Abstract
Programmed remodeling of cell surface glycans by the sequential action of specific glycosyltransferases, can control biological processes by generating or masking ligands for endogenous lectins. Galectins, a family of animal lectins with affinity for β-galactosides, can form multivalent complexes with cell surface glycoconjugates and deliver a variety of intracellular signals to modulate cell activation, differentiation, and survival. Recent efforts involving genetic or biochemical manipulation of O- and N-glycosylation pathways, as well as blockade of the synthesis of endogenous galectins, have illuminated essential roles for galectin-glycoprotein lattices in the control of biological processes including receptor turnover and endocytosis, host-pathogen interactions and immune cell activation and homeostasis.
Keywords: galectins, galectin-glycoprotein lattices, receptor turnover, immunity, inflammation
Introduction
For many systems, the clustering of protein receptors and ligands is required for optimal transmission of signals into a cell. The thermodynamically favorable assembly of ordered arrays of lectins and saccharides on the cell surface may thus be integral to cellular signaling and adhesion [1]. Lectin multivalency enables recognition of multiple binding partners, allowing these glycan-binding proteins to play leading roles in signal transduction in different biological processes as well as in cell-cell and cell-pathogen interactions [2–4].
Galectins are a family of soluble lectins that bind β-galactoside-containing glycans and are defined by a conserved carbohydrate recognition domain (CRD) and a common structural fold [2–4]. Among the various lectin types, galectins are probably the most conserved and ubiquitous family, with members identified in most animal taxa examined so far [4]. As many as fifteen galectins have been identified in mammals and proposed to mediate diverse biological processes involved in the regulation of innate and adaptive immune responses, such as cell activation, differentiation, cytokine secretion and apoptosis [5,6]. Here we discuss recent findings on the biochemistry of galectin-glycoprotein lattices and their functional relevance in the control of receptor endocytosis, host-pathogen interactions and activation and homeostasis of immune cells.
Biochemical aspects of galectin-glycoprotein lattices formation
Lectin-monosaccharide interactions are relatively weak (dissociation constants ~10−4 M), with two to five hydrogen bonds complemented by hydrophobic and van der Waals interactions [4,5,7]. Galectins preferentially bind β-galactoside-containing glycans comprised of repeating units of N-acetyllactosamine (Galβ1,4GlcNAc; LacNAc), either as disaccharide units at the termini of complex N-glycans, or as repeating units in a poly-N-acetyllactosamine chain on N- or O-glycans [5,7,8]. Galectin binding affinities to complex N-glycans are proportional to their LacNAc content and to their GlcNAc branching [5,7–11].
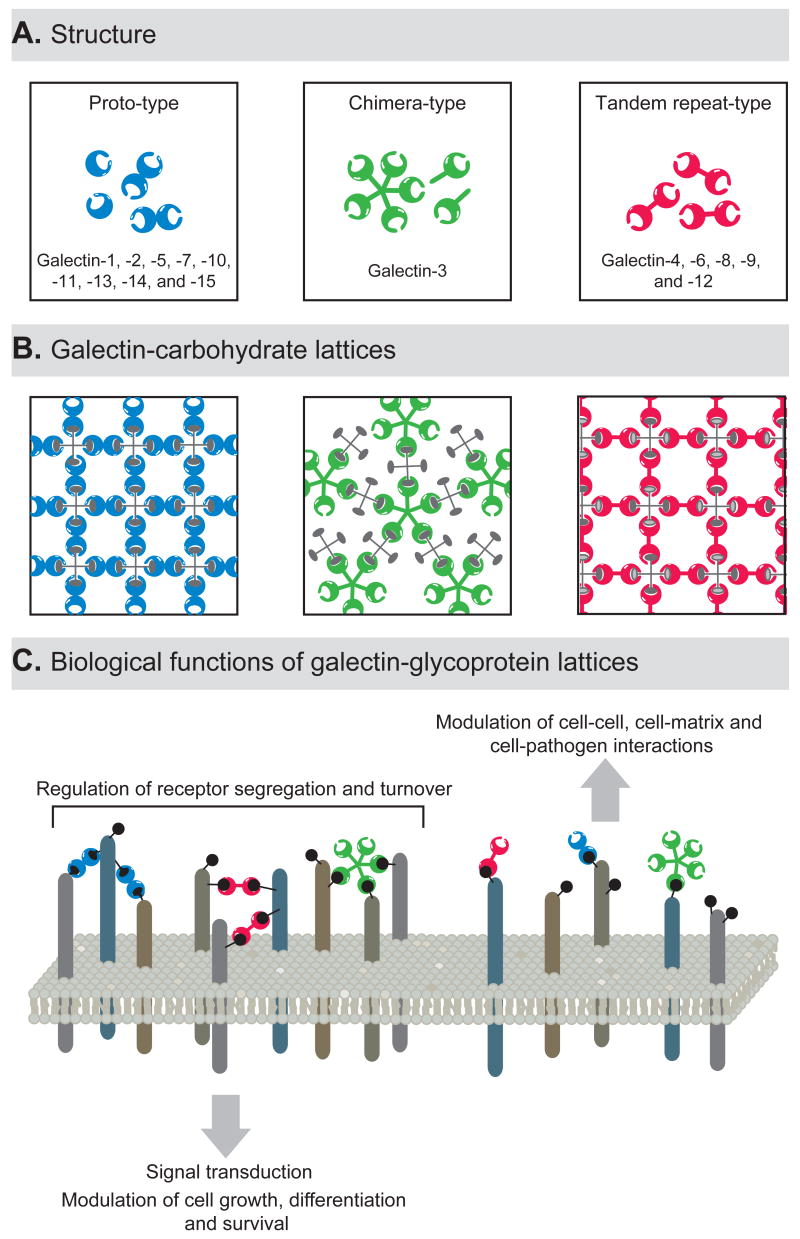
Lattice formation by galectins requires multivalent oligomerization to attain stability and biologic functionality[5]. Based on structural features, galectins have been classified into three types: proto, chimera, and tandem-repeat [4]. Proto-type galectins contain one CRD per subunit and typically dimerize through noncovalent interactions to create functionally bivalent lectins. Proto-type galectin-1 is a dimer in solution and crystallizes as a dimer in cross-linked complexes with a divalent oligosaccharide[12]. The presence of more than one CRD in a galectin-1 homodimer makes it well-suited for mediating cell adhesion, eliciting signaling, and forming lattices [3,5]. The chimera-type galectin-3 has a C-terminal CRD similar to the proto-type, but exhibits an N-terminal domain that is responsible for interactions between subunits, facilitating its oligomerization [3]. Galectin-3 monomers are in equilibrium with higher order oligomers in solution, and galectin-3 precipitates as a pentamer with multivalent oligosaccharides [13]. This lectin binds to multi-glycosylated proteins with positive cooperativity, suggesting that galectin-3 monomers, after ligand binding, recruit additional lectin molecules to form a complex of multivalent interactions [5,13]. The biologic functions attributed to galectin-3 are thus likely to depend upon both ligand cross-linking and oligomerization [6,14–17]. The tandem-repeat type galectins have two CRDs connected by a linker peptide and, thus are bivalent, although the two CRDs may be able to recognize different saccharide ligands [6] (Figure 1).
Figure 1. Biochemistry and functional relevance of galectin-glycoprotein lattices.
(A) Schematic representation of the structure of different monomeric and oligomeric members of the galectin family. Proto-type galectins contain one CRD and exist in solution as homodimers. Chimera-type galectins are thought to undergo a conformational change following carbohydrate ligand binding which enables their oligomerization as pentamers. Tandem-repeat type galectins contain two distinct CRDs in tandem, connected by a linker of up to 70 amino acids, and are thus inherently dimeric. (B) Schematic representation of lattice formation between multivalent galectins and multivalent carbohydrate ligands. (C) Biological relevance of galectin-glycoprotein lattices.
Glycoproteins often bear multiple copies of the saccharide ligands that are recognized by galectins [18]. While galectin binding to a single saccharide ligand is typically a low-affinity interaction (association constants ~104 M−1), the multivalent nature of galectin-saccharide interactions results in high overall avidity (association constants ~106 M−1) [5,9]. This multivalency also allows the formation of lectin-carbohydrate lattices. Both in solution and on the cell surface, multivalent galectins selectively cross-link a single species of glycoprotein to form homogeneous lectin-carbohydrate lattices [5,17,19].
The ability of galectins to reorganize membrane glycoproteins into lipid raft microdomains suggests that multivalent lectin-saccharide interactions occur preferentially in these microdomains [5]. In this regard, the ganglioside GM1 is able to organize microdomains into raft-like structures and is a prominent glycolipid headgroup in such rafts. Galectin-1 binds the GM1 pentasaccharide glycan and these interactions may represent a mechanism by which galectins organize lipid rafts [20]. Since lipid rafts are considered essential for assembling signal transduction components at the plasma membrane [1], association of galectins with lipid rafts is likely to be important for galectin-mediated signaling events.
Biological aspects of galectin-glycoprotein lattices
Galectins and receptor turnover
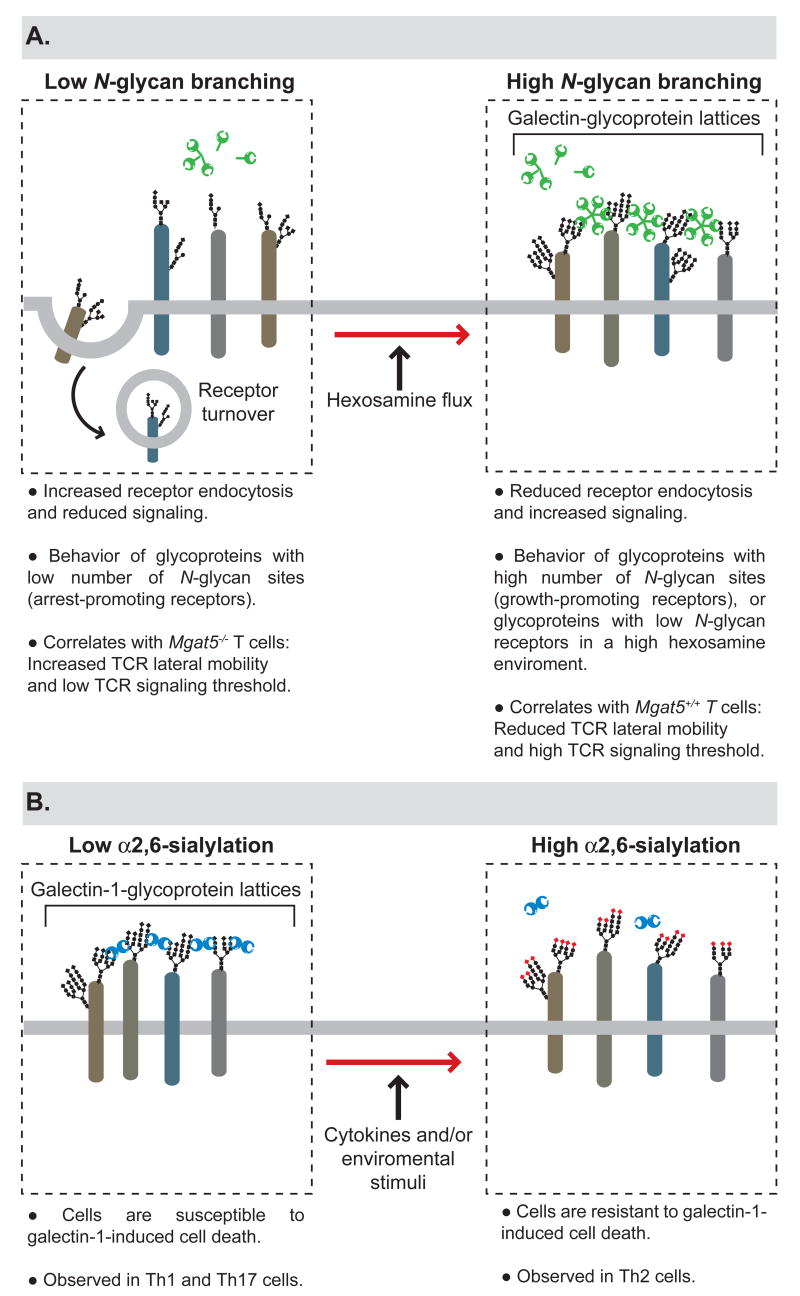
Lattice formation following the binding of complex N-glycans to galectins effectively traps glycoprotein receptors at the cell surface, preventing their endocytosis. Thus, interactions between N-glycans and galectins can regulate the distribution of cell surface receptors as well as the cell’s responsiveness to receptor agonists [11]. Recently, Dennis and colleagues described a link among N-glycan multiplicity, N-glycan branching kinetics, integration of nutrient metabolism and changes between cell growth and arrest [10]. The authors described that N-branching in the Golgi is sensitive to hexosamine flux for its production of complex N-glycans. Whereas arrest-promoting receptors (e.g., e.g., TGFβR and CTLA-4) have few N-glycosylation sites and show a ‘switch-like’ responses to hexosamine concentrations, growth-promoting receptors (e.g., EGFR, IGFR, FGFR, and PDGFR) with high numbers of N-glycans exhibit hyperbolic responses to hexosamine [10]. Therefore, increased nutrient flux stimulated by growth-promoting receptors, will ultimately activate cellular arrest and differentiation programs by increasing surface levels of glycoreceptors with low numbers of N-glycans. The authors report that increasing UDP-GlcNAc leads to increased branching of N-glycans, increased receptor association with cell surface galectin-3 and enhanced signaling [10]. Thus, galectin-carbohydrate lattices can regulate the decision between cell growth and arrest by regulating receptor turnover (Figure 2).
Figure 2. Galectin-glycoprotein lattices in the regulation of receptor turnover, cell signaling and survival.
(A) The degree of N-glycan branching controls galectin-glycoprotein lattice formation, which in turn modulates receptor turnover and signaling. (B) Differential sialylation of cell surface glycoproteins selectively influences the formation of galectin-glycoprotein lattices in distinct T-helper cells, thus regulating their susceptibility to galectin-1-induced cell death.
A second biological example of galectin-mediated control over receptor endocytosis is the galectin-9-glucose transporter 2 (GLUT-2) system. All vertebrate glucose transporters have a single conserved N-glycan site. Cell-type and glycoprotein-specific N-glycans attached by N-acetylglucosaminyltransferase IVa (GlcNAcT-IVa) are needed to maintain the glucose transporter GLUT-2 on the surface of pancreatic β cells [21]. GlcNAcT-IVa-dependent glycosylation increases the cell-surface half-life of GLUT-2, suggesting that interactions involving the GLUT-2 N-glycan structure may suppress its endocytosis. Since GLUT-2 and galectin-9 normally co-localize in pancreatic β cells in a GlcNAcT-IVa-dependent manner [21], it is surmised that galectin-9 acts to retain glucose receptors on the cell surface.
Also by interfering with receptor endocytosis, N-acetylglucosaminyltransferase V (GlcNAcT-V) expression-dependent galectin lattices, such as galectin-3-TGFβR lattices, maintain growth-factor receptor densities at levels that promote invasive phenotypes in transformed cells [11,22]. In addition, galectin-3 interactions with GlcNAcT-V-modified N-glycans stimulate α5β1 integrin activation, focal adhesion remodeling and phosphatidylinositol 3-kinase activation, thus promoting tumor cell motility [23].
Galectin-carbohydrate lattices in host-pathogen interactions
Galectins interact with β-galactoside-enriched glycoconjugates present in several pathogens [24–26]. While the nature of these interactions is not well-characterized, galectin oligomerization and/or lattice formation are likely to play a role.
It has been demonstrated that galectin-1 inhibits envelope-mediated cell-cell fusion of some paramyxoviruses by binding to specific N-glycans on viral glycoproteins and inducing its oligomerization [27]. However, galectin-1 can also promote human immunodeficiency virus infectivity by stabilizing viral attachment to host cells and cross-linking viral glycoproteins with the target cells [28].
Specific interactions have been described between galectin-3 and -9 and the intracellular protozoan Leishmania. Galectin-3 binds the lipophosphoglycan (LPG) of L. major [26]. This binding leads to the proteolytic removal of the galectin-3 N-terminal domain, preventing oligomerization. In this way, L. major destroys galectin-3 lattice formation, leading to a decreased threshold for signal transduction [26]. Galectin-9 also recognizes L. major by binding to LPG and promotes L. major-macrophage interactions, which may be critical for the clinical outcome of leishmaniasis [25].
Recently, a novel galectin type with four CRDs was discovered in the eastern oyster, Crassostrea virginica. This molecule binds to endogenous ligands at the surface of the oyster’s phagocytic cells and recognizes exogenous carbohydrate ligands on microbial pathogens and phytoplankton components [29]. Although this novel galectin binds β-galactosyl residues, it exhibits broader saccharide specificity than mammalian galectins, which may confer upon this oyster galectin biological functions involved in immune recognition, as well as in feeding and intracellular digestion [29]. Thus galectin-carbohydrate lattices may have evolved to ensure host-pathogen interactions during the initiation and resolution of microbial infections.
Galectin-glycoprotein lattices in innate immunity
Galectin-carbohydrate lattices may also modulate the biology of innate immune cells at inflammatory foci [6,15,16]. Galectin-3-mediated ligand clustering triggers neutrophils to phagocytose, produce reactive oxygen species, release proteases, and secrete interleukin (IL)-8 [15,16,30]. In addition, galectin-3 induces mast cell degranulation: recent studies have revealed a critical role for this protein in mast cell function since galectin-3-deficient mast cells show reduced histamine release and IL-4 secretion [14].
Also critical for innate immune responses, macrophages require GlcNAcT-V-dependent galectin-glycoprotein lattice formation to maintain sufficient cell-surface cytokine receptor density to drive motility and phagocytosis [11]. Moreover, by interacting with specific saccharide ligands, galectin-1 differentially regulates Fcγ receptor I-dependent phagocytosis and inhibits major histocompatibility complex (MHC)-II-dependent antigen presentation by monocytes/macrophages [31].
Recently, fluorescence resonance energy transfer (FRET) was employed to visualize physiological galectin-3 oligomerization on the surface of neutrophils and endothelial cells [15]. Removal of the N-terminal domain of galectin-3 by proteolytic cleavage prevented oligomerization. These studies further suggested that galectin-3 lattices are robust, stable, and rigid, with slow lateral movement on the surface of cells, and could thus easily restrict receptor clustering and modulate cell signaling [15].
Galectin-glycoprotein lattices in T cell functions
Cross-linkage of T-cell surface receptors by galectins can trigger different transmembrane signaling events through which diverse processes such as survival, activation, and cytokine secretion are modulated [6].
The T-cell receptor (TCR) α/β is decorated by GlcNAcT-V -modified N-glycans which restrict nonspecific TCR aggregation through binding to galectins. Multivalent galectin-3-TCR complex lattices limit TCR clustering at the immune synapse by restricting lateral TCR movement within the plane of the membrane, thus increasing agonist threshold for TCR signaling [32,33]. Conversely, deficiency in GlcNAcT-V lowers T-cell activation threshold by enabling TCR clustering and signaling characterized by increased TCR-dependent tyrosine phosphorylation and proliferation [32,33].
The authors recently extended their observations, showing that β1,6GlcNAc-branched N-glycans on T cells are regulated by the nutrient environment and metabolite supply of the hexosamine pathway. Thus the production of high affinity ligands for galectins is controlled in T cells by the availability of key metabolic intermediates [34]. Increasing β1,6GlcNAc-branched N-glycans in T cells by hexosamine supplementation suppresses TCR signaling, CTLA-4 endocytosis, Th1 differentiation, and development of autoimmune inflammation [34] (Figure 2).
A critical process for dampening potential harmful T-cell responses is the fine-tuning of T-cell survival. Galectin-1, -2, -3 and -9 bind distinct cell surface glycoprotein receptors and trigger distinct intracellular signaling pathways to promote T-cell death [17,19,35–37]. Remarkably, a number of factors determine the responsiveness of cells to galectin-mediated signals which include the repertoire of glycosylated molecules expressed on the cell surface and the activities of specific glycosyltransferases, which are responsible for creating or masking galectin ligands [5,6,18]. These variables can dramatically change during thymic-development and peripheral activation and differentiation of T cells. [5,6,18,38–41].
Compelling evidence indicates that galectin-1 treatment suppresses chronic inflammation, modulates T-cell survival and skews the balance towards a Th2 cytokine profile in vitro [42] and in vivo in animal models, including experimental autoimmune uveitis [43] and autoimmune diabetes [44]. Furthermore, selective blockade of galectin-1 in tumor tissue results in increased Th1-mediated anti-tumor responses, suggesting potential involvement of this protein in tumor-immune escape [45]. Recent work provides a molecular explanation for galectin-1-mediated Th2 skewing, demonstrating that Th1 and Th17 effector cells express the repertoire of cell surface glycans that are essential for the formation galectin-glycoprotein lattices. In contrast Th2 cells are protected from galectin-1 binding through differential α2,6-sialylation of cell surface glycoproteins (Figure 2). Galectin-1-deficient mice consistently developed greater antigen-specific Th1 and Th17 responses compared to wild-type mice [40]. In addition, other galectin members may contribute to this immunoregulatory effect, including galectin-9 which acts as a specific binding partner of Tim-3, a Th1-specific receptor, and selectively eliminates Th1 cells in vivo [46]. Furthermore, it has been proposed that phosphatidylserine exposure induced by galectins may serve as an alternative homeostatic mechanism, to favor phagocytosis, modulate secretion of anti-inflammatory cytokines and influence the resolution of inflammatory responses [47]. In contrast to the inhibitory actions of galectin-1, galectin-4 contributes to exacerbated intestinal inflammation by promoting CD4+ T cell activation and favoring IL-6 secretion through a protein kinase Cθ-dependent mechanism [48].
In addition to activation-induced cell death, avoidance of collateral damage to the host is also achieved by active immunosuppression mediated by regulatory T cells. We found that treatment with recombinant galectin-1 in the efferent phase of autoimmune ocular inflammation results in increased IL-10 and TGF-β production and expansion of regulatory T cells in vivo [43]. Interestingly, recent studies demonstrated that galectin-1 and -10 are over-expressed in regulatory T cells, and are critical for the suppressive activity of these cells [49,50]. Further studies are needed to establish a role of galectin-carbohydrate lattices at synapse formation between regulatory and effector T cells.
Galectin-glycoprotein lattices in B-cell functions
VpreB, a surrogate immunoglobulin light chain that functions in early stages of B-cell receptor (BCR) maturation in pre-B cells, interacts with galectin-1 to modulate essential B-cell maturation activities [51]. An immune developmental synapse is formed between pre-B and stromal cells in a galectin-1-dependent manner: pre-BCR binding to stromal cells depends upon galectin-1 binding to glycosylated α4β1, α5β1, and α4β7 integrins [51,52]. Pre-B cell integrins and their stromal cell ligands, together with pre-BCR and galectin-1, form a homogeneous lattice at the contact area between pre-B and stromal cells [52]. The resulting synapse formation initiates intracellular tyrosine kinase activity and signal transduction from the pre-BCR [51,52]. In mature B cells, the B cell-specific transcriptional coactivator OCA-B, important for B cell activation and germinal center formation, interacts with galectin-1. The authors showed that galectin-1 negatively regulates B-cell proliferation and tyrosine phosphorylation upon BCR stimulation [53]. Finally, anergic B cells show up-regulated expression of galectin-1 and -3, suggesting a possible role for these lectins in the control of B-cell tolerance [54].
Conclusions
Research over the past few years has illuminated critical functions of galectin-glycoprotein lattices in receptor turnover and cell signaling, thus dictating the choice among cell proliferation, differentiation and survival, and serving as “on-an-off switch” that controls the decision between immune cell responsiveness and tolerance. Given the broad spectrum of immunoregulatory effects in autoimmune diseases and cancer, galectin-carbohydrate lattices are postulated as targets of novel anti-inflammatory and anti-cancer therapies. However, before galectin- or glycan-based therapeutic strategies can be fully realized, a more thorough understanding of the mechanisms by which galectin-carbohydrate lattices modulate cell function is required. To what extent is there functional redundancy and specificity of action within the galectin family? What is the precise explanation of the different functions exerted by the same galectin in different environmental contexts? What are the levels of galectins attained in vivo during an inflammatory reaction, infectious process or tumor dissemination? Increased understanding of the biochemistry and biology of galectin-glycoprotein lattices will provide insights into how the regulation of galectin expression and activity can be exploited for therapeutic purposes.
Acknowledgments
We thank members of the Rabinovich and Vasta laboratories for critical comments and discussion. We apologize to the many authors whose excellent papers could not be cited in this review for space limitations. Work in G.A.R’s laboratory is supported by The Cancer Research Institute “Elaine R. Shepard Memorial Investigator”, National Agency for Promotion of Science and Technology (PICT 2003-05-13787), University of Buenos Aires (M091), and a Program of Fundación Sales/CONICET. Work in G.R.V’s laboratory is supported by grants R01 GM070589-01 from the National Institutes of Health, IOB 0618409 from the National Science Foundation, and NA05NMF4571243 from the National Oceanic and Atmospheric Administration. S.S.J. is supported by grant F32GM083352 from the National Institute of General Medical Sciences.
Abbreviations
- BCR
B cell receptor
- CRD
Carbohydrate recognition domain
- CTLA-4
Cytotoxic T-lymphocyte-associated protein-4
- EGFR
Epidermal Growth Factor Receptor
- FGFR
Fibroblast Growth Factor
- FRET
Fluorescence resonance energy transfer
- GLUT-2
Glucose transporter-2
- IGFR
Insulin-like growth factor receptor
- IL
Interleukin
- LacNAc
N-acetyllactosamine
- LPG
Lipophosphoglycan
- GlcNAcT-IVa
N-acetylglucosaminyltransferase IVa
- GlcNAcT-V
N-acetylglucosaminyltransferase V
- MHC
Major histocompatibility complex
- PDGFR
Platelet-derived growth factor receptor
- TCR
T cell receptor
- TGF-β
Transforming growth factor-β
- TGFβR
Transforming growth factor-β receptor
- Th
T helper
- Tim-3
T cell immunoglobulin mucin-3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miceli MC, Moran M, Chung CD, Patel VP, Low T, Zinnanti W. Co-stimulation and counter-stimulation: lipid raft clustering controls TCR signaling and functional outcomes. Semin Immunol. 2001;13:115–128. doi: 10.1006/smim.2000.0303. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DN. Galectinomics: finding themes in complexity. Biochim Biophys Acta. 2002;1572:209–231. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 3.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 4.Vasta GR, Ahmed H, Odom EW. Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Curr Opin Struct Biol. 2004;14:617–630. doi: 10.1016/j.sbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12:616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 6.Toscano MA, Ilarregui JM, Bianco GA, Campagna L, Croci DO, Salatino M, Rabinovich GA. Dissecting the pathophysiologic role of endogenous lectins: glycan-binding proteins with cytokine-like activity? Cytokine Growth Factor Rev. 2007;18:57–71. doi: 10.1016/j.cytogfr.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, et al. Oligosaccharide specificity of galectins: ra search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik SK, Potvin B, Carlsson S, Sturm D, Leffler H, Stanley P. Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology. 2006;16:305–317. doi: 10.1093/glycob/cwj063. [DOI] [PubMed] [Google Scholar]
- 9.Dam TK, Gabius HJ, Andre S, Kaltner H, Lensch M, Brewer CF. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 2005;44:12564–12571. doi: 10.1021/bi051144z. [DOI] [PubMed] [Google Scholar]
- 10.Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. ●● An elegant study showing a fine-tuning mechanism for switching from growth to arrest in cells based on the flux of UDP-GlcNAc through the Golgi and the extent of N-glycan branching of growth factor receptors. [DOI] [PubMed] [Google Scholar]
- 11.Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 12.Morris S, Ahmad N, Andre S, Kaltner H, Gabius HJ, Brenowitz M, Brewer F. Quaternary solution structures of galectins-1, -3, and -7. Glycobiology. 2004;14:293–300. doi: 10.1093/glycob/cwh029. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 14.Chen HY, Sharma BB, Yu L, Zuberi R, Weng IC, Kawakami Y, Kawakami T, Hsu DK, Liu FT. Role of galectin-3 in mast cell functions: galectin-3-deficient mast cells exhibit impaired mediator release and defective JNK expression. J Immunol. 2006;177:4991–4997. doi: 10.4049/jimmunol.177.8.4991. [DOI] [PubMed] [Google Scholar]
- 15.Nieminen J, Kuno A, Hirabayashi J, Sato S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem. 2007;282:1374–1383. doi: 10.1074/jbc.M604506200. ●● An innovative study demonstrating the oligomerization of galectin-3 using fluorescence resonance energy transfer (FRET). The authors detected FRET signals during galectin-3-lattice formation on neutrophils and endothelial cells. [DOI] [PubMed] [Google Scholar]
- 16.Nieminen J, St-Pierre C, Sato S. Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. J Leukoc Biol. 2005;78:1127–1135. doi: 10.1189/jlb.1204702. [DOI] [PubMed] [Google Scholar]
- 17.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 18.Daniels MA, Hogquist KA, Jameson SC. Sweet 'n' sour: the impact of differential glycosylation on T cell responses. Nat Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez JD, Nguyen JT, He J, Wang W, Ardman B, Green JM, Fukuda M, Baum LG. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J Immunol. 2006;177:5328–5336. doi: 10.4049/jimmunol.177.8.5328. [DOI] [PubMed] [Google Scholar]
- 20.Siebert HC, Andre S, Lu SY, Frank M, Kaltner H, van Kuik JA, Korchagina EY, Bovin N, Tajkhorshid E, Kaptein R, et al. Unique conformer selection of human growth-regulatory lectin galectin-1 for ganglioside GM1 versus bacterial toxins. Biochemistry. 2003;42:14762–14773. doi: 10.1021/bi035477c. [DOI] [PubMed] [Google Scholar]
- 21.Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. doi: 10.1016/j.cell.2005.09.041. ●● The authors describe a novel mechanism, based on galectin-9-glycoprotein lattice formation, by which glycosylation controls cell-surface expression of the glucose transporter 2 (GLUT-2) on the surface of β-cells, providing a link among receptor glycosylation, glucose metabolism and insulin production. [DOI] [PubMed] [Google Scholar]
- 22.Park HJ, Partridge E, Cheung P, Pawling J, Donovan R, Wrana JL, Dennis JW. Chemical enhancers of cytokine signaling that suppress microfilament turnover and tumor cell growth. Cancer Res. 2006;66:3558–3566. doi: 10.1158/0008-5472.CAN-05-2542. [DOI] [PubMed] [Google Scholar]
- 23.Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol. 2006;26:3181–3193. doi: 10.1128/MCB.26.8.3181-3193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabinovich GA, Gruppi A. Galectins as immunoregulators during infectious processes: from microbial invasion to the resolution of the disease. Parasite Immunol. 2005;27:103–114. doi: 10.1111/j.1365-3024.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier I, Hashidate T, Urashima T, Nishi N, Nakamura T, Futai M, Arata Y, Kasai K, Hirashima M, Hirabayashi J, et al. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J Biol Chem. 2003;278:22223–22230. doi: 10.1074/jbc.M302693200. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier I, Sato S. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J Biol Chem. 2002;277:17663–17670. doi: 10.1074/jbc.M201562200. [DOI] [PubMed] [Google Scholar]
- 27.Levroney EL, Aguilar HC, Fulcher JA, Kohatsu L, Pace KE, Pang M, Gurney KB, Baum LG, Lee B. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J Immunol. 2005;175:413–420. doi: 10.4049/jimmunol.175.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J, Sato S, Tremblay MJ. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol. 2005;174:4120–4126. doi: 10.4049/jimmunol.174.7.4120. [DOI] [PubMed] [Google Scholar]
- 29.Tasumi S, Vasta G. A galectin of unique domain organization from hemocytes of the eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J immunol. 2007 doi: 10.4049/jimmunol.179.5.3086. In press. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez GC, Ilarregui JM, Rubel CJ, Toscano MA, Gomez SA, Beigier Bompadre M, Isturiz MA, Rabinovich GA, Palermo MS. Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: involvement of alternative MAPK pathways. Glycobiology. 2005;15:519–527. doi: 10.1093/glycob/cwi026. [DOI] [PubMed] [Google Scholar]
- 31.Barrionuevo P, Beigier-Bompadre M, Ilarregui JM, Toscano MA, Bianco GA, Isturiz MA, Rabinovich GA. A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J Immunol. 2007;178:436–445. doi: 10.4049/jimmunol.178.1.436. [DOI] [PubMed] [Google Scholar]
- 32.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 33.Morgan R, Gao G, Pawling J, Dennis JW, Demetriou M, Li B. N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J Immunol. 2004;173:7200–7208. doi: 10.4049/jimmunol.173.12.7200. [DOI] [PubMed] [Google Scholar]
- 34.Grigorian A, Lee SU, Tian W, Chen IJ, Gao G, Mendelsohn R, Dennis JW, Demetriou M. Control of T cell mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J Biol Chem. 2007 doi: 10.1074/jbc.M701890200. [DOI] [PubMed] [Google Scholar]
- 35.Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
- 36.Lu LH, Nakagawa R, Kashio Y, Ito A, Shoji H, Nishi N, Hirashima M, Yamauchi A, Nakamura T. Characterization of galectin-9-induced death of Jurkat T cells. J Biochem (Tokyo) 2007;141:157–172. doi: 10.1093/jb/mvm019. [DOI] [PubMed] [Google Scholar]
- 37.Sturm A, Lensch M, Andre S, Kaltner H, Wiedenmann B, Rosewicz S, Dignass AU, Gabius HJ. Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J Immunol. 2004;173:3825–3837. doi: 10.4049/jimmunol.173.6.3825. [DOI] [PubMed] [Google Scholar]
- 38.Daniels MA, Devine L, Miller JD, Moser JM, Lukacher AE, Altman JD, Kavathas P, Hogquist KA, Jameson SC. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15:1051–1061. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez JD, Klein J, Van Dyken SJ, Marth JD, Baum LG. T-cell activation results in microheterogeneous changes in glycosylation of CD45. Int Immunol. 2007;19:847–856. doi: 10.1093/intimm/dxm053. [DOI] [PubMed] [Google Scholar]
- 40.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, et al. Differential glycosylation of T(H)1, T(H)2 and T(H)-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 41.Comelli EM, Sutton-Smith M, Yan Q, Amado M, Panico M, Gilmartin T, Whisenant T, Lanigan CM, Head SR, Goldberg D, et al. Activation of murine CD4+ and CD8+ T lymphocytes leads to dramatic remodeling of N-linked glycans. J Immunol. 2006;177:2431–2440. doi: 10.4049/jimmunol.177.4.2431. [DOI] [PubMed] [Google Scholar]
- 42.van der Leij J, van den Berg A, Harms G, Eschbach H, Vos H, Zwiers P, van Weeghel R, Groen H, Poppema S, Visser L. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol Immunol. 2007;44:506–513. doi: 10.1016/j.molimm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176:6323–6332. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 44.Perone MJ, Bertera S, Tawadrous ZS, Shufesky WJ, Piganelli JD, Baum LG, Trucco M, Morelli AE. Dendritic cells expressing transgenic galectin-1 delay onset of autoimmune diabetes in mice. J Immunol. 2006;177:5278–5289. doi: 10.4049/jimmunol.177.8.5278. [DOI] [PubMed] [Google Scholar]
- 45.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. ●● An innovative work identifying galectin-9 as a novel binding partner for Tim-3 and describing the relevance of this interaction in the regulation of Th1-mediated autoimmune inflammation. [DOI] [PubMed] [Google Scholar]
- 47.Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hokama A, Mizoguchi E, Sugimoto K, Shimomura Y, Tanaka Y, Yoshida M, Rietdijk ST, de Jong YP, Snapper SB, Terhorst C, et al. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity. 2004;20:681–693. doi: 10.1016/j.immuni.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 50.Kubach J, Lutter P, Bopp T, Stoll S, Becker C, Huter E, Richter C, Weingarten P, Warger T, Knop J, et al. Human CD4+CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007 doi: 10.1182/blood-2007-01-069229. ● Recent studies demonstrating the contribution of galectins to the suppressive activity of CD4+CD25+ regulatory T cells. [DOI] [PubMed] [Google Scholar]
- 51.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A. 2002;99:13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossi B, Espeli M, Schiff C, Gauthier L. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J Immunol. 2006;177:796–803. doi: 10.4049/jimmunol.177.2.796. ● The authors describe the relevance of galectin-1-glycoprotein lattices in pre-B/stromal cell synapse formation, pre-BCR receptor segregation and activation. [DOI] [PubMed] [Google Scholar]
- 53.Yu X, Siegel R, Roeder RG. Interaction of the B cell-specific transcriptional coactivator OCA-B and galectin-1 and a possible role in regulating BCR-mediated B cell proliferation. J Biol Chem. 2006;281:15505–15516. doi: 10.1074/jbc.M509041200. [DOI] [PubMed] [Google Scholar]
- 54.Clark AG, Chen S, Zhang H, Brady GF, Ungewitter EK, Bradley JK, Sackey FN, Foster MH. Multifunctional regulators of cell growth are differentially expressed in anergic murine B cells. Mol Immunol. 2007;44:1274–1285. doi: 10.1016/j.molimm.2006.06.001. [DOI] [PubMed] [Google Scholar]