Abstract
A Bayesian method for functional connectivity analysis was adapted to investigate between-group differences. This method was applied in a large cohort of almost 300 children to investigate differences in boys and girls in the relationship between intelligence and functional connectivity for the task of narrative comprehension. For boys, a greater association was shown between intelligence and the functional connectivity linking Broca’s area to auditory processing areas, including Wernicke’s areas and the right posterior superior temporal gyrus. For girls, a greater association was shown between intelligence and the functional connectivity linking the left posterior superior temporal gyrus to Wernicke’s areas bilaterally. A developmental effect was also seen, with girls displaying a positive correlation with age in the association between intelligence and the functional connectivity linking the right posterior superior temporal gyrus to Wernicke’s areas bilaterally. Our results demonstrate a sexual dimorphism in the relationship of functional connectivity to intelligence in children and an increasing reliance on inter-hemispheric connectivity in girls with age.
Introduction
An increasing body of literature points to sexual dimorphism in the neuroanatomical correlates of intelligence, hypothesized to be the result of women possessing more neuronal processes but fewer neurons compared to men (de Courten-Myers, 1999), as well as an 8–10% smaller overall brain size (Nopoulos et al., 2000; Rabinowicz et al., 1999). These factors appear to contribute to a greater association in women between white matter properties and intelligence (Gur et al., 1999). In a voxel-based morphometry (VBM) study (Haier et al., 2005), women showed more areas in which white matter density was correlated with IQ in comparison to men, who displayed more areas with gray matter density associated with IQ. Magnetic resonance spectroscopy (MRS) studies have found regions in which N-acetyl-aspartate (NAA) concentrations were only associated with intelligence in women but not in men, including the left occipito-parietal white matter (Jung et al., 2005), and left prefrontal cortex (Pfleiderer et al., 2004). A recent functional MRI (fMRI) study (Schmithorst and Holland, 2006) showed a developmental aspect to this sexual dimorphism, hypothesized to be associated with gray matter pruning (Casey et al., 2000; Courchesne et al., 2000; Giedd et al., 1999), as well as disparate trajectories between boys and girls in overall brain maturation and growth (Lynn, 1999; 1994; Lynn et al., 2000). Girls showed a trend in development toward an increasing association with age of functional connectivity with intelligence, at least in the left hemisphere; while the opposite trend was shown in boys, with an increasing association with age of localized cortical function with intelligence. That study, however, utilized a silent verb-generation task, which predominantly recruits the left hemisphere of the brain for most subjects (Holland et al., 2001).
Sex differences associated with cognitive performance have been found in the splenium of the corpus callosum (Davatzikos and Resnick, 1998), leading us to expect a similar effect (e.g. increasing reliance with age on inter-hemispheric functional connectivity in girls, with the opposite effect in boys) in a task which recruits both hemispheres. In order to investigate this hypothesis we re-analyzed data obtained from a large-scale fMRI study in children investigating narrative comprehension (Schmithorst et al., 2006). Since investigation of sex-related differences, especially in children, likely requires a very large sample size (Plante et al., 2006) due to the small effect size, a reanalysis of this dataset, using a specially tailored data analysis strategy, was determined to be the best way to investigate the above hypothesis. The particular dataset used is ideal for investigation of sex differences in the relationship of inter-hemispheric functional connectivity and intelligence, and any developmental effect, due to the large sample size (>300 children), the age range (5–18 years), and the specific task employed (narrative processing), which from our previous analysis involving Independent Component Analysis has been shown to recruit bilateral networks in the brain (Schmithorst et al., 2006).
We employ a newly-developed Bayesian methodology for modeling functional connectivity (Patel et al., 2006a; b) which is completely data-driven and hypothesis unconstrained. The method eliminates the necessity of providing a predetermined model of connectivity as demanded by Structural Equation Modeling (SEM) (McIntosh and Gonzalez-Lima, 1994; Solodkin et al., 2004) or Dynamic Causal Modeling (DCM) (Friston et al., 2003; Penny et al., 2004a; b). We have modified the method in order to be able to investigate between-subjects and between-groups differences. The benefit of the Bayesian methodology is that it allows the calculation of a posterior probability that the hypothesis of interest is true, rather than simply a rejection of the null hypothesis, as in standard frequentist statistics; moreover, the Bayesian methodology provides an upper bound on the rate of false discovery of significant functional connections (Friston and Penny, 2003).
Materials and Methods
As described above, the present paper re-analyzes fMRI data originally analyzed using Independent Component Analysis (Schmithorst et al., 2006). The task used was that of narrative comprehension, and the subjects consisted of normal children ages 5–18. We refer the reader to our previous paper for a complete description of the task, subjects, and data pre-processing but provide a detailed summary here.
The fMRI scan paradigm consisted of a 30-s on–off block design. One story, read by an adult female speaker, was presented during each 30-s task period. (A complete transcript of one of the stories is given in (Schmithorst et al., 2006).) Each story contained 9, 10, or 11 sentences of contrasting syntactic constructions (e.g., conjoined sentences versus center embeddings). The inclusion of complex syntactic structures was designed to increase the relative processing load for this aspect of language. During each 30-s control period, pure tones of 1 s duration were presented at unequal intervals of 1–3 s. The frequency of each tone was randomly selected from a choice of 150, 200, 250, 500, 700, 900, or 1000 Hz. The control condition was designed to control for sublexical auditory processing. The subject was instructed to listen to the stories so that he or she could answer questions about them after the scans. Performance data were obtained at the end of the scanning session by asking the subject to answer two multiple choice questions about each story (transcript of questions for one of the stories is also given in (Schmithorst et al., 2006)).
Normal children ages 5–18 were recruited after Institutional Review Board approval was obtained for the study. Informed consent was obtained from each child’s parent or guardian (assent was also obtained from subjects eight years and older). Exclusion criteria were as follows: previous neurological illness, learning disability, head trauma with loss of consciousness, current or past use of psychostimulant medication, pregnancy, birth at 37 weeks gestational age or earlier, or abnormal findings at a routine neurological examination performed by an experienced pediatric neurologist. All subjects were native monolingual English speakers.
The current study involves the subset of 303 (151M, 152F) out of the original 313 subjects for which both performance data (post-scan recall) and Wechsler full-scale IQ scores were available. Subjects received the Wechsler Intelligence Scale for Children, Third Edition (WISC-III), or the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III). The IQ (mean ± std.) of boys was 111.6 ± 13.4; the IQ (mean ± std.) of girls was 111.1 ± 14.9. The age (mean ± std.) of boys was 11.8 ± 3.5 yrs; the age (mean ± std.) of girls was 12.0 ± 3.9 yrs. Out of 10 multiple-choice questions asked after the scanning session to test post-scan recall, the performance of boys (mean ± std.) was 7.68 ± 2.08; the performance of girls was 7.84 ± 2.02. The Bayesian connectivity analysis (detailed below) was however restricted to a subset of subjects (296; 146M, 150F) since magnetic susceptibility artifacts prevented extraction of functional time courses from at least one region of interest (ROI) for 7 subjects. For this subset, age (mean ± std.) of boys was 11.7 ± 3.5 yrs, age (mean ± std.) of girls was 12.0 ± 4.0 yrs; IQ (mean ± std.) of boys was 111.7 ± 13.6, IQ (mean ± std.) of girls was 111.3 ± 14.7; performance (mean ± std.) of boys was 7.64 ± 2.09, performance (mean ± std.) of girls was 7.88 ± 1.95. A further analysis was restricted to the subset of subjects where performance on post-scan recall was better than a chance level (p < 0.02), corresponding to correctly answering 6 or more out of the 10 multiple (4)-choice questions. In this subset, age (mean ± std.) of boys was 11.9 ± 3.4 yrs, age (mean ± std.) of girls was 12.5 ± 3.9 yrs; IQ (mean ± std.) of boys was 112.4 ± 13.7, IQ (mean ± std.) of girls was 111.2 ± 14.3; performance (mean ± std.) of boys was 8.22 ± 1.3, performance (mean ± std.) of girls was 8.48 ± 1.21. A complete age and gender breakdown of the subset of subjects for each analysis is detailed in Table 1.
Table 1.
Age (years) and gender breakdown for: the 303 subjects used for the voxelwise fMRI analysis (top table); the 296 subjects incorporated in the Bayesian functional connectivity analysis (middle table); the subset of 259 subjects incorporated in the Bayesian functional connectivity analysis whose task performance was better than a chance (p < 0.02) level (bottom table).
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| M | 7 | 8 | 10 | 15 | 10 | 10 | 17 | 16 | 16 | 9 | 10 | 9 | 11 | 3 |
| F | 6 | 10 | 15 | 11 | 12 | 10 | 8 | 10 | 20 | 9 | 10 | 10 | 10 | 11 |
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| M | 7 | 8 | 10 | 15 | 10 | 10 | 17 | 16 | 15 | 9 | 8 | 8 | 11 | 2 |
| F | 6 | 10 | 15 | 11 | 12 | 10 | 8 | 10 | 18 | 9 | 10 | 10 | 10 | 11 |
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| M | 3 | 6 | 7 | 15 | 10 | 10 | 15 | 14 | 13 | 9 | 7 | 8 | 10 | 2 |
| F | 4 | 6 | 11 | 9 | 10 | 9 | 8 | 10 | 15 | 8 | 10 | 9 | 10 | 11 |
Data were processed using in-house software written in IDL (Research Systems Inc., Boulder, CO). Nyquist ghosts and geometric distortion due to B0 field inhomogeneity were corrected for during reconstruction using a multi-echo reference scan (Schmithorst et al., 2001). Data were corrected for subject motion using a pyramid iterative algorithm (Thevenaz and Unser, 1998); all data sets met the criterion of median voxel displacement at the center of the brain < 2 mm. The fMRI data were subsequently transformed into stereotaxic space (Talairach and Tournoux, 1988) using a linear affine transformation, previously validated for the age range in our study (Muzik et al., 2000; Wilke et al., 2002).
The fMRI data were analyzed using a standard General Linear Model (GLM) method to find regions correlated with full-scale IQ. The analysis was restricted to voxels with p < 1e-6 (Bonferroni-corrected) for significant within-group activation. A three-way ANCOVA was performed, with independent variables of full-scale IQ, subject age, and sex; the main effect of full-scale IQ was the contrast of interest. The Z-scores from the ANCOVA were filtered with a Gaussian filter of width 4 mm, and a threshold of Z > 4 and extent threshold of 25 contiguous voxels was used; these thresholds were found to correspond to a corrected p < 0.05 using a Monte Carlo analysis similar to that described in (Ledberg et al., 1998); spatial autocorrelations were estimated by principal component analysis (PCA). There were six regions found (Table 2): the superior temporal gyrus (STG) bilaterally (corresponding to classical Wernicke’s areas); the posterior aspects of the superior temporal gyrus bilaterally; the left inferior frontal gyrus (classical Broca’s area); and the superior medial frontal gyrus. Maps were also constructed for the sex-X-IQ and age-X-sex-X-IQ interactions.
Table 2.
Regions showing correlations of activation with Full-Scale IQ used in the Bayesian Connectivity Analysis. (X, Y, Z in Talairach coordinates.) Regions correspond to the colored areas in Figure 2.
| Region | Colored Region in Figure 2 | # Voxels | BA | X,Y,Z |
|---|---|---|---|---|
| Left Inferior Frontal Gyrus (Broca’s Area) | Red | 37 | 44/45 | −47, 21, 10 |
| Left Posterior Superior Temporal Gyrus | Green | 85 | 22 | −48, −50, 19 |
| Left Superior Temporal Gyrus (Wernicke’s Area) | Blue | 301 | 22 | −50, −24, 3 |
| Superior Medial Frontal Gyrus | Cyan | 36 | 8/9 | −2, 46, 33 |
| Right Posterior Superior Temporal Gyrus | Magenta | 29 | 22 | 44, −49, 19 |
| Right Superior Temporal Gyrus (Wernicke’s Area) | Yellow | 193 | 22 | 46, −19, 1 |
Regions of interest (ROIs) were defined for voxels having correlation with full-scale IQ, and fMRI time courses were extracted from each subject from these ROIs. As described above, magnetic susceptibility artifacts prevented the extraction of time courses from all ROIs for 7 subjects, which were excluded from the Bayesian connectivity analysis. The Bayesian connectivity analysis was performed both on this subset of 296 subjects and on a further subset of 259 subjects whose post-scan recall of the narratives was better than chance.
The Bayesian methodology for connectivity analysis is described in detail in previous papers (Patel et al., 2006a; b) and is only summarized here, together with the modifications made for the specific analyses desired for this study (notation will differ somewhat from (Patel et al., 2006a) for clarity and brevity). The time courses from each subject and ROI were corrected for baseline drift using a quadratic function, and normalized to percent change from the mean. Assuming a baseline-drift-corrected fMRI timecourse Y the standard General Linear Model is Y = Xβ + ε where X is the “design matrix” consisting of the contrasts of interest and confounds, β is the matrix of parameters, and ε contains the residuals. From each time course a vector of binary values A is constructed according to whether elevated activity occurs during the narrative comprehension period: A = I(Y > c σ) where σ is the standard deviation of ε. Since the fMRI paradigm in the present study is a block-design, the technique is simplified as follows: the time courses were split into resting and active epochs, with the first 3 time points in each epoch discarded to allow sufficient time for the hemodynamic delay. σ was calculated as the standard deviation of the points in the resting epochs. (Temporal pre-whitening is recommended by (Patel et al., 2006a); this step was omitted in the present study as it was a block design and elevated activation during the active (narrative comprehension) epoch is unlikely to be affected by autocorrelated noise.) (Patel et al., 2006a) uses an arbitrary choice of c = 1; however, a data-driven approach to selecting the threshold is more likely to result in significant between-groups differences. Hence a histogram was made of over all time points (in the narrative comprehension epoch) and all subjects. The histogram was spline-smoothed and the mode of the histogram (corresponding to c = 0.626) was selected as the threshold to maximize the separation between “active” and “inactive” time points during the active period.
For each pair of ROIs a and b in each subject s the joint activation probabilities are derived as:
where the indicator m denotes all time points during the active (narrative comprehension) epochs, and I is the indicator function. Since there are 6 ROIs of interest, there are 15 pairs of ROIs for which the above computation is performed. The above differs from (Patel et al., 2006a) in that there the sum is also taken over the subject index; however, this is not done in the present analysis since it is desired to test for between-group differences. With that difference, the methodology remains essentially the same.
The multinomial likelihood of the data is:
Where
The probabilities, unlike as in (Patel et al., 2006a), are not assumed the same for each subject; thus allowing the modeling of inter-subject variability. The Bayesian formalism allows for the incorporation of priors; for this study we simply use flat priors and thus the posterior (Dirichlet) distribution is
We then sample repeatedly from this posterior Dirichlet distribution, which incorporates inter-subject variability and allows the generation of posterior distributions for parameters reflecting between-group differences, such as sex and IQ.
Two derived parameters are proposed in (Patel et al., 2006a):κ, a measure of association related to the joint activation probability, and τ, a measure of functional ascendancy (i.e. one region active at the same time while another region is inactive). However we wished to test for between-groups differences in functional connectivity, not merely measures of connectivity or ascendancy in a single subject. As a measure of connectivity we use the conditional joint activation probability assuming
We subject this parameter to another level of analysis, testing for between-groups differences. For each sample taken from the posterior distribution, a three-way ANCOVA with independent variables of sex, age, and IQ is performed; thus generating posterior distributions for the regression coefficients for IQ (main effect), sex-X-IQ interaction, and age-X-sex-X-IQ interaction. Bayesian posterior inferences are then drawn from the posterior distribution for each coefficient. (For clarity, throughout the paper frequentist probabilities will be denoted by lowercase p, while Bayesian posterior probabilities will be denoted by capitalized P.) A connection was deemed significant if the posterior probability was P > 0.99 for the regression coefficients tested (e.g. main effect of IQ, sex-X-IQ interaction, and sex-X-IQ-X-age interaction) for the analysis involving subset of subjects whose task performance was better than chance, and the posterior probability was P > 0.95 for the analysis involving all subjects. The rationale for this set of criteria is explained in the Discussion section.
Results
For the voxelwise fMRI analysis involving all 303 subjects, and for the Bayesian connectivity analysis involving 296 subjects, there was no significant difference between boys and girls for full-scale IQ (p > 0.7, independent T-test), age (p > 0.4, independent T-test), or performance (p > 0.29, Mann-Whitney U-test). There was a significant association between performance and full-scale IQ for the voxelwise fMRI analysis and for the Bayesian connectivity analysis involving 296 subjects (Spearman’s R = 0.27; p < 1e-5), and this association remained present, albeit with reduced significance, in the subset of 259 subjects where task performance was better than a chance level (Spearman’s R = 0.23; p < 0.001). For this subset of subjects, there was no significant difference between boys and girls for full-scale IQ (p > 0.9, independent T-test) or age (p > 0.24, independent T-test). There is also no significant difference in performance, although it is approaching a trend (p > 0.13, Mann-Whitney U-test).
Results of all active regions are shown in Figure 1; results of regions correlated with full-scale IQ are shown in Figure 2, comprising Wernicke’s area bilaterally, the posterior superior temporal gyrus bilaterally, Broca’s area in the left hemisphere, and the superior medial frontal gyrus (details listed in Table 2). A sagittal view is given in Figure 3, showing the delineation of Wernicke’s area from the most posterior aspect of the superior temporal gyrus. Regions significant for the IQ-X-sex interaction (girls > boys) are shown in Figure 4, and are limited to regions in Figure 2 in the left hemisphere. Regions significant for the IQ-X-sex-X-age (boys > girls) interaction are shown in Figure 5, showing regions with sex differences in the correlation of functional activation with intelligence moderated by age.
Figure 1.
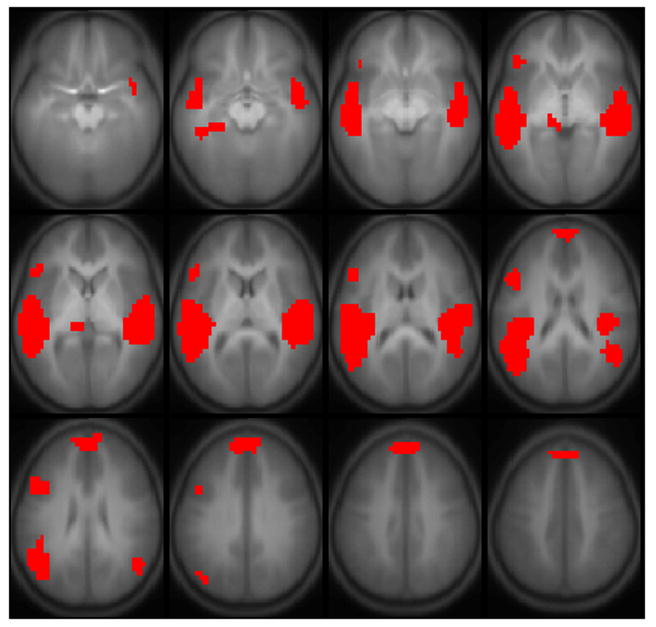
Regions activated during the fMRI paradigm of narrative comprehension for a cohort of 303 normal children ages 5–18. Regions significant with p < 1e-6 (corrected). Slice location: Z = −15 mm to Z = +40 mm. All images in neurologic orientation.
Figure 2.
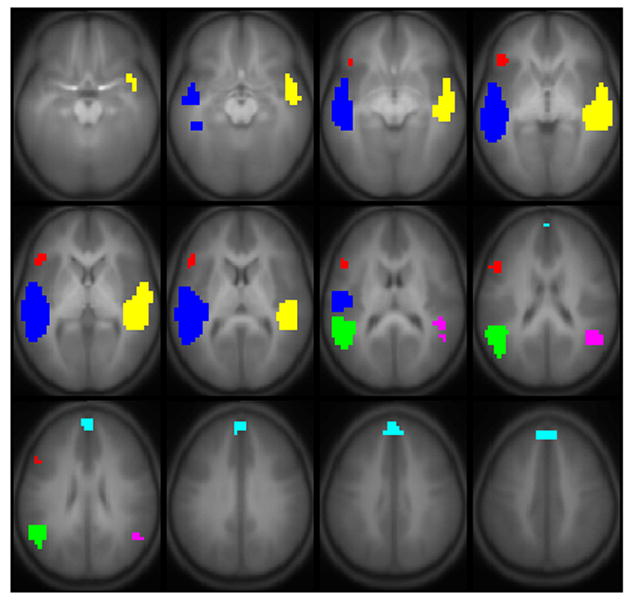
Regions with a significant positive correlation of Wechsler Full-Scale IQ with BOLD activation for the fMRI paradigm of narrative comprehension performed by a cohort of 303 normal children ages 5–18. Regions significant with p < 0.01 (corrected). Slice location: Z = −15 mm to Z = +40 mm. All images in neurologic orientation. The color legend is shown in Table 2.
Figure 3.
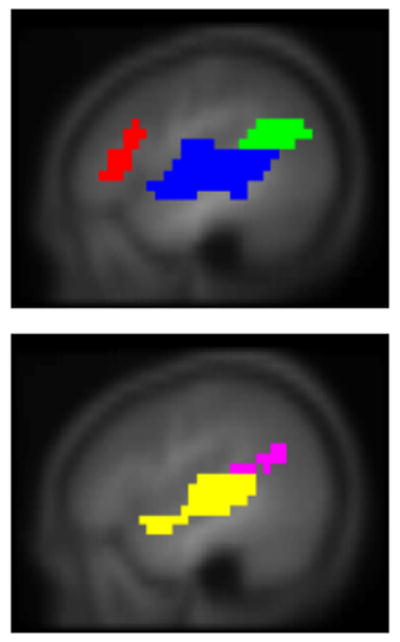
Sagittal view of regions in Figure 2 (slice locations: top, X = −45 mm; bottom, X = +45 mm) showing delineation of Wernicke’s area from posterior superior temporal gyrus. Color legend shown in Table 2.
Figure 4.
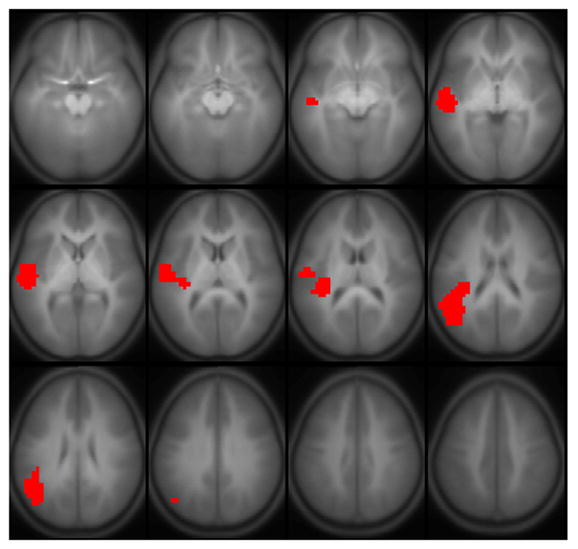
Regions with a significant IQ-X-sex interaction (girls > boys) on BOLD activation for the fMRI paradigm of narrative comprehension performed by a cohort of 303 normal children ages 5–18. Regions significant with p < 0.01 (corrected). Slice location: Z = −15 mm to Z = +40 mm. All images in neurologic orientation.
Figure 5.
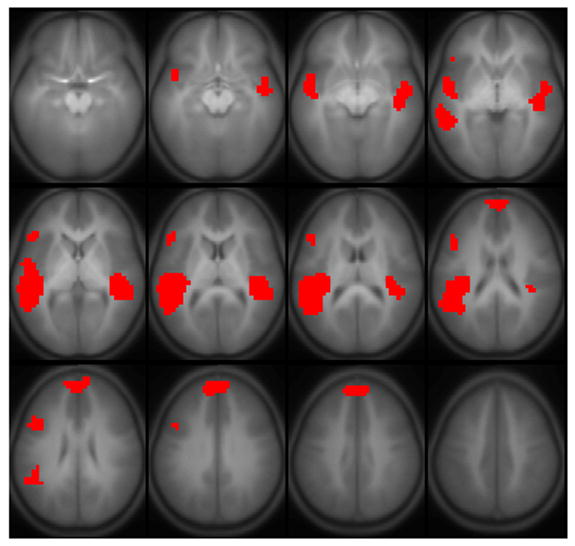
Regions with a significant IQ-X-sex-X-age interaction (boys > girls) on BOLD activation for the fMRI paradigm of narrative comprehension performed by a cohort of 303 normal children ages 5–18. Regions significant with p < 0.01 (corrected). Slice location: Z = −15 mm to Z = +40 mm. All images in neurologic orientation.
For the Bayesian connectivity analysis, a significant positive main effect for IQ was seen for the functional connections diagrammed in Figure 6. Specifically, the significant connections include those from Wernicke’s area in each hemisphere to the other areas in the left hemisphere (Broca’s area and the left posterior superior temporal gyrus), and the superior medial frontal gyrus; an additional intra-hemispheric connection between Broca’s area and the left posterior superior temporal gyrus; and an inter-hemispheric connection between Wernicke’s areas in both hemispheres. Bayesian posterior credible intervals and posterior medians for each connection are listed in Table 3.
Figure 6.
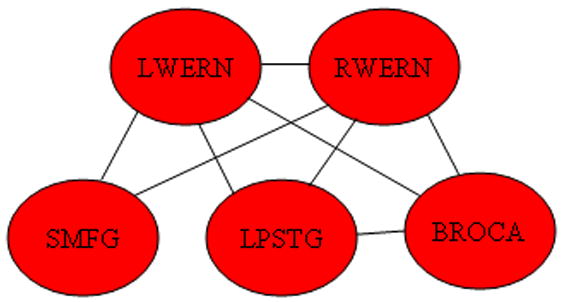
Regions exhibiting a positive main effect of connectivity with IQ. (Abbreviations: LWERN = Left Wernicke’s Area; RWERN = Right Wernicke’s Area; SMFG = Superior Medial Frontal Gyrus; LPSTG = Left Posterior Superior Temporal Gyrus; BROCA = Broca’s Area.)
Table 3.
Bayesian posterior 99% credible intervals (posterior medians in parentheses) for the regression parameter ( 1e3) corresponding to the main effect of IQ on functional connectivity between the regions in the horizontal and vertical rows of the matrices.
| BROCA | LPSTG | LWERN | SMFG | RPSTG | RWERN | |
| BROCA | -- | 0.08 – 2.13 (1.09) | 0.57 – 2.54 (1.59) | −0.58 – 1.82 (0.63) | −0.49 – 1.75 (0.61) | 0.27 – 2.30 (1.30) |
| LPSTG | 0.08 – 2.13 (1.09) | -- | 0.47 – 2.43 (1.42) | −0.62 – 1.64 (0.53) | −1.21 – 1.00 (−0.09) | 0.53 – 2.57 (1.57) |
| LWERN | 0.57 – 2.54 (1.59) | 0.47 – 2.43 (1.42) | -- | 0.37 – 2.30 (1.28) | −0.23 – 1.65 (0.69) | 0.31 – 2.02 (1.17) |
| SMFG | −0.58 – 1.82 (0.63) | −0.62 – 1.64 (0.53) | 0.37 – 2.30 (1.28) | -- | −0.75 – 1.64 (0.50) | 0.18 – 2.31 (1.26) |
| RPSTG | −0.49 – 1.75 (0.61) | −1.21 – 1.00 (−0.09) | −0.23 – 1.65 (0.69) | −0.75 – 1.64 (0.50) | -- | −0.10 – 1.93 (0.92) |
| RWERN | 0.27 – 2.30 (1.30) | 0.53 – 2.57 (1.57) | 0.31 – 2.02 (1.17) | 0.18 – 2.31 (1.26) | −0.10 – 1.93 (0.92) | -- |
| BROCA | LPSTG | LWERN | SMFG | RPSTG | RWERN | |
| BROCA | -- | 0.30 – 2.36 (1.33) | 0.93 – 2.80 (1.85) | −0.29 – 1.92 (0.80) | −0.13 – 1.92 (0.90) | 0.41 – 2.33 (1.39) |
| LPSTG | 0.30 – 2.36 (1.33) | -- | 0.75 – 2.54 (1.62) | −0.47 – 1.57 (0.59) | −0.33 – 1.76 (0.68) | 0.77 – 2.64 (1.71) |
| LWERN | 0.93 – 2.80 (1.85) | 0.75 – 2.54 (1.62) | -- | −0.09 – 1.70 (0.78) | 0.12 – 1.87 (1.00) | 0.67 – 2.33 (1.51) |
| SMFG | −0.29 – 1.92 (0.80) | −0.47 – 1.57 (0.59) | −0.09 – 1.70 (0.78) | -- | −0.59 – 1.59 (0.48) | −0.13 – 1.86 (0.90) |
| RPSTG | −0.13 – 1.92 (0.90) | −0.33 – 1.76 (0.68) | 0.12 – 1.87 (1.00) | −0.59 – 1.59 (0.48) | -- | −0.01 – 1.96 (0.94) |
| RWERN | 0.41 – 2.33 (1.39) | 0.77 – 2.64 (1.71) | 0.67 – 2.33 (1.51) | −0.13 – 1.86 (0.90) | −0.01 – 1.96 (0.94) | -- |
Top matrix = subset of data where subjects performed at a better than chance level (p < 0.02) for post-scan recall; bottom matrix = full dataset. Connections with P > 0.99 for a positive main effect are given in bold black font; connections with P > 0.95 in the bottom matrix are given in bold gray font. (Abbreviations: LWERN = Left Wernicke’s Area; RWERN = Right Wernicke’s Area; SMFG = Superior Medial Frontal Gyrus; LPSTG = Left Posterior Superior Temporal Gyrus; BROCA = Broca’s Area.)
Significant sex-X-IQ interactions (girls > boys) were seen for the functional connectivity of Wernicke’s areas (both hemispheres) to the left posterior superior temporal gyrus (Figure 7). Bayesian posterior credible intervals and posterior medians are listed in Table 4. Post-hoc Bayesian analyses were performed by splitting the data between boys and girls and generating posterior distributions (using a two-way ANCOVA with independent variables of age and IQ) of the regression parameter for main effect of IQ as a function of marginal joint probability for each group (boys and girls) separately. This analysis revealed that for each connection there was a significant (P > 0.99) positive association of connectivity with IQ in girls (denoted in the diagram by coloring the lines pink), while no significant effect was seen for boys.
Figure 7.
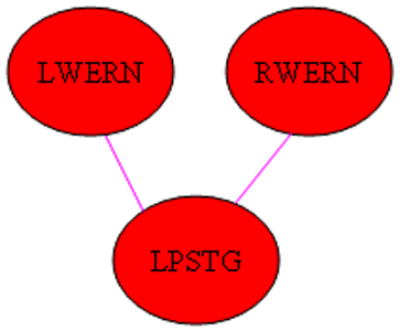
Regions exhibiting a significant IQ-X-sex (girls > boys) interaction on functional connectivity. (Pink line indicates connectivity has positive main effect for girls. Abbreviations: LWERN = Left Wernicke’s Area; RWERN = Right Wernicke’s Area; LPSTG = Left Posterior Superior Temporal Gyrus.)
Table 4.
Bayesian posterior 99% credible intervals (posterior medians in parentheses) for the regression parameter ( 1e3) corresponding to the sex-X-IQ interaction on functional connectivity between the regions in the horizontal and vertical rows of the matrices.
| BROCA | LPSTG | LWERN | SMFG | RPSTG | RWERN | |
| BROCA | -- | −5.03 – −0.41 (−2.75) | −5.66 – −1.67 (−3.56) | −3.86 – 1.16 (−1.35) | −7.04 – −2.41 (−4.78) | −5.48 – −1.17 (−3.46) |
| LPSTG | −5.03 – −0.41 (−2.75) | -- | 0.11 – 4.07 (2.08) | −1.47 – 2.82 (0.66) | −4.19 – 0.48 (−1.88) | 0.49 – 4.53 (2.43) |
| LWERN | −5.66 – −1.67 (−3.56) | 0.11 – 4.07 (2.08) | -- | −2.08 – 1.86 (−0.15) | −4.42 – −0.49 (−2.53) | −3.36 – 0.12 (−1.49) |
| SMFG | −3.86 – 1.16 (−1.35) | −1.47 – 2.82 (0.66) | −2.08 – 1.86 (−0.15) | -- | −3.36 – 1.49 (−1.00) | −1.38 – 2.99 (0.76) |
| RPSTG | −7.04 – −2.41 (−4.78) | −4.19 – 0.48 (−1.88) | −4.42 – −0.49 (−2.53) | −3.36 – 1.49 (−1.00) | -- | −4.53 – −0.46 (−2.56) |
| RWERN | −5.48 – −1.17 (−3.46) | 0.49 – 4.53 (2.43) | −3.36 – 0.12 (−1.49) | −1.38 – 2.99 (0.76) | −4.53 – −0.46 (−2.56) | -- |
| BROCA | LPSTG | LWERN | SMFG | RPSTG | RWERN | |
| BROCA | -- | −3.15 – 1.08 (−1.09) | −3.86 – −0.20 (−2.05) | −2.48 – 2.20 (−0.19) | −4.33 – 0.13 (−2.09) | −3.50 – 0.51 (−1.54) |
| LPSTG | −3.15 – 1.08 (−1.09) | -- | 1.38 – 4.89 (3.16) | −1.17 – 2.98 (0.88) | −2.83 – 1.24 (−0.72) | 1.02 – 4.80 (2.82) |
| LWERN | −3.86 – −0.20 (−2.05) | 1.38 – 4.89 (3.16) | -- | −1.89 – 1.84 (0.06) | −3.26 – 0.35 (−1.45) | −1.55 – 1.61 (0.00) |
| SMFG | −2.48 – 2.20 (−0.19) | −1.17 – 2.98 (0.88) | −1.89 – 1.84 (0.06) | -- | −2.27 – 2.05 (−0.10) | −0.95 – 2.99 (1.01) |
| RPSTG | −4.33 – 0.13 (−2.09) | −2.83 – 1.24 (−0.72) | −3.26 – 0.35 (−1.45) | −2.27 – 2.05 (−0.10) | -- | −3.26 – 0.56 (−1.28) |
| RWERN | −3.50 – 0.51 (−1.54) | 1.02 – 4.80 (2.82) | −1.55 – 1.61 (0.00) | −0.95 – 2.99 (1.01) | −3.26 – 0.56 (−1.28) | -- |
Top matrix = subset of data where subjects performed at a better than chance level (p < 0.02) for post-scan recall; bottom matrix = full dataset. Connections with P > 0.99 for a significant effect (girls > boys) are given in bold pink font; Connections with P > 0.99 for a significant effect (boys > girls) are given in bold blue font. In the bottom matrix, connections with P > 0.95 for a significant effect (girls > boys) are given in normal pink font; connections with P > 0.95 for a significant effect (boys > girls) are given in normal blue font. (Abbreviations: LWERN = Left Wernicke’s Area; RWERN = Right Wernicke’s Area; SMFG = Superior Medial Frontal Gyrus; LPSTG = Left Posterior Superior Temporal Gyrus; BROCA = Broca’s Area.)
Significant sex-X-IQ interactions (boys > girls) were seen for the functional connectivity of Broca’s area to Wernicke’s areas and the right posterior superior temporal gyrus (Figure 8); as well as the functional connectivity between the right posterior superior temporal gyrus and left Wernicke’s area. Bayesian posterior credible intervals and posterior medians are also listed in Table 4. The post-hoc Bayesian analysis revealed a significant positive association of connectivity with IQ in boys for each connection (denoted in the diagram by coloring the lines blue), and a significant negative association of connectivity with IQ in girls only for the connection between Broca’s area and the right posterior superior temporal gyrus (denoted by coloring the line pink).
Figure 8.
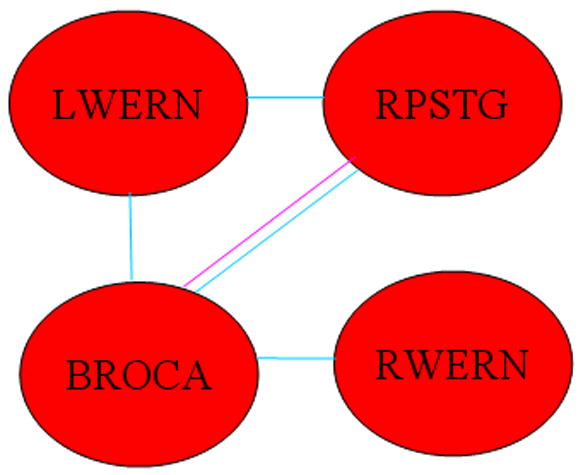
Regions exhibiting a significant IQ-X-sex (boys > girls) interaction on functional connectivity. (Blue line indicates connectivity has positive main effect for boys; pink line indicate connectivity has negative main effect for girls. (Abbreviations: LWERN = Left Wernicke’s Area; RWERN = Right Wernicke’s Area; SMFG = Superior Medial Frontal Gyrus; LPSTG = Left Posterior Superior Temporal Gyrus; RPSTG = Right Posterior Superior Temporal Gyrus; BROCA = Broca’s Area.)
Significant three-way sex-X-age-X-IQ interactions (girls > boys) were seen for the functional connectivity of Wernicke’s areas (both hemispheres) with the right posterior superior temporal gyrus (Figure 9). Bayesian posterior credible intervals and posterior medians are listed in Table 5. Post-hoc Bayesian analyses were performed as above, splitting the data into boys and girls; from the model regression parameters, posterior distributions were generated for the slope of IQ vs. marginal joint probability at 60 months (5 years) and 216 months (18 years) of age, corresponding to the youngest and oldest subjects. For the youngest girls, there was a significant negative association of connectivity with IQ (denoted by the dashed pink lines); while for the oldest girls, there was a significant positive association of connectivity with IQ (denoted by the solid pink lines). For the youngest boys, there was a positive association of connectivity with IQ (denoted by the dashed blue lines); no significant effect was found in the oldest boys. These results indicate a developmental progression in girls for these connections impeding cognitive function (in the youngest girls) to being associated with improved cognitive function (in the oldest girls); while in boys the results indicate a developmental progression with age resulting in less reliance on these connections for improved cognitive function.
Figure 9.
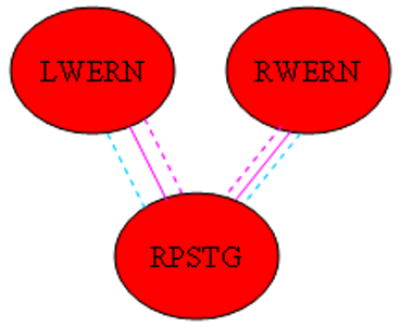
Regions exhibiting a significant IQ-X-age-X-sex (girls > boys) interaction on functional connectivity. (Dashed blue line indicates connectivity has positive effect for youngest (5 years) boys; dashed pink line indicates connectivity has negative effect for youngest (5 years) girls; solid pink line indicates connectivity has positive effect for oldest (18 years) girls. Abbreviations: LWERN = Left Wernicke’s Area; RWERN = Right Wernicke’s Area; RPSTG = Right Posterior Superior Temporal Gyrus.)
Table 5.
Bayesian posterior 99% credible intervals (posterior medians in parentheses) for the regression parameter ( 1e5) corresponding to the sex-X-IQ-age interaction on functional connectivity between the regions in the horizontal and vertical rows of the matrices.
| BROCA | LPSTG | LWERN | SMFG | RPSTG | RWERN | |
| BROCA | -- | −5.83 – 4.33 (−0.61) | −6.31 – 2.98 (−1.60) | −6.07 – 5.56 (−0.38) | −2.64 – 7.83 (2.61) | −7.89 – 1.70 (−3.09) |
| LPSTG | −5.83 – 4.33 (−0.61) | -- | −3.59 – 5.38 (0.55) | −7.63 – 3.37 (−2.06) | −1.32 – 9.53 (4.14) | −2.28 – 6.81 (2.06) |
| LWERN | −6.31 – 2.98 (−1.60) | −3.59 – 5.38 (0.55) | -- | −8.62 – 0.78 (−3.85) | 2.32 – 11.59 (6.79) | −6.57 – 1.69 (−2.48) |
| SMFG | −6.07 – 5.56 (−0.38) | −7.63 – 3.37 (−2.06) | −8.62 – 0.78 (−3.85) | -- | −4.39 – 6.32 (1.04) | −7.29 – 2.50 (−2.49) |
| RPSTG | −2.64 – 7.83 (2.61) | −1.32 – 9.53 (4.14) | 2.32 – 11.59 (6.79) | −4.39 – 6.32 (1.04) | -- | 0.93 – 10.31 (5.66) |
| RWERN | −7.89 – 1.70 (−3.09) | −2.28 – 6.81 (2.06) | −6.57 – 1.69 (−2.48) | −7.29 – 2.50 (−2.49) | 0.93 – 10.31 (5.66) | -- |
| BROCA | LPSTG | LWERN | SMFG | RPSTG | RWERN | |
| BROCA | -- | −5.93 – 3.28 (−1.45) | −6.34 – 2.27 (−2.26) | −6.51 – 4.29 (−1.18) | −2.70 – 6.83 (1.95) | −7.57 – 1.62 (−3.20) |
| LPSTG | −5.93 – 3.28 (−1.45) | -- | −5.60 – 2.56 (−1.50) | −8.15 – 1.11 (−3.84) | −1.16 – 8.89 (3.82) | −3.87 – 4.97 (0.54) |
| LWERN | −6.34 – 2.27 (−2.26) | −5.60 – 2.56 (−1.50) | -- | −9.88 – −1.58 (−5.59) | 1.80 – 10.04 (5.95) | −4.34 – 3.24 (−0.75) |
| SMFG | −6.51 – 4.29 (−1.18) | −8.15 – 1.11 (−3.84) | −9.88 – −1.58 (−5.59) | -- | −3.49 – 6.10 (1.20) | −8.29 – 1.33 (−3.33) |
| RPSTG | −2.70 – 6.83 (1.95) | −1.16 – 8.89 (3.82) | 1.80 – 10.04 (5.95) | −3.49 – 6.10 (1.20) | -- | 0.11 – 8.74 (4.48) |
| RWERN | −7.57 – 1.62 (−3.20) | −3.87 – 4.97 (0.54) | −4.34 – 3.24 (−0.75) | −8.29 – 1.33 (−3.33) | 0.11 – 8.74 (4.48) | -- |
Top matrix = subset of data where subjects performed at a better than chance level (p < 0.02) for post-scan recall; bottom matrix = full dataset. Connections with P > 0.99 for a significant effect (girls > boys) are given in bold pink font; Connections with P > 0.99 for a significant effect (boys > girls) are given in bold blue font. In the bottom matrix, connections with P > 0.95 for a significant effect (girls > boys) are given in normal pink font; connections with P > 0.95 for a significant effect (boys > girls) are given in normal blue font. (Abbreviations: LWERN = Left Wernicke’s Area; RWERN = Right Wernicke’s Area; SMFG = Superior Medial Frontal Gyrus; LPSTG = Left Posterior Superior Temporal Gyrus; BROCA = Broca’s Area.)
Discussion
Previous studies investigating possible sex-related differences in the cortical activation subserving language processing have found disparate results (Sommer et al., 2004). Some studies have found moderate differences in adults (Kansaku et al., 2000; Phillips et al., 2001; Pugh et al., 1996; Shaywitz et al., 1995); however a different study (Frost et al., 1999) failed to find any significant differences, even with a relatively large sample size. A recent study performed on children (Plante et al., 2006) suggested that these disparate results may be the result of sampling variability and that large sample sizes are necessary to reliably detect sex differences, especially in children. Therefore, performing a separate analysis on our large-scale (>300 children) dataset previously analyzed using Independent Component Analysis (Schmithorst et al., 2006) was determined to be the most suitable approach for the investigation of possible sex-related differences in language processing in children relating to intelligence.
While the existence of sex-related differences in language lateralization in fact remains a topic of controversy (Sommer et al., 2004), it has been proposed that sex-related differences are task-dependent (Kitazawa and Kansaku, 2005), with boys showing greater lateralization for tasks which isolate phonological processing (e.g. (Jaeger et al., 1998; Pugh et al., 1996; Shaywitz et al., 1995)) as compared with those which involve semantic processing at the word level (e.g. (Frost et al., 1999; Kansaku and Kitazawa, 2001)). Significant sex differences have also been previously found for story listening tasks (Kansaku et al., 2000; Phillips et al., 2001). It has been hypothesized (Ringo et al., 1994) that sex-related differences in laterality of activation may reflect differences in interhemispheric communication between posterior language areas; this hypothesis has been corroborated by an event-related potential (ERP) study (Nowicka and Fersten, 2001). There is some support via post-mortem analysis and structural MRI studies for sex differences in the shape or size of posterior areas of the corpus callosum (e.g. (Steinmetz et al., 1992; Steinmetz et al., 1995; Witelson, 1989)) and it may even relate to testosterone exposure (Moffat et al., 1997), although differences in corpus callosum size detected MRI have been shown to be highly dependent on methodology (Bermudez and Zatorre, 2001). Diffusion tensor imaging (DTI) has also shown possible sex differences in corpus callosum microstructure (Shin et al., 2005; Westerhausen et al., 2004). Female-specific correlation of cognitive performance with bulbosity and size of the splenium of the corpus callosum has been demonstrated (Davatzikos and Resnick, 1998). Male-specific lateralization has also been previously found in anterior areas (the inferior frontal gyrus) for story processing (Kansaku et al., 2000) and phonological processing (Jaeger et al., 1998; Pugh et al., 1996; Shaywitz et al., 1995).
The difficulty level of the task was selected such that children as young as 5 years old would be able to perform it. It was not designed to recruit specific areas of intelligence such as executive function or working memory. Hence, as a low-g task, the results do not provide the same information as a study which contrasts a high-g task with a low-g or control task (Duncan et al., 2000; Esposito et al., 1999; Gray et al., 2003; Prabhakaran et al., 2001; Prabhakaran et al., 1997). Brain regions involved in intelligence, according to the method of correlated vectors (Jensen, 1998), should not show intelligence-related correlations with activity when performing a low-g task. Rather, it has been hypothesized that differences found in brain activation related to intelligence during performance of low-g tasks relate to differences in information processing mechanisms (Haier et al., 2003). For instance, subjects with higher performance on the Raven’s Advanced Progressive Matrices (RAPM) test of cognitive performance (Raven et al., 1998) showed greater activation in a posterior visual association area during the viewing of videos (Haier et al., 2003), hypothesized to be related to integrating higher-order language concepts or audio components with incoming visual information. Our previous fMRI study in children (Schmithorst and Holland, 2006) involving silent verb generation corroborated this interpretation, with IQ-related increases in activation found in regions associated with semantic processing and visualization, connected with areas associated with executive function.
In the current study we also found increases in brain activation related to intelligence in areas associated with information processing (Figure 2). Wernicke’s areas have been hypothesized to fulfill a variety of different roles in narrative comprehension (Schmithorst et al., 2006). Activation in these regions has been previously reported for processing of semantic anomalies (e.g. (Friederici et al., 2003; Kuperberg et al., 2000; Ni et al., 2000)). Access to word meaning, however, may not be contained in classical Wernicke’s areas (Scott et al., 2000); instead, they may be involved with spectral and temporal processing of auditory input, with information projected to amodal higher-order association cortex. Activation in Wernicke’s area was not found when subjects read, rather than listened to, sentences with semantic anomalies (Newman et al., 2001), and differential activation was found when subjects listened to words versus nonwords, or words versus reversed speech (Binder et al., 2000). Based on our earlier results showing a significant increase in activation with age, we hypothesized the STG to be more involved with spectral and temporal processing rather than word meaning, since the stories only used vocabulary at the level of very young children. The posterior superior temporal gyrus has been hypothesized to be involved in non-domain specific higher-order integration (Friederici et al., 2003; Schmithorst et al., 2006), mapping different types of information, whether semantic, syntactic, or pragmatic, onto each other. The delayed rise in the hemodynamic response relative to the beginning of the narratives shown in our earlier paper (Schmithorst et al., 2006) corroborates this interpretation.
In addition, activation increases with IQ were seen in Broca’s area and the superior medial frontal gyrus. Broca’s area has been hypothesized to be related to syntactic working memory rather than syntactic complexity (Fiebach et al., 2001; Fiebach et al., 2005). During the processing of syntactically complex sentences, the displaced element must be maintained in working memory over a longer distance (Friederici et al., 2003). Bi-directional connectivity with Broca’s area has also been proposed (Matsumoto et al., 2004; Schmithorst et al., 2006), in line with novel “connectionist” models of language processing (McClelland and Rogers, 2003; Nadeau, 2001). The left prefrontal cortex has been hypothesized be involved with a domain-special system for syntactic processing in sentence comprehension (Sakai et al., 2003), as opposed to domain-general working memory. The superior medial frontal gyrus has been implicated in semantic processing in normal adults (Harris et al., 2006) and in children (Holland et al., 2001), although it may also be related to Theory of Mind (Fletcher et al., 1995; Gallagher and Frith, 2003).
Agreeing with previous results for a semantic verb generation task (Schmithorst and Holland, 2006), a significant sex-X-IQ interaction (girls > boys) was seen for the left Wernicke’s area and posterior superior/middle temporal gyrus (Figure 4). The activation intensities for these regions were separated out by boys and girls and a three-way ANOVA was performed with independent variables of age, IQ, and performance. This post-hoc analysis showed that both regions displayed a significant positive correlation of activation intensity with IQ in girls; a negative correlation of activation intensity with IQ in boys was seen in the posterior left superior/middle temporal gyrus. These results corroborate a previous study which demonstrated sex and task differentiation (Neubauer et al., 2002) in “neural efficiency” (Haier et al., 1992), which postulates that more intelligent individuals will utilize fewer neuronal resources to perform at a given cognitive level (Gray and Thompson, 2004). In addition, there was also a three-way sex-X-age-X-IQ interaction (boys > girls) seen (Figure 5) in the superior temporal gyri bilaterally, the medial frontal gyrus, and Broca’s area. The same post-hoc analysis was performed on the activation intensities in these areas as described above. In the right superior temporal gyrus, there was a negative IQ-x-age interaction in girls, while in boys there was a positive IQ-x-age interaction in Broca’s area and the medial frontal gyrus. These results point to an increasing specialization to the left hemisphere in more intelligent girls and boys with age, with however a different mechanism (increased right hemisphere inhibition in girls, increased left hemisphere activation in boys); future research will be necessary to investigate this possibility further.
Intelligence (and sex-related differences in intelligence) appears to depend on functional connectivity or, conversely, localization of cognitive function (Gray and Thompson, 2004; Schmithorst and Holland, 2006), and hence an analysis only of absolute activation patterns is unlikely to give a complete picture. As it has been suggested that “one of the main insights of cognitive neuroscience is that the ‘functional units’ of higher cognition are networks of brain areas, not single areas” (Gray and Thompson, 2004), in addition to investigating associations between activation in individual brain regions and IQ we investigated functional connectivity using a Bayesian analysis procedure. In contrast to the previously published original analysis of this dataset (Schmithorst et al., 2006), which only described the specific networks in the brain used for narrative processing across all subjects, the Bayesian connectivity analysis is able to specifically associate functional connectivity between various regions with inter-subject differences in age, sex, and IQ.
An extensive network was found (Figure 6) relating functionally correlated activity to IQ. Part of this network included functional connectivity between left Wernicke’s area, the left posterior superior temporal gyrus, and Broca’s area. These regions comprise the classical Wernicke-Geschwind pathway (Catani et al., 2005), and our results agree with previous DTI results showing the increased anisotropy in the left arcuate fasciculus with age (Schmithorst et al., 2002), and the correlation of anisotropy with IQ in a population of mainly girls (Schmithorst et al., 2005). Our results provide corroboration for the relation of connectivity in the arcuate fasciculus and between regions in the Wernicke-Geschwind model for both boys and girls. Similar results have been shown relating connectivity in this area with reading performance in adults (Klingberg et al., 2000), and also in children (Beaulieu et al., 2005). However, the results also implicate inter-hemispheric connectivity between right Wernicke’s area and left Wernicke’s area, and thus also between right Wernicke’s area and the left-hemispheric language processing network being associated with IQ. While previous magnetoencephelography (MEG) studies (Papanicolaou et al., 2003; Pardo et al., 1999) have shown evidence for a left-hemispheric specialization of the superior temporal gyrus for speech processing, more intelligent subjects may be more proficient in processing and integrating information likely to be specialized to the right hemisphere (e.g. tone of speech).
With regard to the differences between boys and girls in the relation of brain connectivity to intelligence, a previous paper (Schmithorst and Holland, 2006) involving fMRI of silent verb generation, showed the optimal configuration (at least in the left hemisphere) for boys progressing from more to less connected with age, with the reverse progression in girls, from less to more connected. The results of the current study, however, employing a more sophisticated analysis technique, and with activation on both hemispheres of the brain, show that the actual developmental progression is more involved. Girls show increased reliance on connectivity between Wernicke’s areas and the left posterior superior temporal gyrus (Figure 7) when compared to boys. While it might seem from the results (Figure 8) that boys show increased reliance between left Wernicke’s area and the right posterior superior temporal gyrus, the age-X-sex-X-IQ interaction (Figure 9) shows this effect to be due to the young girls possessing a negative association of this connectivity with IQ. As girls mature, they develop a positive association with IQ of the connectivities between Wernicke’s area and the right posterior superior temporal gyrus, while the reliance of boys on these connections disappears. On the other hand, boys show a greater reliance on the functional connectivity between Broca’s area and Wernicke’s areas; as well as the connectivity between Broca’s area and the right posterior superior temporal gyrus.
Thus from the current study we can demonstrate a greater reliance in more intelligent girls on functional connectivity between regions used for spectral and temporal processing of the sounds, and amodal higher-order associative regions such as the posterior superior temporal gyri. There is however a hemispheric asymmetry; the association with the left posterior superior temporal gyrus is present even in younger girls while the association with the right posterior superior temporal gyrus only comes into place later in the developmental period. The situation is different in boys. There is no association of IQ and connectivity between Wernicke’s areas and the left posterior superior temporal gyrus, or (at least at the end of the developmental period) the right posterior superior temporal gyrus. However there is a greater reliance on connectivity between Wernicke’s areas and the right posterior superior temporal gyrus if one considers the relevant information being mediated or “funneled” through Broca’s area (Figure 8). There is greater reliance on the functional connectivity between Wernicke’s areas (bilaterally) and Broca’s area, and then greater reliance on the functional connectivity between Broca’s area and the right posterior superior temporal gyrus.
These results suggest that sexual dimorphism in the neuroanatomical correlates of intelligence is more complex than a simple statement that females rely more on functionally connected networks, with males relying on increased functional localization, as hypothesized previously (Schmithorst and Holland, 2006). The question is also rather more involved than simply one of males exhibiting increased hemispheric lateralization (Wager et al., 2003) for various tasks, including affect recognition (Cahill et al., 2001; Killgore and Yurgelun-Todd, 2001) and language (Saucier and Elias, 2001; Shaywitz et al., 1995). Rather, we hypothesize that the sexual differences in optimal neuroanatomical configuration reflect a difference in information processing schemes. Our results show that more intelligent boys rely more on feed-forward and feed-back connectivity between Broca’s area and other auditory processing regions. This result corroborates the observation of (Pugh et al., 1996), where it was suggested that the phonological and semantic networks overlap more in women than in men, and that the networks engaged for phonological and lexical-semantic processing are more spatially distinctive for males. It was also observed (Pugh et al., 1996) that the left inferior frontal gyrus activated more in males than for females. A more diffuse network for phonological processing in females, with more hemispheric specialization and use of the left inferior frontal gyrus in boys, was also observed in (Shaywitz et al., 1995). Our results corroborate these previous observations, as more intelligent children will be more proficient in language processing. If phonological processing is more concentrated in the superior temporal gyrus (Wernicke’s area) in boys, while semantic processing is more concentrated in the posterior superior temporal gyrus, more intelligent boys will need to rely more on functional connectivity of the left inferior frontal gyrus with Wernicke’s area and the posterior superior temporal gyrus. Syntactic representations will need to be held in working memory for a longer period of time in order to accomplish the transition between phonological and semantic representations. On the other hand, if the networks for phonological and semantic processing overlap more in girls, there will be less reliance on mediate connectivity with the left inferior frontal gyrus and more reliance on immediate connectivity between Wernicke’s areas and the posterior superior temporal gyri.
Interestingly, the sex difference (favoring girls) in the association of IQ and connectivity between Wernicke’s areas and the left posterior superior temporal gyrus is present throughout the age span. However, there is a developmental effect in the sex difference in the association of IQ and connectivity between Wernicke’s areas and the right posterior superior temporal gyrus, with the difference not becoming present until later in development. Moreover, a sex-related difference (favoring boys) is only shown for mediate connectivity (e.g. “funneled” through Broca’s area) for the right posterior superior temporal gyrus. We hypothesize that this result stems from differences in the cortical demands for word-level semantic processing versus higher-level semantic processing. While sex-related differences have not been shown for some studies involving semantic tasks at the word level, they have been shown for higher-order semantic processing at the sentence level (Kansaku et al., 2000) used in narrative comprehension. In a hierarchical semantic decision task (Baxter et al., 2003) involving categorical knowledge, women displayed greater activation in the right superior temporal areas and posterior superior temporal areas. The authors suggested that previous null results investigating sex-related difference in the performance of semantic tasks were due to methodological differences as to whether the task incorporated higher-order language processing. The results supported a previously hypothesized inter-hemispheric model of sex differences in language processing (McGlone, 1977), but also a previously hypothesized intra-hemispheric model (Kimura, 1983). Our results suggest the inter-hemispheric differences may occur later in development, as more intelligent girls become more proficient at higher-order language processing, and more reliant on the connectivity between the right posterior superior temporal gyrus and other areas.
There was no significant difference in performance between the boys and the girls included in this study. Thus, our results suggest that the age and IQ dependent connectivity differences shown between boys and girls reflect the development of different but equivalent and equally effective neural circuits to comprehend and retain information from auditory narratives, subject to the limitation that our performance measure is based on a post-hoc recall of events in the narrative rather than a direct, real-time measure. This interpretation is in line with previous results showing minimal or no differences in overall intelligence between adult men and women (e.g. (Lynn, 1999; Mackintosh, 1996)), although there may in fact be a small difference in g between the sexes favoring males of about 3–4 IQ points (Jackson and Rushton, 2006). Our results also agree with previous studies showing intelligence to be more associated with white matter in women and gray matter in men (e.g. (Gur et al., 1999; Haier et al., 2005)), and evidence a sexual dimorphism in neural architecture but one resulting in similar cognitive performance.
The novel Bayesian a posteriori post-processing approach, based on that of (Patel et al., 2006a), allows the investigation of functional connectivity associated with individual differences and has several advantages over other methods. The regions of interest were restricted to those regions which were shown to be activated and also correlated with IQ in order to obviate difficulties associated with selecting seed points. The method is completely data-driven and hypothesis-unconstrained. Bayesian posterior probabilities are generated, removing the necessity of correcting for multiple comparisons. While the method is only able to investigate functional connectivity, rather than effective connectivity, and thus no inferences on possible causation can be drawn, it may be useful nonetheless for construction of hypotheses to test in a hypothesis-constrained method such as Linear Structural Equation Modeling (LSEM) (Solodkin et al., 2004). It is also straightforward to extend the method to investigate individual differences in functional ascendancy, as described in (Patel et al., 2006a), as well as simple functional connectivity.
A limitation of the study is that differences in functional connectivity due to IQ may be confounded with those due to task performance. Inattentive or uncooperative subjects will not activate the necessary functional connections between regions as will normally-performing subjects. However, as differing IQ will cause a change in task performance (as demonstrated by the highly significant correlation between IQ and performance) as well as functional connectivity, attempting to covary out performance in the analysis would also remove effects due to IQ. Hence the procedure detailed in the Materials and Methods section was employed to account for task performance yet retain sensitivity in investigating changes in connectivity due to IQ. The initial analysis was restricted to subjects who performed better than chance on the post-scan recall, ensuring that the subjects were not inattentive or uncooperative but were in fact listening to and comprehending the narratives. This restriction however could possibly result in a spurious effect due to selection bias favoring higher-IQ subjects. Thus the connections found to have a significant (P > 0.99) main effect of IQ, sex-X-IQ interaction, or sex-X-IQ-X-age interaction, were then validated and deemed significant if there was also a significant (P > 0.95) result from the analysis involving the complete dataset. All connections found significant using the subset of data were validated from the analysis involving the complete dataset with the exception (Table 4) of two ipsilateral connections showing a sex-X-IQ interaction. There were also some connections with significant effects from the analysis involving the complete dataset that did not replicate in the analysis involving the subset; these effects are likely spurious, stemming from differences in task performance (e.g. uncooperative/inattentive subjects) rather than IQ.
Conclusion
Possible sex differences in the neuroanatomical bases for intelligence in children were investigated in a large cohort of children ages 5–18 using the fMRI paradigm of passive story listening. To investigate functional connectivity differences a recently-published Bayesian technique for modeling functional connectivity was modified for the investigation of individual differences. Girls showed a greater reliance on connectivity between the left posterior superior temporal gyrus and Wernicke’s areas bilaterally, and a developing greater reliance with age on connectivity between the right posterior superior temporal gyrus and Wernicke’s areas bilaterally. Boys displayed a greater reliance on connectivity between Broca’s area and auditory processing areas including Wernicke’s areas and the right posterior superior temporal gyrus. We hypothesize that the optimal configuration for boys and girls is not simply a matter of females developing greater reliance on functional connectivity with age and males developing greater reliance on functional localization but that the optimal configurations are modulated by specific information processing demands.
Acknowledgments
This research was supported by a grant by the U.S. National Institutes of Health, National Institute of Child Health and Development, #R01-HD38578. The authors thank Dr. Anna Byars, Ph.D., for assistance in the administration of the Wechsler Full-Scale IQ tests, Drs. Richard Strawsburg, M.D. and Mark Schapiro, M.D., for performing the neurological examination, and Dr. William Ball, M.D., for reading the structural scans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter LC, Saykin AJ, Flashman LA, Johnson SC, Guerin SJ, Babcock DR, Wishart HA. Sex differences in semantic language processing: a functional MRI study. Brain Lang. 2003;84:264–72. doi: 10.1016/s0093-934x(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–71. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Bermudez P, Zatorre RJ. Sexual dimorphism in the corpus callosum: methodological considerations in MRI morphometry. Neuroimage. 2001;13:1121–30. doi: 10.1006/nimg.2001.0772. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–28. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–82. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Resnick SM. Sex differences in anatomic measures of interhemispheric connectivity: correlations with cognition in women but not men. Cereb Cortex. 1998;8:635–40. doi: 10.1093/cercor/8.7.635. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM. The human cerebral cortex: gender differences in structure and function. J Neuropathol Exp Neurol. 1999;58:217–26. doi: 10.1097/00005072-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science. 2000;289:457–60. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain. 1999;122 ( Pt 5):963–79. doi: 10.1093/brain/122.5.963. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Friederici AD. Syntactic working memory and the establishment of filler-gap dependencies: insights from ERPs and fMRI. J Psycholinguist Res. 2001;30:321–38. doi: 10.1023/a:1010447102554. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca’s area in sentence processing: Syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–7. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny W. Posterior probability maps and SPMs. Neuroimage. 2003;19:1240–9. doi: 10.1016/s1053-8119(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122 ( Pt 2):199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–22. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nat Rev Neurosci. 2004;5:471–82. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–72. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. The neuroanatomy of general intelligence: sex matters. Neuroimage. 2005;25:320–7. doi: 10.1016/j.neuroimage.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Jr, MacLachlan A, Soderling E, Lottenberg S, Buchsbaum MS. Regional glucose metabolic changes after learning a complex visuospatial/motor task: a positron emission tomographic study. Brain Res. 1992;570:134–43. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- Haier RJ, White NS, Alkire MT. Individual differences in general intelligence correlate with brain function during nonreasoning tasks. Intelligence. 2003;31:429–441. [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, McGrath L, Condouris K, Tager-Flusberg H. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI Brain Activation Patterns in Children Performing a Verb Generation Task. Neuroimage. 2001;14:837–43. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Rushton JP. Males have greater g: Sex differences in general mental ability from 100,000 17- to 18-year olds on the Scholastic Achievement Test. Intelligence. 2006;34:479–486. [Google Scholar]
- Jaeger JJ, Lockwood AH, Van Valin RD, Jr, Kemmerer DL, Murphy BW, Wack DS. Sex differences in brain regions activated by grammatical and reading tasks. Neuroreport. 1998;9:2803–7. doi: 10.1097/00001756-199808240-00022. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The g factor: the science of mental ability. Praeger; Westport: 1998. [Google Scholar]
- Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, Sibbitt WL, Brooks WM. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. Neuroimage. 2005;26:965–72. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Kansaku K, Kitazawa S. Imaging studies on sex differences in the lateralization of language. Neurosci Res. 2001;41:333–7. doi: 10.1016/s0168-0102(01)00292-9. [DOI] [PubMed] [Google Scholar]
- Kansaku K, Yamaura A, Kitazawa S. Sex differences in lateralization revealed in the posterior language areas. Cereb Cortex. 2000;10:866–72. doi: 10.1093/cercor/10.9.866. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex differences in amygdala activation during the perception of facial affect. Neuroreport. 2001;12:2543–7. doi: 10.1097/00001756-200108080-00050. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex differences in cerebral organization for speech and praxic functions. Can J Psychol. 1983;37:19–35. doi: 10.1037/h0080696. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kansaku K. Sex difference in language lateralization may be task-dependent. Brain. 2005;128:E30. doi: 10.1093/brain/awh460. author reply E31. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright IC, Lythgoe DJ, Williams SC, David AS. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci. 2000;12:321–41. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8:113–28. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- Lynn R. Sex differences in intelligence and brain size: a developmental theory. Intelligence. 1999;27:1–12. [Google Scholar]
- Lynn R. Sex differences in intelligence and brain size: a paradox resolved. Personality and Individual Differences. 1994;17:257–271. doi: 10.1016/j.paid.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn R, Allik J, Must O. Sex differences in brain size, stature and intelligence in children and adolescents: some evidence from Estonia. Personality and Individual Differences. 2000;29:555–560. [Google Scholar]
- Mackintosh NJ. Sex differences and IQ. Journal of Biosocial Science. 1996;28:559–572. doi: 10.1017/s0021932000022586. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–30. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci. 2003;4:310–22. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- McGlone J. Sex differences in the cerebral organization of verbal functions in patients with unilateral brain lesions. Brain. 1977;100:775–93. doi: 10.1093/brain/100.4.775. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2:2–22. [Google Scholar]
- Moffat SD, Hampson E, Wickett JC, Vernon PA, Lee DH. Testosterone is correlated with regional morphology of the human corpus callosum. Brain Res. 1997;767:297–304. doi: 10.1016/s0006-8993(97)00614-8. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12:538–49. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Nadeau SE. Phonology: a review and proposals from a connectionist perspective. Brain Lang. 2001;79:511–79. doi: 10.1006/brln.2001.2566. [DOI] [PubMed] [Google Scholar]
- Neubauer A, Fink A, Schrausser DG. Intelligence and neural efficiency: The influence of task content and sex on the brain-IQ relationship. Intelligence. 2002;30:515–536. doi: 10.1016/j.cogbrainres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Newman AJ, Pancheva R, Ozawa K, Neville HJ, Ullman MT. An event-related fMRI study of syntactic and semantic violations. J Psycholinguist Res. 2001;30:339–64. doi: 10.1023/a:1010499119393. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, Shaywitz BA, Gore JC, Shankweiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci. 2000;12:120–33. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Nowicka A, Fersten E. Sex-related differences in interhemispheric transmission time in the human brain. Neuroreport. 2001;12:4171–5. doi: 10.1097/00001756-200112210-00061. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Castillo E, Breier JI, Davis RN, Simos PG, Diehl RL. Differential brain activation patterns during perception of voice and tone onset time series: a MEG study. Neuroimage. 2003;18:448–59. doi: 10.1016/s1053-8119(02)00020-4. [DOI] [PubMed] [Google Scholar]
- Pardo PJ, Makela JP, Sams M. Hemispheric differences in processing tone frequency and amplitude modulations. Neuroreport. 1999;10:3081–6. doi: 10.1097/00001756-199909290-00038. [DOI] [PubMed] [Google Scholar]
- Patel RS, Bowman FD, Rilling JK. A Bayesian approach to determining connectivity of the human brain. Hum Brain Mapp. 2006a;27:267–76. doi: 10.1002/hbm.20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RS, Bowman FD, Rilling JK. Determining hierarchical functional networks from auditory stimuli fMRI. Hum Brain Mapp. 2006b;27:462–70. doi: 10.1002/hbm.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004a;22:1157–72. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Modelling functional integration: a comparison of structural equation and dynamic causal models. Neuroimage. 2004b;23(Suppl 1):S264–74. doi: 10.1016/j.neuroimage.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Ohrmann P, Suslow T, Wolgast M, Gerlach AL, Heindel W, Michael N. N-acetylaspartate levels of left frontal cortex are associated with verbal intelligence in women but not in men: a proton magnetic resonance spectroscopy study. Neuroscience. 2004;123:1053–8. doi: 10.1016/j.neuroscience.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Lowe MJ, Lurito JT, Dzemidzic M, Mathews VP. Temporal lobe activation demonstrates sex-based differences during passive listening. Radiology. 2001;220:202–7. doi: 10.1148/radiology.220.1.r01jl34202. [DOI] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006;44:1210–21. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Rypma B, Gabrieli JD. Neural substrates of mathematical reasoning: a functional magnetic resonance imaging study of neocortical activation during performance of the necessary arithmetic operations test. Neuropsychology. 2001;15:115–27. doi: 10.1037//0894-4105.15.1.115. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JA, Desmond JE, Glover GH, Gabrieli JD. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven’s Progressive Matrices Test. Cognit Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119 ( Pt 4):1221–38. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Dean DE, Petetot JM, de Courten-Myers GM. Gender differences in the human cerebral cortex: more neurons in males; more processes in females. J Child Neurol. 1999;14:98–107. doi: 10.1177/088307389901400207. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford Psychologists Press; Oxford, England: 1998. [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4:331–43. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Sakai KL, Homae F, Hashimoto R. Sentence processing is uniquely human. Neurosci Res. 2003;46:273–9. doi: 10.1016/s0168-0102(03)00122-6. [DOI] [PubMed] [Google Scholar]
- Saucier DM, Elias LJ. Lateral and sex differences in manual gesture during conversation. Laterality. 2001;6:239–45. doi: 10.1080/713754416. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–9. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. Neuroimage. 2006;31:1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Cognitive Modules Utilized for Narrative Comprehension in Children: A Functional Magnetic Resonance Imaging Study. Neuroimage. 2006;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive Functions Correlate with White Matter Architecture in a Normal Pediatric Population: A Diffusion Tensor MR Imaging Study. Human Brain Mapping. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of White Matter Diffusivity and Anisotropy Changes with Age During Childhood: A Cross-Sectional Diffusion Tensor Imaging Study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–6. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, Gore JC. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–9. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Shin YW, Kim DJ, Ha TH, Park HJ, Moon WJ, Chung EC, Lee JM, Kim IY, Kim SI, Kwon JS. Sex differences in the human corpus callosum: diffusion tensor imaging study. Neuroreport. 2005;16:795–8. doi: 10.1097/00001756-200505310-00003. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14:1246–55. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127:1845–52. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Jancke L, Kleinschmidt A, Schlaug G, Volkmann J, Huang Y. Sex but no hand difference in the isthmus of the corpus callosum. Neurology. 1992;42:749–52. doi: 10.1212/wnl.42.4.749. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Staiger JF, Schlaug G, Huang Y, Jancke L. Corpus callosum and brain volume in women and men. Neuroreport. 1995;6:1002–4. doi: 10.1097/00001756-199505090-00013. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Thevenaz P, Unser M. A Pyramid Approach to Subpixel Registration Based on Intensity. IEEE Transactions on Image Processing. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res Cogn Brain Res. 2004;21:418–26. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Human Brain Mapping. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112 ( Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]


