Abstract
The immune system is constantly exposed to dying cells, most of which arise during central tolerance and from effete circulating immune cells. Under homeostatic conditions, phagocytes (predominantly macrophages and dendritic cells) belonging to the innate immune system, rapidly ingest cells and their debris. Apoptotic cell removal requires recognition of altered self on the apoptotic membrane, a process which is facilitated by natural antibodies and serum opsonins. Recognition, may be site and context specific. Uptake and ingestion of apoptotic cells promotes an immunosuppressive environment that avoids inflammatory responses to self antigens. However, it does not preclude a T cell response and it is likely that constant exposure to self antigen, particularly by immature dendritic cells, leads to T cell tolerance. Tolerance occurs by several different mechanisms including anergy and deletion (for CD8+ T cells) and induction of T regulatory cells (for CD4+ T cells). Failed apoptotic cell clearance promotes immune responses to self antigens, especially when the cellular contents are leaked from the cell (necrosis). Inflammatory responses may be induced by nucleic acid stimulation of toll like receptors and other immune sensors, specific intracellular proteins and non protein (uric acid) stimulation of inflammasomes.
Keywords: Anergy, Apoptosis, Dendritic cells, Macrophages, Tolerance
Apoptotic load and sites of ingestion of apoptotic cells
Within the immune system alone, more than 109 apoptotic cells are removed from the body each day. These apoptotic cells are generated in vast numbers in the central lymphoid organs such as the thymus and bone marrow by out of frame rearrangements of antigen receptors, negative selection, or simple “neglect.” A significant load of apoptotic cells is produced in the peripheral immune system because of the relatively short life span of lymphocytes and myeloid cells and secondary selection of high-affinity B cells in germinal centers. The specialized sites of selection (i.e. thymus, bone marrow, and lymphoid follicles) have remarkably efficient phagocytes that rapidly remove the dying cells so that dying cells are difficult to detect.
Ingestion of apoptotic cells is likely a continuous process that occurs directly by macrophages in solid tissues as well as by dendritic cells in draining lymph nodes. Sites such as the lung, spleen and liver that have large numbers of macrophages and relatively easy access to circulating cells. The marginal zone of the spleen plays a special role in this regard in that it contains two populations of macrophages – marginal metallophic macrophages, which are located in the inner region of the marginal zone and which express siglec-1 (sialoadhesin, CD169), and the marginal zone (MZ) macrophages, which express MARCO and SR-A and are known to ingest particulate matter from the circulation. When normal mice receive a pulse of apoptotic cells, the cells become concentrated in splenic marginal zones at early time points (e.g. 4 hr post-injection) (Fig. 1). Tingible body macrophages (CD68+, F4/80−) located in the white pulp ingest apoptotic centroblasts during the process of B cell selection in germinal centers.
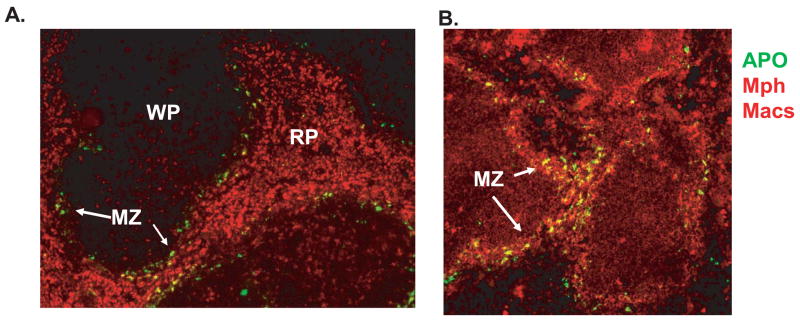
Figure 1. Apoptotic cells are removed from the circulation by macrophages in the marginal zone of the spleen.
Apoptotic cells (labeled green) injected into normal mice are observed in the marginal zones in spleens harvested 4 hrs after injection. In A, cells were stained with anti-CD68 which recognizes all macrophage populations and in B, macrophages were stained with anti-CD169 which recognizes marginal metallophilic (Mph) macrophages. Frozen sections were analyzed by immunofluorescence at 10x magnification. WP= white pulp, RP =red pulp; MZ = marginal zone.
Immunological significance of recognition of apoptotic cells
Since apoptotic cells need to be removed rapidly by phagocytosis, recognition and engulfment must occur either by neighboring cells (as occurs in embryogenesis) or elements of the innate immune system (as occurs in developing lymphoid tissue). Innate means to exist from origin, and represents the first or “original” immune response. There is no memory response involved in the innate immune response to pathogen; therefore the cells of the innate immune system must be able to recognize and respond to a broad spectrum of pathogens. Once a pathogen invades a tissue, it can be opsonized by components of the innate immune system. Depending on the site, specialized proteins such as mannose binding lectin (MBL), C1q and C3 or lung surfactants bind to pathogens, activate complement, resulting in opsonization and clearance by macrophages or the elicitation of an inflammatory response and destruction of the pathogen. Tissue macrophages or dendritic cells (DCs) may themselves recognize pathogens through Toll like receptors (TLR) and initiate an inflammatory response. Other cells involved in the initial response to pathogens, such as neutrophils and monocytes, are called in as support by way of macrophage chemokine production.
Surprisingly, recent evidence suggests that mechanisms similar to those used by the innate immune system to recognize and dispose of pathogens are also used to recognize and dispose of apoptotic cells. Many of the proposed systems function as scavenger receptors or receptors for bacterial components, reinforcing the idea that the innate immune system has evolved to scavenge both self as well as non-self material. What happens after clearance dictates how the innate immune system and, indeed, the adaptive immune system respond to the ingested particles. For more discussions of innate and adaptive immune responses and their relationship to loss of tolerance, a more detailed discussion can be found in several recent papers published in the Journal of Autoimmunity [1–3].
Natural antibodies and serum opsonins facilitate apoptotic cell clearance by binding to modified lipids on the apoptotic cell surface
Modification of two lipid structures, phosphatidylserine (PS) and phosphorylcholine (PC) play important roles in the clearance of apoptotic cells. The flip of PS from the inside to the outside of the cell membrane is a very early event that is caused by the reduced function of a translocase and possibly by activation of a lipid scramblase. Unmodified PS probably does not directly serve as a ligand for binding to a receptor, although oxidation of PS or phosphatidylcholine is reported to engage the scavenger receptor, CD36 [4]. Translocation of PS to the cell surface membrane leads to binding by a number of serum opsonins such as Annexin I, Gas6, beta-2 glycoprotein 1, and milk fat globule epidermal growth factor 8 (MFG-E8) and is therefore a critical signal for apoptotic cell recognition (Fig. 2) Why so many opsonins are involved is unknown, but may allow multistep adhesion and signal transduction, engagement of different receptors under baseline versus inflammatory conditions or at different tissue sites. .
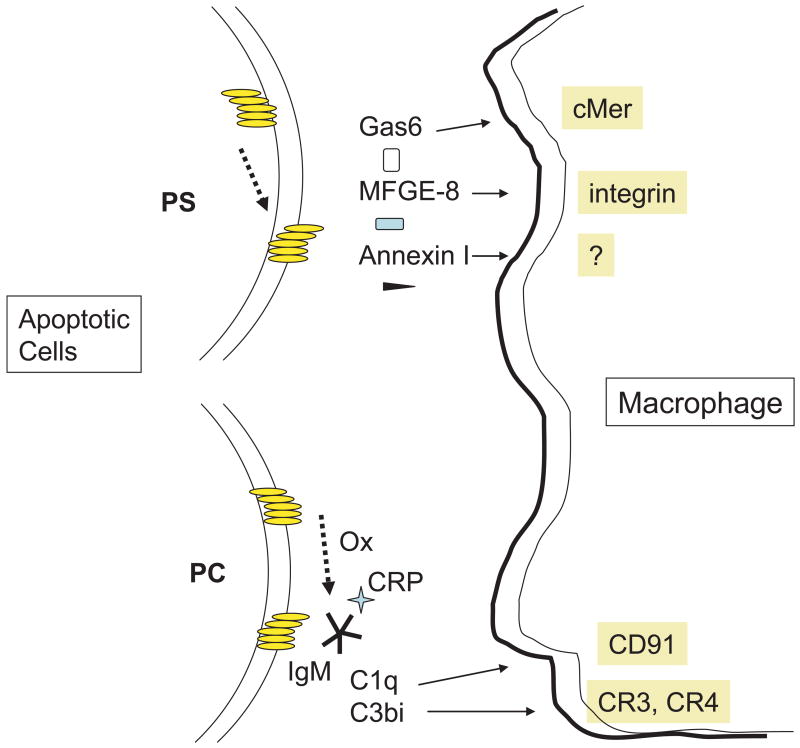
Figure 2. Role of modified lipids in the recognition and clearance of apoptotic cells.
Upper left. Translocation of phosphatidylserine (PS) from the inside to outside of the apoptotic cell membrane allows serum opsonins to bind to PS. Different opsonins interact with different ligands on the phagocyte allowing compartment specific uptake to occur.
Lower left. Phosphorylcholine, which is situated on the outside of the cell membrane may be biochemically modified in several ways on apoptotic cells. Caspase 3 activates the calcium independent phospholipase A2 (iPLA2) to cleave the fatty acid from PC, yielding lysoPLA2. LysoPLA2 acts as both an antigen for natural IgM antibodies as well as a chemoattractant for macrophages. PC may be oxidized by reactive oxygen (ROI) intermediates as well. Binding of natural antibodies to apoptotic cells explains, in part, activation of the classical pathway of complement and deposition of C3bi on the cell surface. C3bi serves as a ligand for CR3 and CR4 on phagocytes.
Unlike PS, PC is normally found on the outside of the plasma membrane of cells and is therefore normally recognized as self. However, when a cell undergoes apoptosis, PC is modified by one of several mechanisms. We have shown that, following apoptosis induction, caspase 3 cleaves the calcium independent phospholipase A2 (iPLA2), which in turn, removes the fatty acid from the sn-2 position of PC generating lyso-PC [5]. IgM antibodies and acute phase proteins such as CRP, have been shown to bind to lyso-PC [6, 7] (Fig. 2). Other investigators have also shown that certain anti-PC monoclonal antibodies that bear the T15 idiotype bind to oxidatively modified determinants of PC on apoptotic cells [8].
Antibodies reactive with PC were described decades ago and act as a first line of defense against S. pneumoniae and other Gram positive organisms that contain PC as a component of the outer cell wall. All strains of mice produce idiotypically restricted anti-PC antibodies of the T15 family that are synthesized predominantly by B1 B cells. The antibody response to PC is therefore largely T cell- independent and thought to be part of the ‘natural repertoire’ [9]. Most of these antibodies belong to the IgM or IgG3 subclass.
Unlike conventional bone marrow derived B2 cells, CD5+ B-1 cells develop from liver fetal cells and utilize a limited set of VDJ gene combinations to form their receptors. For example, anti-PC antibody is encoded by either VH11and Vk9 or by VH12 and Vk4 [10]. This restricted antibody repertoire suggests that B-1 cells are a component of innate immunity preserved to respond to self and other natural antigens. Although these antibodies may be stimulated by exposure to bacterial PC as discussed, a significant percentage of normal IgM is found to have anti-PC specificity in the serum of germ free mice [11], suggesting that this specificity is selected by self antigen. In other contexts, B-1 cells also differ from B-2 cells in their response to self antigens- instead being deleted, B-1 cells are positively selected [12]. This may explain why CD5+ B1 cells are most abundant in the peritoneum, a “privileged site” that insulates them from being fully activated by self antigens.
A second B cell population that shares many attributes with B-1 cells is marginal zone (MZ) B cells (reviewed in [13]). As the progeny of bone marrow precursors, MZ B cells reside in spleen. Their unique location makes them likely to have frequent encounters with blood born pathogens. More importantly, these cells may provide a rapid response to antigens and their expression of CD1 like MHC class I on their surface make MZ B cells potential stimulators of T cell subsets with specificity for lipids. Whether or not they interact with apoptotic cells or fragments thereof exposed on MZ macrophages or DCs, remains to be determined.
We have provided evidence that both IgM and CRP facilitate apoptotic cell clearance through activation of the classical complement pathway [6, 7, 14] (Fig. 2). Activation of the classical complement pathway leads to C3b deposition on the cell surface which is rapidly inactivated to C3bi. Full assembly of the C3 convertase C3b, Bb is prevented by the action of cell membrane complement regulatory proteins, CD55 (DAF), CD46 (MCP) and/or recruitment of the potent serum complement inhibitor, Factor H [6]. C3bi on the apoptotic cell is recognized by CR3 and CR4 on macrophages and possibly dendritic cells. Others have provided strong supporting in vivo evidence that clearance of apoptotic cells is, in part, C3 dependent [15].
Other serum opsonins or bridging molecules include thrombospondin that bridges the αvβ3 and CD36 receptors [16] and collectins (mannose binding protein, C1q and surfactant proteins). Collectin binding receptors are controversial (see[17] for discussion). The ER protein, calreticulin (CRT), is unique in that it is translocated from the ER to the cell surface of apoptotic cells but can also be detected at low concentrations on live cells [18]. CRT on the apoptotic cells binds to CD91 on the phagocyte, although precise signaling mechanisms remain to be determined (Fig. 2).
Non opsonic receptors and ligands implicated in phagocytosis of apoptotic cells
Despite the detection of only limited chemical alterations on the apoptotic cell membrane, blockade of a large and diverse number of receptors on phagocytes can impair the uptake of apoptotic cells (Fig. 2). This diversity may in part be explained by the different cells and conditions used for phagocytic assays, but it undoubtedly also reflects the overlapping and partially redundant function of each individual receptor as well as specialized requirements at different anatomic sites. For example, MFG-E8 facilitates apoptotic cell clearance in germinal centers [19] whereas C1q deficiency leads to apoptotic cell accumulation in the kidney [20].
Many of the phagocytic receptors are integrins comprising the vitronectin receptor, αvβ3, αvβ5 [21], complement receptors 3 (CD11b/CD18) and 4 (CD11c/CD18)[22], and class A (CD36) and B scavenger receptors. Non-integrin receptors include the ATP-binding cassette transporter (ABC1) [23], CD14 [24] and the closely related Tyro 3 family receptor tyrosine kinases, c-Mer, TYRO and Axl [25]. CD91 (LDL-receptor related protein) a multifunctional receptor, recognizes 30 different ligands, calreticulin being one [18]. According to the “tether and tickle” model[26], some receptors, such as CD14 or CR3, serve as recognition structures and contribute to adhesion, others like CD91 convey signals for engulfment and yet others, such as SIRP-α, prevent uptake.
Effect of phagocytosis of apoptotic cells on innate immune function
The ingestion of apoptotic cells has significant effects on the phagocyte and, potentially on the T cell response to ingested antigens. Because some peptides derived from apoptotic cells can be presented to lymphocytes by DCs and possibly by macrophages through cross-priming [21, 27], a question of critical importance to studies of autoimmunity is whether self peptides are presented after phagocytosis of apoptotic cells and under what conditions they induce tolerance or immunity. We will first consider the effect of apoptotic cells on both macrophages and DCs (Fig. 3).
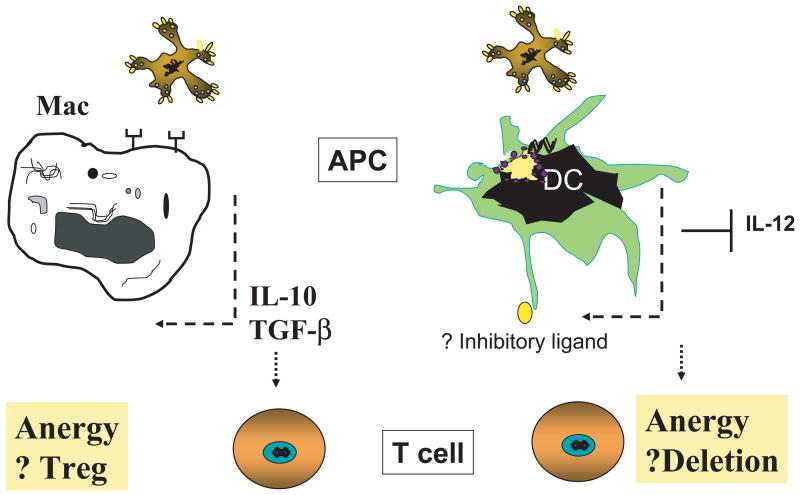
Figure 3. Effect of apoptotic cell ingestion on innate and adaptive immunity.
Left panel. Ingestion of apoptotic cells by macrophages induces the expression of anti-inflammatory cytokines such as TGF-β and IL-10. Both of these cytokines are known to promote the expression of T regulatory cells (Treg).
Right panel. Uptake of apoptotic cells by immature dendritic cells (iDC) leads to suppression of IL-12 production as well as possible changes in the cells surface expression of inhibitory ligands. Interaction of iDC with potentially autoreactive CD8+ T cells leads to anergy and/or deletion of CD8+ T cells. See text for details.
Macrophages
As shown in Fig. 1, splenic macrophages readily ingest particulates including apoptotic cells in the circulation but the functional outcome of this interaction in vivo is unclear. In vitro [28, 29] and some in vivo [30] studies suggest that uptake of apoptotic cells by macrophages induces the expression of immunosuppressive cytokines such as transforming growth factor–β1 (TGF-β1), prostaglandin E2, and possibly IL-10 by macrophages. These cytokines tend to dampen an immune response to self antigens. Significantly, opsonic ligands such as Gas6 and protein S on apoptotic cells that engage the tyrosine kinase receptor, c-Mer, suppress production of IL-12 or TNF-α by macrophages.
Macrophages are heterogeneous populations of cells. Different subsets are identified by their location, cell surface staining characteristics as well as their prior exposure to cytokines and growth factors [31]. Amongst the best known murine subsets are the M1 and M2 (classical and alternative macrophage populations M1 and M2 respectively). M1 macrophages are stimulated by IFN-g, produce iNOS and inflammatory cytokines such as TNF-a and IL-6. M2 macrophages are activated by IL-4 and IL-13 and produce arginase and the anti-inflammatory cytokine, IL-10. Recently, it was shown that M-CSF derived human macrophages that produce IL-10 preferentially ingest apoptotic cells compared to “inflammatory” type macrophages stimulated by Gm-CSF [32]. The implications of these observations are that ingestion of apoptotic cells by IL-10 producing macrophages will maintain IL-10 production and immunosuppression whereas ingestion of apoptotic cells by ‘inflammatory” macrophages leads to a reduction of inflammatory cytokines.
Dendritic Cells
Stuart et al [33] first reported that following ingestion of apoptotic cells, DCs suppress the expression of IL-12, the critical activator of IFN-g. We have confirmed these observations and isolated a specific transcriptional repressor of IL-12, GC-BP [34]. In addition, we have also identified a co-inhibitory molecule that is upregulated on DCs following ingestion of apoptotic cells and that facilitates suppression of T cell responses to antigens contained within apoptotic cells (manuscript in preparation).
T cell responses to apoptotic cells
To date, most studies have focused on the ability of apoptotic cells to prime T cells in mouse models. Using ovalbumin (OVA) as a pseudo self antigen, many groups have attempted to identify the DC subsets that activate CD8+ T cells. Different experimental designs have given rise to different results. In one approach, apoptotic cells were injected in vivo, different types of APCs purified and examined for their ability to stimulate OT-I T cells in vitro. Here, it was concluded that CD8a+ CD11c DCs were the sole population that cross-presented apoptotic cell derived antigen [35, 36]. In a second approach, APCs were sorted and incubated with apoptotic cells and OT-I T cells in vitro. This study concluded that both CD8a+ and CD8a− DCs can cross-present, though the efficacy was greater for CD8a+CD11c DCs [37]. In order to reconcile the results, one has to consider the immediate activation of DCs in vitro and their high death rate after overnight culture. To minimize these potential in vitro artifacts, future studies will need to utilize adoptive transfer of apoptotic cell loaded DCs into hosts that are deficient in protein processing or antigen presentation so that only the transferred DC are able to present antigen.
Some studies have used human monocyte derived DCs in vitro to investigate the T cell responses to cross-presentation. The original observation made by Albert et.al [38] revealed the critical role of DCs in presentation of apoptotic material. Blander and Medzhitov proposed that apoptotic cells and bacteria are contained in segregated compartments of DCs and that the endosomes containing self antigens from apoptotic cells are totally degraded and not presented efficiently [39]. Though this study provides a satisfying explanation of how autoimmunity is averted, the conclusions do not explain responses to endogenously expressed antigens. For example, numerous studies have transferred beta cell specific BDC2.5 T cells into NOD mice or OVA specific T cells into RIP-mOVA mice. In the absence of any significant TLR signals, these T cells are activated and proliferate in draining lymph nodes - presumably in response to antigen released by apoptotic cells from the pancreas [40]. In the case of infections, TLR stimuli are inevitably bundled with dead and dying cells but normal individuals rarely develop autoimmunity after infection. Thus, co-localization of TLR stimulus with apoptotic self antigen cannot be a sufficient explanation for autoimmunity. A second difficulty of generalizing the afore-mentioned conclusion is uncertainty as to which subset(s) of in vivo DCs bone marrow derived DCs truly represent. Of note, Naik et al [41] suggested monocyte derived DCs only appear in vivo under certain inflammatory conditions.
The fate of T cells following presentation or cross-presentation of apoptotic cells
The T cell response to antigen is dependent upon 3 signals: TCR engagement (signal 1), co-stimulation (signal 2, that includes CD4 T cell help by IL-2 production) and inflammatory signals (signal 3) [42]. In response to an infectious agent, the TCR response is usually strong, co-stimulatory molecules are unregulated and inflammatory cytokines secreted. In addition, cytokines such as IL-2, Type I IFN, IL-7, IL-15 released during infection are critical for maintaining a sizable and functional memory CD8 T cell pool. In contrast, during cross-presentation of apoptotic cells (self antigens), T cells will encounter a weak signal 1, a weak perhaps inhibitory signal 2 and an absence of a positive signal 3. As a result, T cells will likely be partly activated [43], become anergic and eventually be deleted [44] (Fig. 3).
Therapeutic immunosuppression by apoptotic cells
As discussed above and illustrated in Fig. 3, uptake of apoptotic cells by APC leads to the production of TGF-b and IL-10 in vitro. Both of these cytokines have been implicated in the induction or maintenance of T regulatory cells (Treg) (reviewed in [45]). Although not known at the time, the first beneficial use of apoptotic cells was the administration of UV-A irradiated peripheral blood cells previously exposed to 8-methoxypsoralen (8-MOP) (PUVA) for treatment of cutaneous lymphomas in humans. This treatment has subsequently been used for psoriasis and other autoimmune disorders. Experimental studies in mice revealed that intravenous administration of PUVA treated apoptotic cells attenuated T cell mediated contact hypersensitivity [46]. Suppression could be adoptively transferred and was dependent on DC induction of CD4+ CD25+ Treg. In a related study, Kleinclauss et al [47] reported that intravenous apoptotic cell infusion was efficient at the suppression of graft versus host disease (GVHD). In this case, suppression was also mediated by Treg which were verified to be Foxp3 mRNA positive but the suppression was abrogated by macrophage depletion. Thus, both macrophages and DCs have been implicated in CD4 T cell immunosuppression by Treg in clinically relevant situations.
Consequences of failed apoptotic cell clearance
Engulfment of apoptotic cells or necrotic cell debris is the first step of the “clean up” process, but swift degradation of cellular contents both within the phagocyte and extracellularly is equally important. Failure to degrade these molecules likely explains why the debris from necrotic cells induces type 1 IFN, TNF-α and other pro-inflammatory cytokines [28, 48].
There are multiple mechanisms for debris clean up. Extracellularly, opsonins such as CRP functions as a scavenger not only for the intact apoptotic cell, but also for nucleoproteins. Serum contains a potent DNase, DNase 1, as well as abundant RNases that help remove nucleic acids released from cells. Within the cell, a specific acid-activated DNase, DNase II, resides in lysosomes and degrades ingested DNA, TREX1 is a 3′ DNA exonuclease and multiple different RNases have been described. Conclusively illustrating the vital importance of these nucleases, deficiencies are associated with autoimmunity. DNase 1 deficiency causes lupus on a susceptible strain background, selective deficiency of DNase II in macrophages led to arthritis in mice [49] and TREX1 mutations are associated with chilblain lupus [50].
The consequences of failed apoptotic cell clearance and relationship to induction of autoimmune responses have been discussed in detail elsewhere [51]. Here we will briefly summarize the relevant molecular pathways. Self nucleic acids may be potent inducers of type 1 interferons (IFN) and other inflammatory cytokines through activation of both Toll Like Receptors (TLR 3, 7,8 and 9) and TLR-independent pathways (reviewed in [52]). A number of cellular constituents have also been implicated in inflammation including HMGB1 and uric acid. Of note, it was recently reported that highly purified non-recombinant HMGB-1 was non inflammatory but when bound to DNA could induce cellular activation [53]. In addition, once tolerance is broken and autoantibodies generated, the antibodies binding to nucleoprotein antigens amplify the immune response through engagement of B cell receptors and ingestion by DCs, particularly, plasmacytoid DC (pDCs).
Conclusions
Removal of apoptotic cells has only been studied in depth over the last decade. Apoptotic cells undergo specific changes on their cell membrane which are recognized by natural antibodies and serum opsonins. Under homeostatic conditions, the three potential TCR activating signals are weak resulting in T cell anergy, deletion (CD8 T cells) and or Tregulatory cell production (CD4+ T cells). The products of apoptotic cells likely continuously tolerize T cells. Deficiency of specific opsonins leads to accumulation of apoptotic cells at specific sites implicating specialized regional clearance pathways. Defective clearance of nucleic acids appears to play a critical role in breaking tolerance, particularly if the nucleic acid engages intracellular sensors. The data and discussion herein can be compared and contrasted with other papers in this volume which discuss related issues that lead to loss of tolerance [54-.
Acknowledgments
This work was supported by research grants K08-AR052804 from NIAMS and HHMI Physician-Scientist Early Career Award (DAM) and AR48796 (KBE) from NIAMS. We thank current and past laboratory members for contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbas AK, Lohr J, Knoechel B. Balancing autoaggressive and protective T cell responses. J Autoimmun. 2007;28:59–61. doi: 10.1016/j.jaut.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blank M, Shoenfeld Y. B cell targeted therapy in autoimmunity. J Autoimmun. 2007;28:62–8. doi: 10.1016/j.jaut.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien BA, Geng X, Orteu CH, Huang Y, Ghoreishi M, Zhang Y, Bush JA, Li G, Finegood DT, Dutz JP. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun. 2006;26:104–15. doi: 10.1016/j.jaut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–25. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med. 2002;196:655–65. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive Protein Binds to Apoptotic Cells, Protects the Cells from Assembly of the Terminal Complement Components, and Sustains an Antiinflammatory Innate Immune Response. Implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim G, Jun JB, Elkon KB. Necessary role of phosphatidylinositol 3-kinase in transforming growth factor beta-mediated activation of Akt in normal and rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2002;46:1504–11. doi: 10.1002/art.10314. [DOI] [PubMed] [Google Scholar]
- 8.Shaw PX, Goodyear CS, Chang MK, Witztum JL, Silverman GJ. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J Immunol. 2003;170:6151–7. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 9.Kearney JF. Immune recognition of OxLDL in atherosclerosis. J Clin Invest. 2000;105:1683–5. doi: 10.1172/JCI10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa K, Hardy RR. Development and function of B-1 cells. Curr Opin Immunol. 2000;12:346–53. doi: 10.1016/s0952-7915(00)00098-4. [DOI] [PubMed] [Google Scholar]
- 11.Grandien A, Coutinho A, Viale AC, Freitas A, Andersson J, Marcos M. On the origin of natural IgM in immunoglobulin transgenic mice. Intl Immunol. 1992;4:1153–60. doi: 10.1093/intimm/4.10.1153. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 13.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–35. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 14.Ogden CA, Kowalewski R, Peng YF, Montenegro V, Elkon KB. IgM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 15.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, Wang Z, Watkins SC, Falo LD, Jr, Thomson AW. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuart LM, Henson PM, Vandivier RW. Collectins: opsonins for apoptotic cells and regulators of inflammation. Curr Dir Autoimmun. 2006;9:143–61. doi: 10.1159/000090778. [DOI] [PubMed] [Google Scholar]
- 18.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 20.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 21.Albert ML, Pearce SFA, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alpha-v-beta-5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mevorach D, Mascarenhas J, Gershov DA, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp, Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luciani MF, Chimini G. The ATP binding cassette transporter, ABC1, is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 1996;15:226–235. [PMC free article] [PubMed] [Google Scholar]
- 24.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 26.Somersan S, Bhardwaj N. Tethering and tickling: a new role for the phosphatidylserine receptor. J Cell Biol. 2001;155:501–4. doi: 10.1083/jcb.200110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA. Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol. 1997;159:5391–5399. [PubMed] [Google Scholar]
- 28.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppresive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 30.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF- beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–44. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten C. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 2006;107:4930–7. doi: 10.1182/blood-2005-10-4144. [DOI] [PubMed] [Google Scholar]
- 33.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–35. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 34.Kim SJ, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 35.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci U S A. 2006;103:10729–34. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 39.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–12. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 40.Kurts C, Carbone FR, Barnden M, Blanas E, Allison J, Heath WR, Miller JF. CD4+ T cell help impairs CD8+ T cell deletion induced by cross- presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–62. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–71. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 42.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–70. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 44.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–7. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 46.Maeda A, Schwarz A, Kernebeck K, Gross N, Aragane Y, Peritt D, Schwarz T. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174:5968–76. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 47.Kleinclauss F, Perruche S, Masson E, de Carvalho Bittencourt M, Biichle S, Remy-Martin JP, Ferrand C, Martin M, Bittard H, Chalopin JM, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 49.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 50.Rice G, Newman WG, Dean J, Patrick T, Parmar R, Flintoff K, Robins P, Harvey S, Hollis T, O’Hara A, et al. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;80:811–5. doi: 10.1086/513443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin DA, Elkon KB. Apoptosis. In: Hahn BH, editor. Dubois’ Lupus. 2007. [Google Scholar]
- 52.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–32. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Z-H, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz-Argüelles A, Brito GJ, Reyes-Izquierdo P, Pérez-Romano B, Sánchez-Sosa S. Apoptosis of melanocytes in vitiligo results from antibody penetration. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.012. in press. [DOI] [PubMed] [Google Scholar]
- 56.Zelenay S, Fontes MFM, Fesel C, Demengeot J, Coutinho A. Physiopathology of natural auto-antibodies: The case for regulation. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.011. in press. [DOI] [PubMed] [Google Scholar]
- 57.Papadimitraki ED, Bertsias G, Boumpas DT. Toll like receptors and autoimmunity: A critical appraisal. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.09.001. in press. [DOI] [PubMed] [Google Scholar]
- 58.Lutz HU. Homeostatic roles of naturally occurring antibodies. An overview. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.007. in press. [DOI] [PubMed] [Google Scholar]
- 59.Cohen IR. Biomarkers, self-antigens and the immunological homunculus. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.016. in press. [DOI] [PubMed] [Google Scholar]
- 60.Pasquali J-L, Soulas-Sprauel P, Korganow A-S, Martin T. Auto-reactive B cells in transgenic mice. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.006. in press. [DOI] [PubMed] [Google Scholar]
- 61.Lang KS, Burow A, Kurrer M, Lang P, Recher M. Balance of the innate immune response in autoimmune disease. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.018. in press. [DOI] [PubMed] [Google Scholar]
- 62.Vollmers HP, Brändlein S. Natural antibodies and cancer. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.013. in press. [DOI] [PubMed] [Google Scholar]
- 63.Rowley B, Tang L, Shinton S, Hayakawa K, Hardy RR. Autoreactive B-1 B cells: Constraints on natural autoantibody B cell antigen receptors. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryan KR, Patel SD, Stephens LA, Anderton SM. Death, adaptation and regulation: the three pillars of immune tolerance restrict the risk of autoimmune disease caused by molecular mimicry. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.014. in press. [DOI] [PubMed] [Google Scholar]
- 65.Avrameas S, Ternynck T, Tsonis IA, Lymperi P. The immune system as emerges from studies on natural autoantibodies. J Autoimmun. 2007 in press. [Google Scholar]
- 66.Milner J, Ward J, Keane-Myers A, Min B, Paul WE. Repertoire-dependent immunopathology. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lan RY, Mackay IR, Gershwin ME. Regulatory T cells in the prevention of mucosal inflammatory diseases: Patrolling the border. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]