Brodmann’s areas; obj, object; spl, spatial.
Indicates practice X task interaction such that spatial trials decreased disproportionately compared with object trials
Regional analysis
For our analysis of practice effects at the regional level, we first collapsed across all brain regions for each trial period. Repeated-measures ANOVAs with factor Practice (runs 1 - 5) and Task (object, spatial) for all three trial periods revealed a significant linear trend of run for the target period only, indicating that activation during this period decreased significantly over the course of the five runs (Target, p < 0.001; Delay, p = 0.128; Probe, p = 0.079). There were no cubic or 4th order (i.e. non-linear) changes in activation for any trial period, and no region showed a main effect of task or significant interaction with task during any trial period.
Next, cortical region types were examined in order to investigate our hypothesis that primary, unimodal, and multimodal regions would show increasing sensitivity to practice. Repeated-measures ANOVAs with factors Practice (runs 1 - 5) and Region Type (primary, unimodal, multimodal) were conducted for all trial periods and for all multimodal subregions (lateral frontal, medial frontal, parietal, and temporal). Effect sizes (partial eta squared) of the linear decrease across runs for different regions types is shown in Figure 4. The individual ROIs contributing to these parameter estimates in the figures are shown in Figure 3 and listed in Table 1. During the target, there were statistically significant decreases in activation for unimodal and multimodal regions (effect sizes: unimodal, 0.704; multimodal-lateral frontal, 0.663; multimodal-medial frontal, 0.653; multimodal-parietal, 0.342; multimodal-temporal, 0.392; all p < 0.01), but not for primary regions (0.253). During the delay, there were no significant practice-related effects. During the probe, only multimodal parietal regions showed a significant practice effect (0.418; p = 0.045).
Figure 4.
Effect sizes (partial eta squared) of the linear decrease in signal across runs 1 to 5 are shown for primary, unimodal, and multimodal regions during the three trial periods. Effect sizes that represented statistically significant decreases in activation across runs (p < 0.05) are indicated with an asterisk (*).
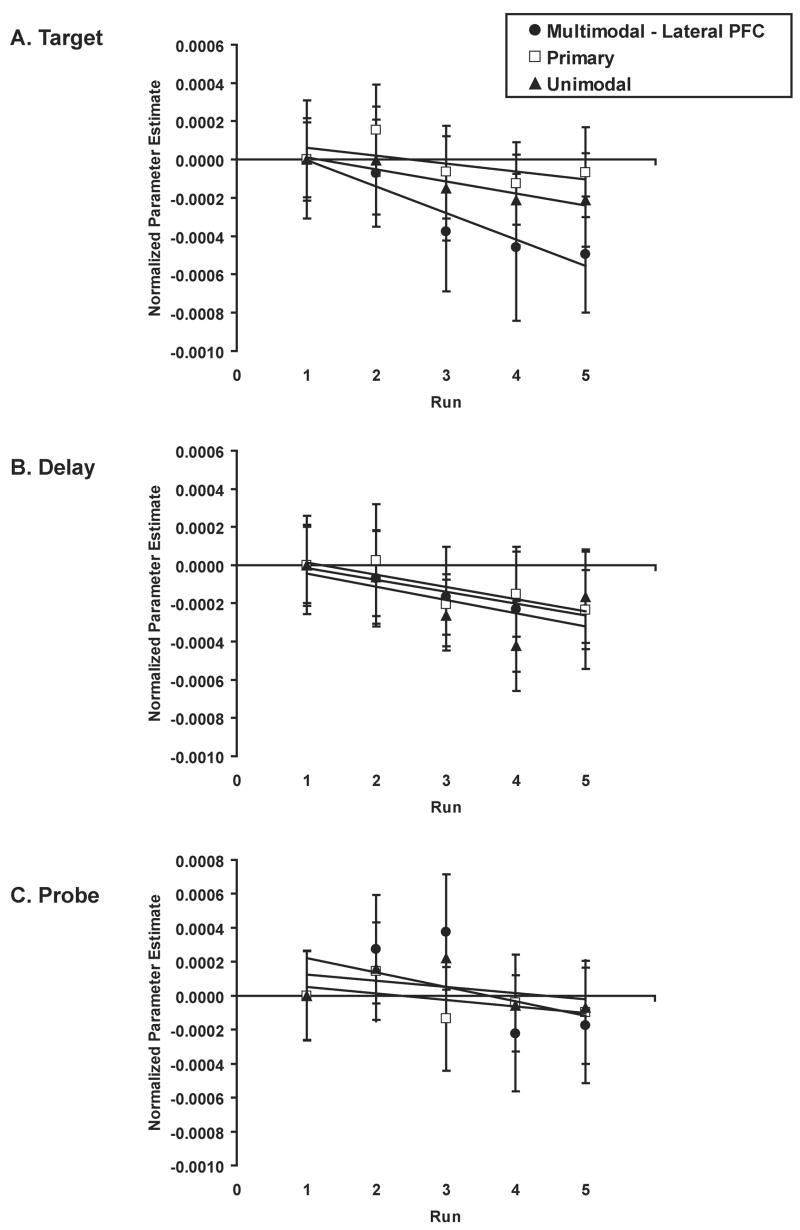
Finally, in order to determine whether different region types decreased at different rates with practice, we were also interested in interactions between Region Type and Practice. During the target, we observed such an interaction, and a follow-up 2-sample t-test revealed that the multimodal lateral PFC regions decreased disproportionately relative to the primary regions [F(1,10)=14.30, p=0.004]. Figure 5 illustrates the decreases across runs for primary, unimodal, and multimodal lateral PFC regions (other multimodal regions are not shown) for all three trial periods. As shown in Figure 5a, multimodal lateral frontal regions decreased from the first to the last run by 39.4%, unimodal regions decreased by 13.6%, and primary regions decreased by 8.1%. During the delay and probe periods, multimodal PFC regions did not decrease disproportionately relative to other region types (Figure 5b,c).
Figure 5.
Changes in parameter estimates across runs and during (a) target, (b) delay, and (c) probe trial periods averaged separately across primary and frontal multimodal subregions (see Experimental procedure). To illustrate relative changes in activation between region types across runs, parameter estimates were shifted so that parameter estimates overlapped at zero for run 1. Only frontal multimodal regions during the target showed a disproportionate practice-related decrease relative to primary regions. ROIs appear in Figure 3 and are listed in Table 1. Unimodal regions and other multimodal subregions in parietal, anterior cingulate, and temporal cortex were examined separately (not shown).
We also examined other multimodal regions for interactions with other regions across runs. During the delay, there were no significant effects of Practice or interactions with Region Type. During the probe, we observed a second Region Type by Practice interaction. A follow-up 2-sample t-test revealed that multimodal temporal regions decreased disproportionately with respect to primary regions [F(1,10)=7.32, p=0.024] (not shown).
Regions of interest (ROI) analysis: Main effect
In addition to collapsing across cortical region types, we characterized the individual ROIs making up those region types with respect to practice. Repeated-measures ANOVAs were carried out for individual ROIs, and the results of this analysis are listed in Table 1. During the target, middle/inferior frontal regions, bilateral precentral, right angular gyrus, right middle temporal gyrus, and right parietal regions showed significant decreases across runs. No significant practice effects were observed in any individual ROI during the delay. During the probe, right anterior cingulate/medial prefrontal regions, left postcentral gyrus, right precentral gyrus, bilateral parietal regions, right occipital regions showed significant decreases across runs.
Regions of interest (ROI) analysis: Task-specific regions
Task-specific regions were identified with the mapwise contrast of object vs. spatial trials. As shown in Figure 6 and listed in Table 2, during the target there were object-specific activations within bilateral occipital and left temporal regions, while there was widespread spatial-specific activation across bilateral frontal and parietal regions. During the delay, there was object-specific activation across a primarily left-lateralized network including motor, occipital, temporal, and inferior frontal regions. Spatial-specific activation was primarily right-lateralized, and was observed in right postcentral gyrus and parietal regions. Finally, during the probe, we observed primarily left-lateralized object-specific activation in left parietal and left superior/medial, and inferior frontal regions. Object-specific activation was bilateral and widespread, and was located bilateral postcentral, occipital, parietal, and temporal regions, medial PFC, and middle frontal gyri.
Figure 6.

Task-specific regions generated from the contrast Object vs. Spatial for target, delay, and probe trial periods. Object-specific regions are shown in red/yellow, spatial-specific regions are shown in blue/light blue. Regions are listed in Table 2.
Table 2. Task-specific regions.
Locations of activation comprising statistical parametric maps of the contrast Object vs. Spatial for each trial period (target, delay, probe). Regions are listed according to location, laterality, Brodmann Area (BA), type of region (primary, unimodal, or multimodal), and cluster size. Also shown are any significant p-values, representing a linear practice-related decrease resulting from the ANOVA (see Experimental procedure).
| BRAIN REGION | R/L | subregion type | BA | #vox | practice |
|---|---|---|---|---|---|
| Target: Object-specific | |||||
|
| |||||
| Mid/inferior occipital gyri | R | unimodal | 18,19 | 131 | |
| Mid/inferior occipital gyri | L | unimodal | 18,19 | 125 | |
| Middle temporal gyrus | L | multimodal – temporal | 37 | 59 | |
|
| |||||
| Target: Spatial -specific | |||||
|
| |||||
| Postcentral gyrus | L | primary | 2 | 16 | |
| Postcentral gyrus | R | primary | 2,3,4 | 272 | |
| Precentral gyrus | R | unimodal | 6 | 247 | 0.014 |
| Mid/inferior occipital gyri | R | unimodal | 19 | 27 | |
| Superior parietal lobules | R | multimodal-parietal | 7 | 50 | |
| Superior/inf parietal lobules | L | multimodal-parietal | 7,40 | 149 | |
| Middle frontal gyrus | L | multimodal-lateral PFC | 9 | 50 | |
| Middle frontal gyrus | R | multimodal-lateral PFC | 9 | 38 | |
| Superior frontal gyrus | R | multimodal -medial PFC | 8 | 37 | |
|
| |||||
| Delay: Object-specific | |||||
|
| |||||
| Postcentral gyrus | L | primary | 2,3,4 | 84 | |
| Mid/inferior occipital gyri | L | unimodal | 18,19 | 231 | |
| Inferior temporal gyrus | L | multimodal – temporal | 37 | 120 | |
| Middle temporal gyrus | R | multimodal – temporal | 21 | 111 | |
| Inferior frontal gyrus | L | multimodal-lateral PFC | 45 | 186 | |
|
| |||||
| Delay: Spatial-specific | |||||
|
| |||||
| Postcentral gyrus | R | primary | 2 | 46 | |
| Superior/inf parietal lobules | R | multimodal-parietal | 7 | 27 | |
|
| |||||
| Probe: Object-specific | |||||
|
| |||||
| Superior frontal/Anterior cingulate gyri | L | multimodal -medial PFC | 8, 32 | 53 | |
| Inferior frontal gyrus | L | multimodal -lateral PFC | 45 | 80 | |
| Inferior parietal lobule | L | multimodal-parietal | 40 | 36 | |
|
| |||||
| Probe: Spatial-specific | |||||
|
| |||||
| Postcentral gyrus | L | primary | 2 | 37 | |
| Postcentral gyrus | R | primary | 2,4 | 173 | |
| Mid/inferior occipital gyri | L | unimodal | 18,19 | 141 | |
| Mid/inferior occipital gyri | R | unimodal | 19 | 114 | +0.060 |
| Superior parietal lobules | L | multimodal-parietal | 7 | 114 | 0.037 |
| Superior/inf parietal lobules | R | multimodal-parietal | 7 | 505 | |
| Superior temporal gyrus | L | multimodal – temporal | 38 | 70 | |
| Superior temporal gyrus | R | multimodal – temporal | 38 | 308 | |
| Anterior cingulate gyrus | L | multimodal -medial PFC | 32 | 20 | |
| Anterior cingulate gyrus | R | multimodal -medial PFC | 8,32 | 163 | |
| Middle frontal gyrus | L | multimodal-lateral PFC | 9,46 | 173 | |
| Middle frontal gyrus | R | multimodal-lateral PFC | 9,46 | 136 | |
L, left; R, right; BA, Brodmann’s areas; obj, object; spl, spatial.
indicates a marginally significant effect (0.05 < p < 0.10)
Repeated-measures analysis of variance (ANOVA) on these task-specific ROIs revealed that most did not show effects of practice (see Table 2). There were two regions that did show practice-related decreases, one spatial-specific region in right precentral gyrus during the target, and the other spatial-specific region in the left superior parietal lobule during the probe. Both of these regions overlapped with main effect ROIs that showed effects of practice as well (Table 1).
3. Discussion
We examined the regional specificity of practice-related changes in activation over the course of a scanning session while participants performed a delayed recognition task with two trial types (object, spatial) and three trial periods (target, delay, probe). When activity was collapsed across all regions of the brain, there appeared to be significant decreases in activation across runs during the encoding stage of the task (e.g. target period). Further examination of different region types (primary, unimodal, multimodal cortex) revealed that multimodal and unimodal regions decreased to a greater extent than primary (e.g. somatosensory and motor) regions (shown in Figure 4). Practice effects interacted with region type such that multimodal lateral frontal regions decreased disproportionately relative to primary regions during the target (shown in Figure 5a), and temporal regions decreased disproportionately relative to primary regions during the probe. Finally, we identified task-specific regions by direct comparison of the spatial and object tasks, and activation in only two spatial-specific regions (target, right premotor; probe, left superior parietal) decreased with practice.
The decreases in activation that we observed are consistent with our predictions that unimodal and multimodal regions would exhibit greater practice effects than primary regions. However, practice-effects were only observed during the target (WM encoding) and probe (WM retrieval), but not during the delay (WM maintenance). Furthermore, the task-specific regions (e.g. object or spatial specific regions) appeared to show a lesser degree of sensitivity to practice, suggesting that these regions may be specialized more for carrying out processing within a particular modality than for learning-related plasticity. Overall, these findings suggest that multimodal and unimodal regions are more sensitive to practice than primary sensory regions, perhaps because they are specially adapted for flexible behavior.
Practice and behavioral performance
Activation changes occurred during both the target, as we have reported previously (Landau et al 2004), and during the probe period, indicating that increased efficiency of both encoding and retrieval processes may underlie the improvements in the speed of task performance. In other words, the improvement in reaction times is most likely primarily due to increasing neural efficiency related to encoding processes, and secondarily, to retrieval processes. However, several studies have shown that practice-related changes in activation are not necessarily accompanied by changes in behavioral performance (Landau et al 2004, Olesen et al 2004, Sayala et al 2006), indicating that it is not possible to determine a clear link between the activation decreases and the faster reaction times.
Characterization of region types
It is important to note that our method of collapsing across large regions of cortex (primary, unimodal, multimodal region types) did involve combining functionally heterogeneous areas, such as extrastriate and premotor cortex. This first type of analysis biased us against identifying effects within precise subregions of our ROIs, such as potential regional specialization of object and spatial processing within dorsal and ventral PFC as has been reported previously (Buchel et al 1999, Courtney et al 1996, Owen et al 1998, Sayala et al 2006). Sayala et al. (2006), for example, examined regions specializing in object and spatial processing for changes with practice, and reported that decreases for spatial-specific regions were more common than decreases for object-specific regions. Consistent with these results, we only idenitified practice-related effects in spatial specific regions (premotor region during the target and parietal regions during the probe). It should be noted, however, that identifying regions that specialize in object or spatial processing is complicated by the possibility of practice-dependent shifts in either laterality, e.g. from right to left PFC (Goldberg et al 1994) or in anatomy, e.g. from medial frontal to insular regions (Petersen et al 1998, Raichle et al 1994).
Nonetheless, our individual main effect ROI analysis was designed to reveal the individual characteristics of the ROIs that contributed to the overall effects we observed across primary, unimodal, and multimodal regions. This analysis revealed, for example, that although decreases were observed at the regional level for unimodal areas (Figure 4), these decreases were clearly driven by the premotor (and not extrastriate) unimodal ROIs (see Table 1). Overall, the ROI analysis was broadly consistent with the regional analysis. However, there were a number of individual ROIs during the probe period that showed significant practice-related decreases (Table 1), whereas only parietal regions reached significance at the regional level. The significance level and effect size of these practice effects, however, was lower than those during the target (see Table 1 and Figure 4), which may explain why they were less robust at the regional level than those during the target.
Practice and regional specificity
At the regional level, our findings support the hypothesis that practice influences brain regions differently depending on their capacity for top-down, adaptive function. Primary sensory and motor regions, which perform the least amount of top-down processing, showed the lowest sensitivity to practice, while unimodal and multimodal regions, which show increasingly higher levels of top-down processing, are maximally flexible. The ROI analysis revealed that premotor cortex was driving the practice effects observed for unimodal cortex overall, a finding that is consistent with the robust practice effects also shown by the neighboring multimodal lateral frontal regions. A spatial-specific region within left premotor cortex was also sensitive to practice in the task-specific ROI analysis.
The analysis of task-specific ROIs (Figure 6; Table 2) also provides some insight into the question of regional specificity. These ROIs differed from our main effect ROIs in that they were reliably active during object trials to a greater extent than spatial trials, and vice versa. Interestingly, the majority of these ROIs did not decrease over the session, even those located in multimodal and unimodal regions. Thus it appears that functional plasticity does not occur uniformly across the multimodal and unimodal network. Object-specific and spatial-specific subregions within this network may operate with less adaptability, since they are specialized for certain kinds of information, and show less sensitivity to practice.
Overall our findings are consistent with the Control System proposed by Schneider and Chein (2003) and with theories suggesting that multimodal regions participate in top-down modulatory and selection processes and are capable of greater flexible change, whereas primary sensory and motor regions participate in bottom-up perceptual and motor processes that may be less susceptible to adaptation (Mesulam 1998). Functional plasticity has also been reported during practice on a number of WM tasks in higher-level associative regions (Garavan et al 2000, Olesen et al 2004).
Electrophysiological experiments have supported the idea that neurons in the prefrontal cortex, in particular, are capable of highly flexible behavior depending on context and task demands (Funahashi 2001, Miller 2000). The disproportionate practice-related decreases in multimodal frontal regions compared with primary regions in our current experiment are a further example of adaptive flexibility to changing task demands. The activation decreases we observed are also consistent with the neural efficiency hypothesis, which proposes that the development of skill results in more efficient use of neural circuits and reduced activation (Haier et al 1992, Landau et al 2004, Neubauer et al 2004, Rypma & D’Esposito 1999). In our task, top-down, goal-driven attention mechanisms that are required during encoding and retrieval of object and spatial information may change as the task becomes well practiced, and this is reflected by decreases in activation. In contrast, perceptual and motor processes, associated with primary visual and motor cortex, are more stable from early to late in practice and thus show little activation change. Following theories of neural efficiency, perceptual regions (compared with multimodal and unimodal regions) may be maximally efficient because they are frequently used across multiple domains. In other words, perceptual regions may be less influenced by experience since they have reached optimal efficiency from consistent use.
Practice and WM processes
Why practice primarily influenced WM encoding and retrieval to a greater extent than maintenance is a question that remains open for further exploration. Early in the session, the task is novel and participants have not developed strategies to encode and retrieve the stimuli with optimal efficiency. As the task is performed repetitively throughout the session, participants learn to identify characteristics of the object locations or spatial positions that allow them to efficiently and effectively retrieve them. This increased efficiency during retrieval is also reflected in our behavioral data, in which subjects had faster RTs from early to late in the session. Thus, task practice and the development of strategies for stimulus encoding strongly influence top-down attentional mechanisms, such as encoding the salient stimulus-specific characteristics. WM maintenance also engages regions involved in top-down processing, but it is possible this processing is less strategic, or that the network may show a different type of practice-related change that we were not able to detect here, such as alterations in connectivity.
The current findings are in agreement with practice-related decreases we observed previously for a similar WM task (face recognition) in that these decreases were also specific to the encoding period (Landau et al 2004). Taken together, these data suggest that changes in activity may represent flexible changes in encoding and retrieval strategies that develop with experience on a variety of stimulus types. The delay period, in contrast, may be less susceptible to experience-dependent change. Furthermore, in the current paradigm, encoding may have been the most demanding phase of the task, since there were two stimuli that had to be attended to and encoded based on the relevant features (object shape or spatial position). During the probe period, only one object was presented for recognition, so the decision may have been more familiarity-based and therefore less dependent on strategy use than during encoding.
Temporal dynamics of experience-dependent WM networks
Our results provide evidence that unimodal and multimodal regions, in particular, play a critical role in top-down modulation of attentional processes related to learning. These processes are susceptible to modulation as strategies are developed over the course of the session and the task becomes well practiced. Additionally, our neuroimaging findings provide insight into the interpretation of our behavioral data in that they suggest the decreases in RT from early to late in the session may be a result of the successful implementation of strategies over the course of the session and subsequent improvement of the efficiency of WM encoding and retrieval.
The examination of changes in activation over time provides some potential methodological challenges and questions about interpretation. For example, we chose not to model changes in global signal as a nuisance variable, as is frequently done in fMRI studies, raising the possibility that fluctuations in scanner gain could have accounted for the practice effects we reported. While we cannot rule this out, we believe that this is unlikely because we did not find practice effects across all regions or across all trial periods, and offsets in scanner gain would have been expected to influence these factors somewhat uniformly.
This study contributes to a growing body of work showing that task experience may alter the contributions of brain regions, depending on their role in the specific or abstract nature of the task. Thus as a task becomes well practiced, strategy development and shifts in attention from specific to more general features may result in a change in the underlying network engaged by the task. Schumacher, Hendricks, and D’Esposito, for example, have reported differential effects of practice for right versus left dorsolateral prefrontal cortex with practice on a choice-reaction task over several scanning sessions (Schumacher et al 2005), suggesting that the functional topography associated with a task depends critically on participants’ level of task skill. The interaction of regional specialization and practice has important implications for WM studies that do not directly consider the role of task experience on neural activation. If unimodal and multimodal regions decrease more than sensory regions with task practice, then the overall task-active network may be biased early in the session toward identifying regions involved in unimodal and multimodal, integrative processing.
The fact that these effects occurred over a single scanning session raises the question of how different time courses of training would influence functional plasticity. Here, a key goal was to investigate the extent to which practice may play a role in “typical” non-practice fMRI studies, when subjects are not intentionally trying to improve their skills, but studies of task learning have used a variety of intentional learning paradigms, sometimes with scanning sessions occurring before and after an extended period of task practice. Studies using both motor and non-motor tasks have shown expansions in the cortical area engaged by the learned task after days or weeks of practice (Karni et al 1995, Olesen et al 2004), which is considerably different from the within-session decreases in activation that we and others have reported (Garavan et al 2000, Landau et al 2004, Sayala et al 2006). Thus, the implications of our results may not extend to longer time frame of practice, since short-term and long-term practice may engage different types of neural mechanisms (Karni et al 1998, Landau & D’Esposito 2006), although this issue clearly warrants further investigation.
In summary, the findings reported here highlight the importance of examining the temporal dynamics of brain activity, as opposed to a static activation “snapshot” of data that is collapsed across time and trial periods of a WM task. Strategies such as examining changes in a network of activation over time (Fletcher et al 1999) and changes in BOLD signal over a small number of trials (Yamaguchi et al 2004) provide insight into neural mechanisms than can be observed by examining a fixed activation profile. Examining dynamic neural properties using these techniques reveals a more complete view of the subtle and complex processes that occur with task repetition, strategy development, and behavioral flexibility. The results presented here complement these strategies and provide insight into the role of regional specialization in functional plasticity.
4. Experimental procedure
Participants
Eleven right-handed participants (mean age = 23.5, 9 male) were recruited from the University of California, Berkeley campus. All participants gave written, informed consent prior to participation in the study. Participants were screened against medical, neurological, and psychiatric illnesses, and for use of prescription medications. Behavioral data for two participants were lost due to technical difficulties.
Behavioral task
Figure 1 illustrates the trial periods of the object and spatial tasks. Each 30-s trial was composed of 1) a 2-second target period, 2) a 12-second delay period, 3) a 2-second probe period and 4) a 14-second inter-trial interval. During the target period, each participant saw two different images presented simultaneously. The objects were irregular polygons designed to be difficult to encode with verbal strategies (Attneave & Arnould 1956, Smith et al 1995). The two objects were presented in two of eight possible positions along the circumference of an imaginary circle that was centered about a fixation cross.
Object and spatial tasks differed only with respect to the instruction screen presented at the beginning of each object or spatial block of trials. For object trials, participants were instructed to attend to the shape of the objects; for spatial trials, participants were instructed to attend to the spatial position of the objects on the screen. During the delay period, a crosshair appeared at the center of the screen. During the probe period, a single object appeared and participants were required to give a motor response indicating whether it matched either one of the stimuli (shape of the stimulus for object trials; spatial position of the stimulus for spatial trials) presented at the target.
In order to ensure that participants’ attended selectively to object and spatial features according to the appropriate task, we manipulated the difficulty of the foil stimuli. There were two levels of foil difficulty: similarity in shape of foils to target stimuli (similar vs. dissimilar) and spatial proximity of foils to target stimuli (near vs. far). The similarity of shape foils were previously determined by Smith et al. based on similarity ratings performed in a pilot study. For object trials, the relevant factor was whether foils were similar or dissimilar to probe stimuli, and for spatial trials, the relevant factor was whether foils were near (in a neighboring stimulus position) or far (not in a neighboring position) relative to probe stimuli. Both factors were varied in both tasks, so the influence of these factors was related only to participants’ attention to either object or spatial features. There were equal numbers of targets and foils, and foil types (i.e., similar, dissimilar, near, far) were randomized and counterbalanced across runs.
Before performing the WM task described above, each participant also performed one fMRI run of a task used to derive an individual hemodynamic response function (HRF). During this run, a central white fixation cross changed briefly (200 ms) to a flickering checkerboard presented to the left or right hemifield every 20 s, cueing the participant to make a bilateral button press. Twenty such events occurred during the 400-s run.
MRI data acquisition
The fMRI scanning session consisted of 10 fMRI runs for each participant, plus an additional run for deriving the HRF as described above. Object and spatial trials were presented in separate blocks of trials. There were five runs for each type of task (object, spatial), and 8 trials per run, totaling 40 object trials and 40 spatial trials. Importantly, object and spatial runs were interleaved in a pseudorandomized way, and the order was fixed across subjects as follows: object (run1), spatial (run2), spatial (run3), object (run4), spatial (run5), object (run6), object (run7), spatial (run8), object (run 9), spatial (run10). Using this sequence of runs, practice-related changes could be examined across series of five runs for each task individually (e.g. object: 1, 4, 6, 7, 9; spatial: 2, 3, 5, 8, 10) and collapsed across tasks (e.g. collapsing across object and spatial trials for each of the five runs).
Functional and structural images were acquired with a Varian INOVA 4.0T scanner and a TEM send-and-receive RF head coil. Head movement was restricted using a foam cushion adjusted for each participant. Participants viewed a back-lit projection screen at their waist from within the magnet bore through a mirror mounted on the head coil.
Functional images were acquired using a 2-shot gradient echo EPI sequence (TR=2180, TE=28ms, matrix size = 64 × 64, FOV = 22.4 cm) was used to acquire data sensitive to the blood oxygen level dependent (BOLD) signal. Twenty axial slices of 3.5 mm voxels (with 0.5 mm interslice gap) were acquired. Each slice was acquired with a 22.4cm² field of view with a 64×64 matrix size resulting in an in-plane resolution of 3.5×3.5mm. This slice prescription allowed for whole-brain coverage. Data were acquired during 10 runs lasting 6 minutes each. Twenty seconds of dummy gradient and RF pulses preceded each scan to approach steady-state tissue magnetization. Two high-resolution structural T1-weighted scans were also acquired for anatomical localization. The first collected 20 axial slices in the same plane as the EPI images (TR = 200 ms, TE = 5msec, matrix size = 256 × 256, FOV = 22.4 cm). The second was a 3-D MPFLASH scan (TR = 9 ms, TE = 4.8 ms, T1 = 300 ms).
MRI data preparation
Off-line data processing was performed using the VoxBo analysis package (www.voxbo.org) and SPM2. Initial data preparation proceeded in the following steps: image reconstruction; sinc interpolation in time (to correct for the fMRI slice acquisition sequence); motion correction (six-parameter, rigid-body, least-squares alignment); slice-wise motion compensation (to remove spatially coherent signal changes via the application of a partial correlation method to each slice in time) (Aguirre et al 1998a, Zarahn et al 1997b).
Each participant’s brain was normalized to the Montreal Neurological Institute reference brain. Spatial normalization was performed as a two-step procedure: first, a structural image acquired to overlay the EPI images was coregistered to the high-resolution MPFLASH anatomical structural image. Second, this structural image was spatially normalized. The two resulting transformations were combined into a single transformation and used to spatially normalize the EPI images directly.
Data modeling
Images were smoothed with a 7mm FWHM Gaussian kernel and masked using a whole-brain mask to remove extraneous signal caused by ghosting. Since fMRI data are temporally auto-correlated under the null-hypothesis (Zarahn et al 1997b), statistical analyses were conducted within the framework of the modified general linear model (GLM) for serially correlated error terms (Worsley & Friston 1995). A time-domain representation of the expected 1/f power structure (Zarahn et al 1997b) and a notch filter that removed frequencies above the 0.24 Hz and below 0.02 Hz (i.e., the portions of highest power in the noise spectrum) were placed in the convolution matrix (Worsley & Friston 1995).
The rationale for empirically deriving a HRF is described elsewhere (Aguirre et al 1998b). A HRF was derived from primary sensorimotor cortex for each participant in the following manner. Each HRF was the trial-averaged response to 20 saccades and manual button presses that occurred during the HRF run as described above. These functional data were modeled using a Fourier basis set of four sines and four cosines. A partial F test was used to evaluate the significance of activity of voxels in primary motor cortex, and a HRF estimate was extracted from the suprathreshold voxels by averaging their time series. This empirical estimate of the HRF was used in subsequent analyses for each participant.
The general linear model (GLM) describes fMRI signal change as a series of amplitude-scaled and time-shifted covariates. Each covariate modeled a series of a brief neural events convolved by the participant’s empirical HRF. A set of covariates were used to model the target, delay, and probe periods for both object and spatial tasks. The covariates for each trial period, convolved with individual HRFs, modeled the data as follows: the target modeled t=0–2 sec of a trial; late target t=4–6 sec; delay t=12–14 sec; and probe t=14–16 sec. Additional nuisance covariates were included to model an intercept, trial-specific effects, late target (t=4–6 sec), and early delay (t=8–10 sec).
The nuisance late target/early delay covariates were included to avoid contamination of delay-related activation by variance that was not captured by the target covariate (Zarahn et al 1997a). Therefore, all delay-related activity reported in this analysis arises from the delay covariate and not the nuisance late target/early delay covariates.
Another set of covariates was used to model the three trial periods separately for each of the ten runs (5 object, 5 spatial) in the scanning session in order to examine incremental changes in signal across the session. Because we were interested in identifying low-frequency changes in signal over the course of the scanning session, additional covariates of no interest modeling the global signal in each run were not included.
Normalized whole-brain maps for each condition were calculated for each participant. Random-effects analyses were carried out by performing voxel-level t-tests on these maps.
Mapwise analyses
We identified a map of task-active regions for each trial period (target, delay, probe) by conducting one-tailed across-participants t-tests for activation relative to fixation baseline. These maps were thresholded using a peak criterion of p < 0.001, uncorrected, and applying a clusterwise correction of 54 voxels, accounting for smoothing (Cao 1999), resulting in an overall threshold of p<0.05.
We also generated a map of task-specific regions (object-specific, spatial-specific) based on the contrast of object vs. spatial trials. Target and probe maps were thresholded with a peak criterion of p < 0.005. Due to lower statistical power to detect activation during the delay period in the absence of stimuli, we used a peak criterion of p < 0.01. The same clusterwise threshold as used above (54 voxels) was applied to all maps.
Regional analyses
Functionally-defined individual ROIs were then defined by delineating local peaks of activity within each Brodmann Areas (BAs) of interest. A normalized template map containing locations of BAs that correspond to stereotaxic coordinates (Drury et al 1999) was used to constrain the ROIs within BAs. This template map was plotted on the MNI/ICBM anatomical template (http://www.psychology.nottingham.ac.uk/staff/cr1/lesion.html#brod) and visualized with MRIcro (Rorden & Brett 2000). Using this method, sets of functionally-defined ROIs based on BAs were defined for each trial period and are listed in the Tables. Mean parameter estimates of voxels within each ROI for each scanning run were obtained for each participant.
To examine differences in practice effects between primary, unimodal, and multimodal regions, we collapsed across ROIs based on the cytoarchitectonic properties of the neurons in particular regions as well as their functional roles (Mesulam 1998). Specifically, we grouped ROIs based on whether they were in primary cortex, which consisted of bilateral primary somatosensory, motor (BAs 2, 3, 4) and visual (BA 17) regions; unimodal cortex, which consisted of premotor (BA 6) and extrastriate (BAs 18, 19) regions; or multimodal cortex. Because of the heterogeneity of multimodal cortex, we carried out analyses separately for multimodal subregions as follows: lateral frontal multimodal cortex included BAs 9, 44, 45, 46, 47; parietal multimodal cortex included BAs 7, 40; medial frontal multimodal cortex included BAs 8, 24, 32; and temporal multimodal cortex included BAs 21, 37, 38.
Repeated measures ANOVAs were all conducted at α = 0.05 on mean parameter estimates as described in the Results. Because we were interested in any kind of systematic change in activation over the course of the session, we examined significant linear and/or higher order (i.e. quadratic, cubic, or fourth order effects) effects of practice for each region. We observed no significant higher order effects, so all reported practice effects are significant linear monotonic decreases.
Regions-of-interest (ROI) analyses
Individual ROIs from both the main effect and object vs. spatial analyses were also probed for effects of practice. As can be seen in Tables 1 and 2, individual ROIs were grouped when they had some general functional commonalities and functional clusters tended to overlap across BAs (e.g. BAs 2,3,4 and BAs 18,19) in order to reduce the number of statistical tests being conducted. ROIs smaller than the extent criterion (54 voxels) sometimes resulted when a region from the mapwise analysis extended between two heterogeneous ROIs (e.g. BAs 2,3,4 and BA 6). Repeated measures ANOVAs were then conducted at α = 0.05 as described in the Results. Again, we observed no significant higher order (i.e. nonlinear) effects of practice.
Acknowledgments
We thank Leon Deouell, John Jinks-Ollinger, and Rayhan Lal for their assistance with analysis of the imaging data. This research was supported by a pre-doctoral fellowship from the National Science Foundation and grants from the National Institute of Health (NS 40813 and MH63901) and Dana Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D’Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage. 1998a;8:302–6. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998b;8:360–9. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Acquisition of cognitive skill. Psychological Review. 1982;89:369–406. [Google Scholar]
- Attneave R, Arnould MD. Methodological considerations in the quantitative study of shape and pattern in perception. Psychological Bulletin. 1956;53:452–71. doi: 10.1037/h0044049. [DOI] [PubMed] [Google Scholar]
- Buchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283:1538–41. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Cao J. The size of the connected components of excursion sets of X2, t, and F fields. Advances in Applied Probability. 1999;31:577–94. [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Drury HA, Van Essen DC, Corbetta M, Snyder AZ. Surface-based analyses of the human cerebral cortex. In: Toga A, editor. Brain Warping. Academic Press; 1999. pp. 337–63. [Google Scholar]
- Fletcher P, Buchel C, Josephs O, Friston K, Dolan R. Learning-related neuronal responses in prefrontal cortex studied with functional neuroimaging. Cereb Cortex. 1999;9:168–78. doi: 10.1093/cercor/9.2.168. [DOI] [PubMed] [Google Scholar]
- Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res. 2001;39:147–65. doi: 10.1016/s0168-0102(00)00224-8. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Podell K, Lovell M. Lateralization of frontal lobe functions and cognitive novelty. Journal of Neuropsychiatry. 1994;6:371–8. doi: 10.1176/jnp.6.4.371. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Jr, MacLachlan A, Soderling E, Lottenberg S, Buchsbaum MS. Regional glucose metabolic changes after learning a complex visuospatial/motor task: a positron emission tomographic study. Brain Res. 1992;570:134–43. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, et al. The acquisition of skilled motor performance: fast and slow experience- driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–8. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human Functional Neuroimaging of Brain Changes Associated with Practice. Cereb Cortex. 2005 doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Brain plasticity and behavior. Annu Rev Psychol. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- Landau SM, D’Esposito M. Sequence learning in pianists and nonpianists: an fMRI study of motor expertise. Cogn Affect Behav Neurosci. 2006;6:246–59. doi: 10.3758/cabn.6.3.246. [DOI] [PubMed] [Google Scholar]
- Landau SM, Schumacher EH, Garavan H, Druzgal TJ, D’Esposito M. A functional MRI study of the influence of practice on component processes of working memory. Neuroimage. 2004;22:211–21. doi: 10.1016/j.neuroimage.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Logan GD. Automaticity, resources, and memory: theoretical controversies and practical implications. Hum Factors. 1988;30:583–98. doi: 10.1177/001872088803000504. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Grabner RH, Freudenthaler HH, Beckmann JF, Guthke J. Intelligence and individual differences in becoming neurally efficient. Acta Psychol (Amst) 2004;116:55–74. doi: 10.1016/j.actpsy.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–9. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci U S A. 1998;95:7721–6. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci U S A. 1998;95:853–60. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. “What”-Then-Where” in visual working memory: an event-related fMRI study. J Cogn Neurosci. 1999;11:585–97. doi: 10.1162/089892999563652. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci U S A. 1999;96:6558–63. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Sayala S, Sala JB, Courtney SM. Increased neural efficiency with repeated performance of a working memory task is information-type dependent. Cereb Cortex. 2006;16:609–17. doi: 10.1093/cercor/bhj007. [DOI] [PubMed] [Google Scholar]
- Schneider W, Chein JM. Controlled and automatic processing: behavior, theory, and biological mechnisms. Cognitive Science. 2003;27:525–59. [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Schumacher EH, Hendricks MJ, D’Esposito M. Sustained involvement of a frontal-parietal network for spatial response selection with practice of a spatial choice-reaction task. Neuropsychologia. 2005;43:1444–55. doi: 10.1016/j.neuropsychologia.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher E, Minoshima S. Spatial versus object working memory: PET Investigations. Journal of Cognitive Neuroscience. 1995;7:337–56. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci U S A. 1998;95:883–90. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–8. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D’Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24:5356–63. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997a;6:122–38. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D’Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions. Neuroimage. 1997b;5:179–97. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]