Abstract
Adoptive cell transfer (ACT) of tumor-reactive lymphocytes has been shown to be an effective treatment for cancer patients. Studies in murine models of ACT indicated that antitumor efficacy of adoptively transferred T cells is dependent on the differentiation status of the cells, with lymphocyte differentiation inversely correlated with in vivo antitumor effectiveness. T-cell in vitro development technologies provide a new opportunity to generate naive T cells for the purpose of ACT. In this study, we genetically modified human umbilical cord blood–derived hematopoietic stem cells (HSCs) to express tumor antigen-specific T-cell receptor (TCR) genes and generated T lymphocytes by coculture with a murine cell line expressing Notch-1 ligand, Delta-like-1 (OP9-DL1). Input HSCs were differentiated into T cells as evidenced by the expression of T-cell markers, such as CD7, CD1a, CD4, CD8, and CD3, and by detection of TCR excision circles. We found that such in vitro differentiated T cells expressed the TCR and showed HLA-A2–restricted, specific recognition and killing of tumor antigen peptide–pulsed antigen-presenting cells but manifested additional natural killer cell–like killing of tumor cell lines. The genetic manipulation of HSCs has broad implications for ACT of cancer.
Introduction
Adoptive cell transfer (ACT) is an effective method for the immunotherapy of patients with metastatic melanoma, with an objective response rate of >50% (1, 2). A limitation of this approach is the requirement that patients have preexisting tumor reactive cells, and in cancers other than melanoma, it is difficult to identify these tumor-reactive lymphocytes. To overcome this limitation, T-cell receptor (TCR) gene transfer can be an alternative to the isolation of naturally occurring antitumor T cells (3–6). We recently reported that transfer of T cells genetically engineered with tumor antigen-specific TCR in patients with malignant melanoma resulted in high sustained levels of circulating engineered cells, and that objective cancer regression was observed (7). The response rate of 13% observed in this TCR gene transfer trial was lower than the 50% response rate previously achieved by the infusion of autologous tumor-infiltrating lymphocytes (TIL). It is possible that the effectiveness of TCR gene transfer of T lymphocytes is limited, in part, by pairing of the introduced TCR α and β chains with endogenous TCR proteins. Transducing TCR genes into hematopoietic stem cells (HSCs), which could be differentiated into T cells in vitro, might prevent endogenous TCR expression via allelic exclusion (8). Furthermore, recent studies in a murine model of ACT indicated that lymphocyte differentiation was inversely correlated with in vivo antitumor effectiveness (9), and in vitro derived T cells would, by definition, be naive cells.
The finding that Notch signals could influence T-cell versus B-cell differentiation suggested that particular Notch ligands provided a unique signal to induce T-lineage commitment in the thymus. By transfecting the delta-like Notch ligand gene Delta-like 1 into a murine bone marrow stromal line OP9 (OP9-DL1), Schmitt et al. found that the OP9-DL1 cell line was no longer capable of supporting B-cell development from murine HSCs, but it gained the striking capacity to induce full T-lineage commitment and differentiation in vitro (10). The OP9-DL1 culture system not only supported the commitment of T cells from murine HSCs, but similar studies reported that human CD34+ HSCs from both umbilical cord blood (UCB) or pediatric bone marrow (11, 12) could be differentiated into T cells in vitro.
In this report, we tested whether functional T lymphocytes could be developed by coculture of OP9-DL1 with HSCs transduced with tumor antigen-specific TCR.
Materials and Methods
Cell cultures
OP9 cell line was purchased from the American Type Culture Collection (Rockville, MD), and OP9-DL1 cells were obtained from Dr. Zuniga-Pflucker (Department of Immunology, University of Toronto, Sunnybrook, Ontario, Canada; ref. 10). CD133+ progenitor cells were enriched from mononuclear cells from UCB (Allcells, Berkeley, CA) by depleting CD3+ cells using Dynabeads human CD3 (Invitrogen, Carlsbad, CA) and followed by positive selection of CD133+ cells using CD133 Cell Isolation kit (Miltenyi Biotec, Gladbach, Germany), which yielded a population of HSCs with the purity over 99% of double CD133+/CD34+ cells. Melanoma lines 888mel and SK23, breast cancer line MDA-MB-231 and osteosarcoma line Saos 2, the human Burkitt lymphoma-derived B cell line Daudi, the human erythromyeloid leukemia cell line K562, and the lymphoblastoid cell line T2 were maintained in culture in RPMI 1640.
Retroviral transduction and in vitro T-cell development
Vector constructs used for the transduction of HSCs were MSGE1APB (NY-ESO-1–specific, HLA-A2–restricted human TCR), MSGV-p53-AIB (human p53–specific, HLA-A2–restricted murine TCR), and MSGIN (expressing eGFP and NeoR) as previously described (3, 6). Purified HSCs were resuspended in Iscove’s modified Dulbecco’s medium supplemented with BIT-9500 (1% bovine serum albumin, 10 μg/mL recombinant human insulin, 20 μg/mL human transferrin, and 100 ng/mL of each recombinant human Flt3-L, stem cell factor, and thrombopoietin; StemCell Technologies, Inc., Vancouver, British Columbia, Canada) and cultured for 48 h before transduction. After stimulation, the cells were transduced as previously described (6), after which the cells were cocultured with OP9-DL1 (or OP9 as control) in complete medium, consisting of α-MEM (Invitrogen) supplemented with 20% FCS (Hyclone, Perbio, Erembodegem-Aalst, Belgium) in the presence of 5 ng/mL interleukin-7 (IL-7) and 5 ng/mL Flt3L (PeproTech, Rocky Hill, NJ). Cocultures were refreshed every 3 to 4 days by recovering the cells with vigorous pipetting followed by filtering the cells through a cell strainer with a mesh of 70 μm and centrifugation (200 × g, room temperature, 5 min). The cell pellet was transferred to a fresh confluent monolayer of OP9-DL1 cells in complete medium as described (11).
Flow cytometry analysis
Flow cytometry was done using a FACSscan instrument, with FITC- or phycoerythrin-conjugated monoclonal antibodies (BD Biosciences, San Jose, CA). Data were live-gated by forward/side light scatter and lack of propidium iodide uptake. Green fluorescent protein (GFP)–expressing OP9-DL1 cells were gated out of the analysis through GFP expression and side scatter exclusion. Frequencies in quadrant corners are given as percentage of gated cells.
Cytokine release and 51Cr release assays
In vitro developed T cells were cocultured for 24 h with T2 cells pulsed with peptide NY-ESO-1, 157–165V (a modification of position 165 from Cys to Val; SLLMWITQV) or p53, 264–272 (LLGRNSFEV) at the indicated concentration, and the reactivity was assayed by ELISA for cytokine release (Endogen, Rockford, IL) as described (6). The ability of the T cells to lyse peptide-pulsed target cells and tumor lines was measured using a 51Cr release assay as described (6).
PCR amplification of TCR excision circles
Genomic DNA was extracted using Wizard Genomic DNA Purification kit (Promega, Madison, WI). PCR was done in 50-μL reactions containing 0.5 μg DNA. Temperature cycling began with a initial hold at 94°C for 2 min; this was followed by 35 cycles of 94°C for 30 s and 60°C for 30 s. The primer sequences were as follows: TREC5, 5′-CATCCCTTTCAACCATGCTGACACCTCT-3′; TREC3, 5′-CGTGAG-AACGGTGAATGAAGAGCAGACA-3′; β-actin5, 5′-GCTCGCCCTTTCTCACT-GG-3′; β-actin3, 5′-CCGCTTTACACCAGCCTCATG-3′. The PCR products were visualized by loading 10 μL of PCR product onto a 2% agarose gel stained with ethidium bromide.
Results and Discussion
In vitro development and transduction of T lymphocytes from HSCs
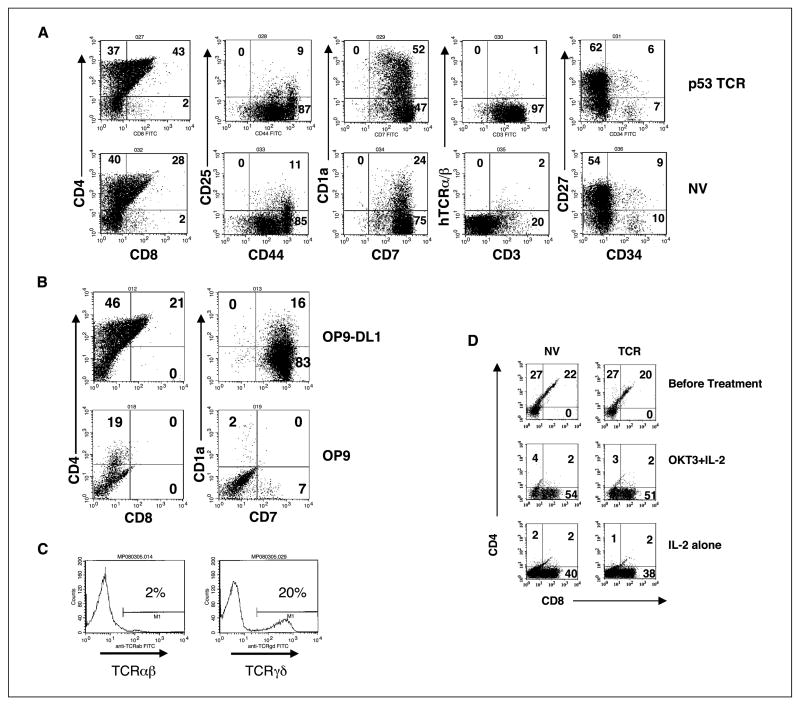
It has been reported that transplantation of TCR-transduced HSCs or bone marrow cells protected against tumor challenge in mice (13, 14). To determine if tumor-reactive T cells could be generated in vitro from human HSCs genetically engineered with tumor antigen-reactive TCR, we first determined if HSC pre-culture stimulation (required for retroviral vector transduction) affected in vitro T-cell development. In a representative experiment, following coculture of both transduced or nontransduced HSCs with OP9-DL1, HSCs could be differentiated into cells expressing T-cell lineage markers, CD1a, CD7, CD25, CD27, CD44, CD3, CD4, and CD4/CD8 double-positive cells (Fig. 1A), with the number of CD4/CD8 double-positive cells increasing over time (data not shown). Cells cocultured with control OP9 that does not express DL1 expressed low levels of CD4 and CD7, and there were no CD4/CD8 or CD7/CD1a double-positive cells (Fig. 1B). CD3 expression on the T cells committed from nontransduced HSCs was mainly TCR γ/δ (20%) with a small amount of TCR α/β (2%; Fig. 1C). CD4/CD8 double-positive T cells can be further differentiated into single-positive CD8 T cells by culture in the presence of IL-2 and/or anti-CD3 antibody OKT3 (Fig. 1D).
Figure 1.
T cells committed from genetically engendered HSCs. A, flow cytometry analysis for T-cell markers for MSGV-p53-AIB–transduced HSCs (p53 TCR) or nontransduced HSCs (NV) cocultured with OP9-DL1 cell line for 29 d. Representative of four experiments. B, flow cytometry analysis for T-cell markers for MSGV-p53-AIB–transduced HSCs cocultured with OP9-DL1 or OP9 for 25 d. Data were gated as in (A). Representative of two experiments. C, HSCs were cocultured with OP9-DL1 for 37 d and stained for human TCR α/β (TCR α/β) or human TCR γ/δ (TCR γ/δ). D, flow cytometry analysis for T-cell markers for MSGV-p53-AIB–transduced HSCs (TCR) or nontransduced HSCs (NV) before and after OKT3/IL-2 treatment. HSCs were cocultured with OP9-DL1 cell line for 44 d (top), and then cells were cultured in medium supplemented with OKT3 + IL-2 (middle) or IL-2 alone (bottom) for additional 5 d. Representative of three experiments.
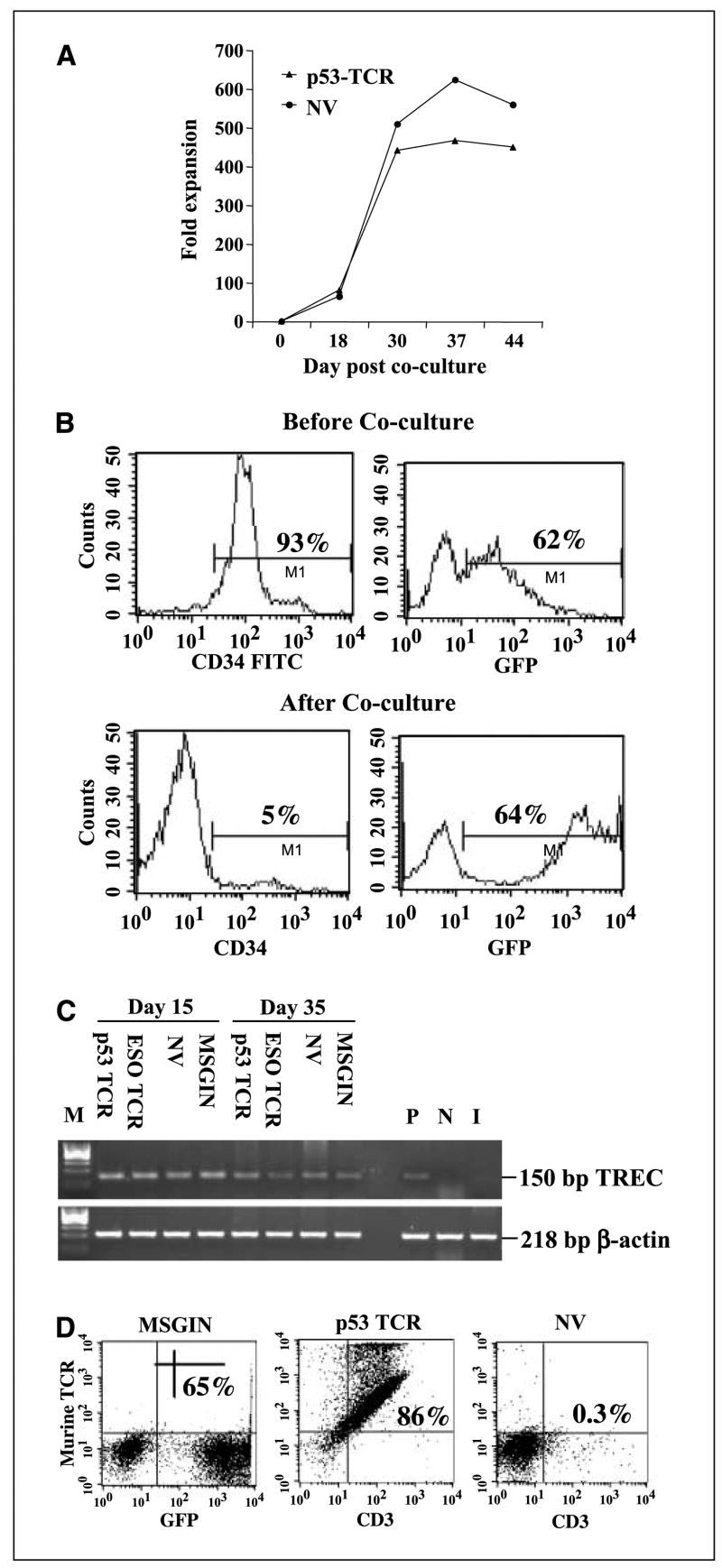
T-cell proliferation increased rapidly up to 5 weeks in culture (yielding approximately a 500- to 600-fold expansion) and decreased thereafter (Fig. 2A). To test the feasibility of genetically engineering HSCs and differentiating these into T lymphocytes, HSCs were transduced with a GFP reporter retroviral vector and then cocultured with OP9-DL1. Sixty-two percent of CD34+ HSCs were transduced with the GFP vector, and this percentage was maintained following differentiation (Fig. 2B). TCR excision circles (TREC) result from δRec-ψJα rearrangement (TCRD deletion) that occurs late during thymopoiesis. As these molecules are lost upon further cell division, their quantification is used to estimate thymic function. TREC were not detected in starting CD34+ HSCs, but detectable TREC were found in all the groups that were cocultured with OP9-DL1 at 15 and 35 days after coculture (Fig. 2C). Although TREC formation was observed, partial allelic exclusion was suggested by the observation of higher levels of CD3 and TCR surface staining in p53-transduced cells in comparison with mock-transduced cells (Fig. 2D).
Figure 2.
Expansion of transgene expression of in vitro developed T cells. A, cell number was counted at different time intervals after MSGV-p53-AIB–transduced HSCs (p53 TCR) were cocultured with OP9-DL1 as indicated. The fold expansion was based on the starting HSC numbers. Nontransduced HSCs (NV) cocultured with OP9-DL1 was used as control. B, expression of CD34 and GFP on transduced HSCs (top) and following coculture for 24 d with OP9-DL1 (bottom). C, PCR detection of TREC in T cells generated from HSCs cocultured with OP9-DL1 for 15 and 35 d. As a control, DNA from purified CD3+ CB was used as a positive control (P). DNA from adult PBLs was used as a negative control (N). DNA isolated from CD34+ HSCs before coculture with OP9-DL1 was shown as input cells (I). D, flow cytometry analysis of MSGIN or MSGV-p53-AIB (p53 TCR)–transduced HSCs cultured with OP9-DL1 for 24 d. Nontransduced HSCs (NV) cocultured with OP9-DL1 cell line for 24 d were used as a control. Data were gated as in (A).
Antigen-specific recognition of TCR-transduced in vitro differentiated T cells
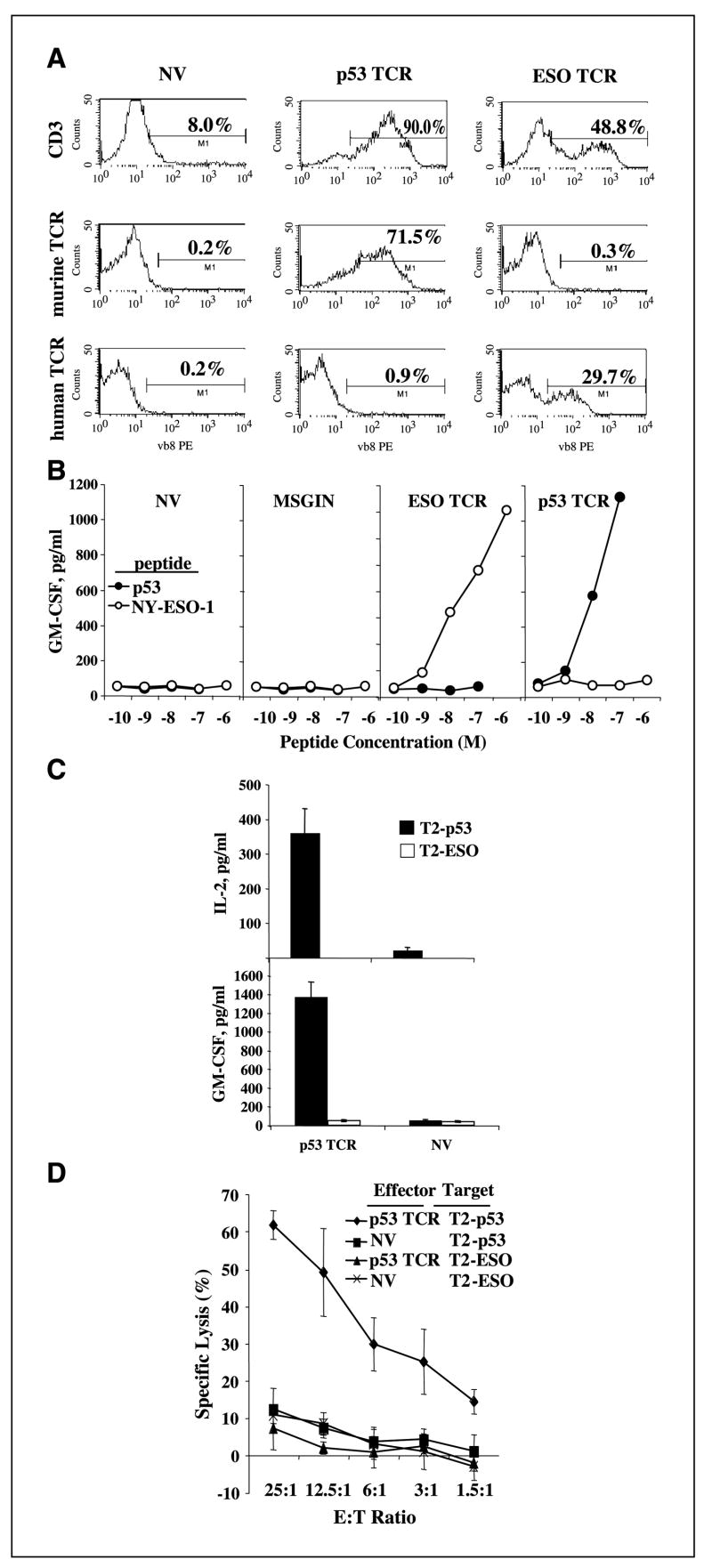
HSCs were transduced with TCR-expressing vectors directed against tumor antigens p53 or NY-ESO-1 (gene transfer, ∼70% and 30%, respectively; Fig. 3A). These differentiated cells, from both transduced and nontransduced HSCs, displayed high level expression of CD62L (37.7 ± 9.6%) and CD56 (60.7 ± 6.5%). To determine if these T cells were capable of recognizing specific tumor antigen epitopes, T cells developed from TCR-transduced HSCs were cocultured with peptide-pulsed antigen-presenting cell line T2. MSGE1APB (NY-ESO-1 TCR)– and MSGV-p53-AIB (p53 TCR)–transduced HSCs specifically recognized T2 pulsed with peptide, causing the secretion of the effector cytokine granulocyte macrophage colony-stimulating factor (GM-CSF; Fig. 3B) with similar results obtained at later times (data not shown). TCR-transduced HSCs specifically recognized peptide concentration of 1 to 10 nmol/L level, which was equivalent with our previous report of these two TCR-transduced peripheral blood lymphocytes (PBL; refs. 3, 6).
Figure 3.
Antigen-specific functional responses of the T cells in vitro developed from tumor antigen TCR–transduced HSCs. A, flow cytometry analysis of CD3 and transduced TCR for MSGV-p53-AIB (p53 TCR, detected by anti-murine TCR), MSGE1APB (ESO TCR, detected by anti-human TCR), or nontransduced (NV) HSCs cocultured with OP9-DL1 for 30 d. Representative of two experiments. B, T cells generated by coculturing MSGV-p53-AIB (p53 TCR)–, MSGE1APB (ESO TCR)–, or MSGIN (MSGIN)–transduced HSCs with OP9-DL1 for 24 d [nontransduced HSCs (NV) were used as control] were cocultured with serial diluted p53 peptide–pulsed T2 cells (p53) or NY-ESO-1 peptide–pulsed T2 cells (NY-ESO-1). Twenty hours later, the supernatant collected from the coculture was subjected to ELISA for the detection of GM-CSF secretion. Representative of two experiments. C, T cells generated by coculturing MSGV-p53-AIB (p53 TCR)–transduced HSCs with OP9-DL1 for 42 d [nontransduced HSCs (NV) were used as control] were cocultured with p53 peptide–pulsed T2 cell line (T2-p53); NY-ESO-1 p156-165V peptide–pulsed T2 cells (T2-ESO) were used as a control. Production of IL-2 and GM-CSF was determined by ELISA. Representative of three experiments. D, T cells generated by coculturing MSGV-p53-AIB–transduced HSCs (p53 TCR) or nontransduced HSCs (NV) with OP9-DL1 for 44 d were cocultured with 51Cr-labeled, p53 peptide–pulsed T2 (T2-p53) or NY-ESO-1 peptide–pulsed T2 (T2-ESO) for 4 h, and 51Cr release was measured. Representative of two experiments.
In an independent experiment (Fig. 3C), antigen-specific response was evidenced by the secretion of both IL-2 and GM-CSF, and the cytokine secretion profile assay (SearchLight Human Cytokine Array, Pierce, Rockford, IL) showed that TCR-transduced in vitro developed T cells specifically produced GM-CSF, IL-2, IL-3, IL-6, IL-18, and IL-13 but not IFN-γ, tumor necrosis factor-α, IL-10, IL-4, and IL-12 (data not shown). Using peptide-pulsed, 51Cr-labeled T2 as target cells, T cells developed from TCR-transduced HSCs (p53 TCR) lysed only p53 peptide–pulsed T2 (T2-p53) but not NY-ESO-1 peptide–pulsed T2 (T2-ESO; Fig. 3D). These results suggest that TCR-transduced HSCs induced to differentiate into T cells in vitro were not only phenotypically similar to thymocyte-derived T cells but manifested antigen-specific cytokine secretion upon specific antigen stimulation and specifically lysed peptide-pulsed target cells.
Natural killer cell–like activity of in vitro differentiated T cells
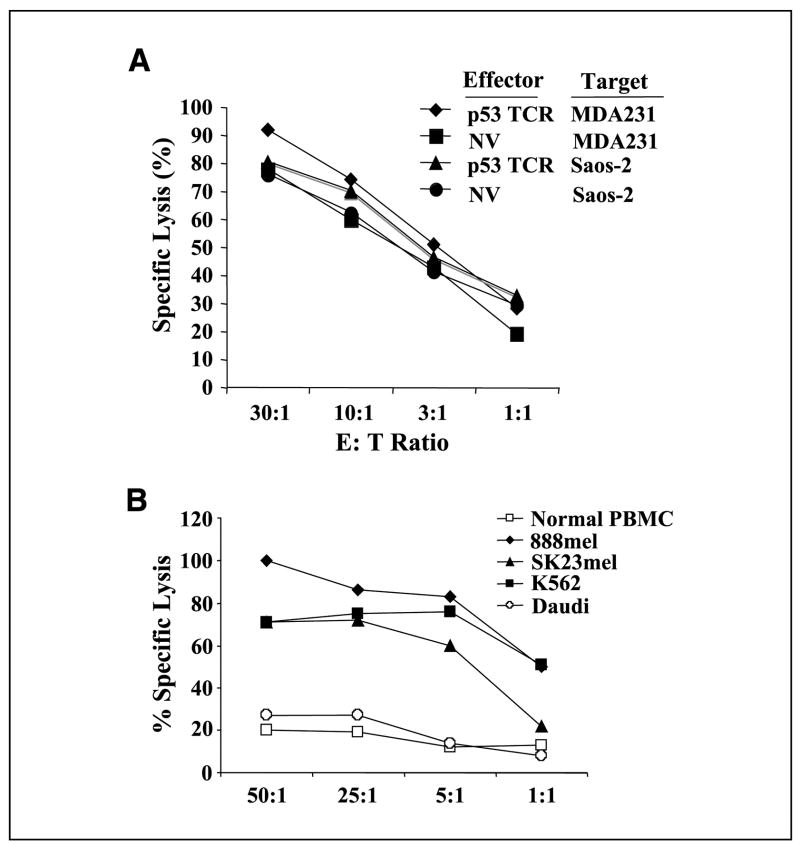
In vitro developed, p53 TCR–transduced T cells were tested for their ability to lyse tumor lines. Unexpectedly, both the T cells developed from TCR-transduced and nontransduced HSCs lysed both specific and nonspecific tumor targets (Fig. 4A) in a natural killer (NK) cell–like manner. To verify this NK cell–like activity, an independent culture of HSCs was in vitro developed without gene transfer, and these cells were also shown to have NK cell–like activity (lysis of NK cell target line K562, but not NK cell–negative target Daudi or normal peripheral blood mononuclear cells; Fig. 4B). In addition to observing NK cell–like activity, in vitro generated T cells expressed abundant CD56, and up to half of these can be CD16+ (data not shown), both of these NK cell–like markers were not reported in the La Motte-Mohs or De Smedt’s publications (11, 12).
Figure 4.
Killing of tumor cell lines by in vitro generated T cells. A, TCR-transduced (p53) or nontransduced (NV) T cells generated by coculture of HSCs with OP9-DL1 for 40 d were cocultured with 51Cr-labeled tumor cell line p53+ MDA231 or p53−Saos-2. 51Cr release was measured after 4 h of coculture. B, in vitro developed T cells from a 40-d coculture with OP9-DL1 were cultured with OKT3/IL-2 (14 d) to stimulate differentiation to CD8 single-positive cells. Fourteen d later, these cells were cocultured with 51Cr-labeled cells as indicated, and the percent lysis was determined at 4 h.
There is evidence indicating that inhibiting notch signaling biases thymocyte development towards the NK cell lineage (15, 16). Although the cells generated from coculture of CD34+ HSCs with OP9-DL1 expressed CD56 and displayed NK cell–like activity, they expressed a panel of T-cell markers that typical NK cells do not express (in particular, CD7). Furthermore, in vitro generated T cells from TCR-transduced HSCs expressed high levels of CD3, which is absent in NK cells. The cytokine secretion profile of these cells is also not consistent with NKT cells, which are known to express IFN-γ and IL-4. The detailed characterization of these T cells merits further investigation. TCR-transduced PBL or TIL recognize and kill tumors in an HLA-restricted manner and cannot kill tumors that have lost expression of HLA (2, 17). The property of in vitro generated T cells reported herein (specific tumor antigen recognition and NK cell–like activity) make them potential candidates for use in ACT of cancer for both HLA-restricted and HLA-independent tumors (perhaps even in an allogeneic setting).
A potential major clinical advantage of targeting HSCs with TCR-expressing vectors is that these in vitro developed T cells are less differentiated than in vitro expanded PBLs currently used for adoptive immunotherapy. Furthermore, in vitro developed T cells genetically engineered with antitumor TCRs via this method displayed little endogenous TCR expression, and a high percentage of the introduced tumor-reactive TCR is expressed on the cell surface. The formation of a correctly paired and functional TCR may be considerably improved by using a TCR gene transfer into HSCs lacking substantial endogenous TCR expression in comparison with mature T cells. Moreover, a higher density of TCR may help to reach the critical threshold for T-cell activation as was previously shown (18). A recent study in a mouse allogeneic HSC transplantation model in which the OP9-DL1–derived T-cell precursors markedly enhanced T-cell reconstitution, resulted in graft-versus-tumor activity without graft-versus-host disease, may suggest a potential beneficial effect of such in vitro generated, genetically modified T cells in the allogeneic transfer setting (19). Although we have been unable to achieve similar results in adult-derived HSCs, the further development of this approach raises the possibility that transplantation of retrovirally transduced in vitro developed T cells in the setting of adoptive immunotherapy in cancer patients may be therapeutically useful.
Acknowledgments
Grant support: Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
We thank Dr. Zuniga-Pflucker for generously providing the OP9-DL1 cell line and for helpful suggestions on appropriate culture conditions.
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen CJ, Zheng Z, Bray R, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RA, Dudley ME, Yu YY, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–95. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanislawski T, Voss RH, Lotz C, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–70. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–23. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bluthmann H, Kisielow P, Uematsu Y, et al. T-cell-specific deletion of T-cell receptor transgenes allows functional rearrangement of endogenous α- and β-genes. Nature. 1988;334:156–9. doi: 10.1038/334156a0. [DOI] [PubMed] [Google Scholar]
- 9.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–56. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 11.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–9. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 12.De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis. 2004;33:227–32. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci U S A. 2005;102:4518–23. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alajez NM, Schmielau J, Alter MD, Cascio M, Finn OJ. Therapeutic potential of a tumor-specific, MHC-unrestricted T-cell receptor expressed on effector cells of the innate and the adaptive immune system through bone marrow transduction and immune reconstitution. Blood. 2005;105:4583–9. doi: 10.1182/blood-2004-10-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan XQ, Lacey D, Hill D, et al. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88:402–9. [PubMed] [Google Scholar]
- 16.De Smedt M, Hoebeke I, Reynvoet K, Leclercq G, Plum J. Different thresholds of Notch signaling bias human precursor cells toward B-, NK-, monocytic/ dendritic-, or T-cell lineage in thymus microenvironment. Blood. 2005;106:3498–506. doi: 10.1182/blood-2005-02-0496. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yang JC, Robbins PF, et al. Cell transfer therapy for cancer: lessons from sequential treatments of a patient with metastatic melanoma. J Immunother. 2003;26:385–93. doi: 10.1097/00002371-200309000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–86. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakrzewski JL, Kochman AA, Lu SX, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–47. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]