Abstract
Electroacupuncture (EA) at the Neiguan-Jianshi acupoints (P5-P6, overlying the median nerve) attenuates sympathoexcitatory reflexes probably through affecting the opioid system. The arcuate nucleus (ARC) within hypothalamus is an important brain area that produces opioid peptides. Current physiological studies have demonstrated that the predominant response to EA is excitation in the ARC and that excitatory projections from the ARC to the ventrolateral periaqueductal gray during EA at P5-P6 contribute to inhibition of sympathoexcitatory cardiovascular reflexes. These data imply that ARC neurons activated by EA also may contain excitatory neurotransmitters. Thus, the present study evaluated activation of the ARC induced by EA at P5-P6, in relation to the opioid system and glutamate, by detecting c-Fos, an immediate early gene, opioid peptides and vesicular glutamate transporter 3 (VGLUT3). To enhance detection of perikarya containing the opioid peptides, colchicine (90–100 µg/kg) was administered in cats 28–30 hours before EA or the sham-operated control. EA was performed at P5-P6 for 30 min. Compared to controls (n=5), c-Fos positive cells and neurons double-labeled with c-Fos and β-endorphin, enkephalin or VGLUT3 in the ARC were significantly increased in EA-treated cats (n=6; all P<0.05). Moreover, neurons triple-labeled with c-Fos, β-endorphin and VGLUT3 were noted in this region following EA stimulation, but not in controls. Thus, EA at P5-P6 activates neurons in the ARC, some of which contain opioids as well as glutamate or both. The results imply that EA at P5-P6 has the potential to influence ARC neurons containing multiple neuronal substances that subsequently modulate cardiovascular function.
Keywords: Acupuncture, arcuate nucleus, β-endorphin, enkephalin, glutamate, c-Fos
1. Introduction
Acupuncture has been employed to treat various diseases in Asian countries for several thousand years. Interest in application of acupuncture as an alternative medical management in the western countries has increased over the last two decades. Acupuncture at the Neiguan-Jianshi acupoints (P5-P6, overlying the median nerve) is used specifically to treat cardiovascular diseases, one of greatest causes of death around the world (Ho et al., 1999; Li et al., 1998; Lin et al., 2001). Our previous studies have demonstrated that electrical stimulation of the P5-P6 acupoints significantly attenuates sympathoexcitatory pressor responses elicited by chemical and mechanical stimulation of viscera, such as gallbladder and stomach (Li et al., 2001 and 2002), suggesting that electroacupuncture (EA) exerts its influence by modulating sympathetic activity (Tjen-A-Looi et al., 2003). Also, our data have shown that EA reduces sympathoexcitatory responses through an influence of β-endorphin and enkephalin on µ and δ opioid receptors in the rostral ventralateral medulla (rVLM), a site where visceral afferents converge on bulbospinal premotor sympathetic neurons (Li et al., 2001). These results suggest the possibility of an opioid mechanism contributing to EA-related cardiovascular regulation.
Recently, our laboratory has found that EA at P5-P6 induces expression of c-Fos, an immediate early gene, in the rVLM and periaquductal gray (PAG; Guo et al., 2004). These two regions control cardiovascular function by regulating autonomic activity (Dampney, 1994). The c-Fos gene has been widely used to identify neuronal activation (Guo and Longhurst, 2003; Morgen et al., 1987). Furthermore, we also have observed that c-Fos positive nuclei co-localize with rVLM perikarya containing enkephalin, and are in close apposition to fibers containing enkephalin or β-endorphin in the rVLM and PAG (Guo et al., 2004). These findings imply a role for these opioid peptides in neurons activated by EA. However, the responses of higher brain regions to P5-P6 stimulation remain unclear. In particular, it is not known if EA at these acupoints activates cell bodies containing enkephalin or β-endorphin in brain regions other than the rVLM and PAG.
The arcuate nucleus (ARC) within hypothalamus is an important site of synthesis of opioid peptides. Perikarya in the ARC contain either β-endorphin or enkephalin, but not both peptides (Bloom et al., 1978; Lantos, et al., 1995). Stimulation of proopiomelanocortin (β-endorphin precursor)-containing neurons in the ARC causes opioid-mediated hypotension (Li et al., 1996). These results suggest that opioids-containing neurons in the ARC might regulate sympathetic outflow and the hemodynamic responses to EA (Li et al., 1996).
Our recent physiological studies have demonstrated that the predominant response of ARC neurons to stimulation of P5-P6 acupoints is excitation. Furthermore, excitatory projections from the ARC to the ventrolateral PAG (vlPAG) contribute to inhibition of sympathoexcitatory cardiovascular reflexes induced by EA at P5-P6 (Li et al., 2006). These findings indicate that EA likely activates ARC neurons that contain excitatory neurotransmitters in addition to modulatory neurotransmitters like opioid peptides. Glutamate is an important excitatory neurotransmitter in the brain. There is evidence showing glutamatergic neurons are present in the ARC (Kiss et al., 2005). Although some glutamate positive neurons contain β-endorphin in the ARC (Kiss et al., 2005), it is uncertain if EA activates neurons contain both neuronal substances in this region.
In the present study, we evaluated the neuroanatomical substrates in the ARC that are activated during P5-P6 stimulation specifically with respect to the opioid system and glutamate. We proposed the following hypotheses: 1) stimulation of the P5-P6 acupoints increases c-Fos expression in the ARC. 2) cells demonstrating c-Fos activity in the ARC following P5-P6 stimulation co-localize with neurons containing β-endorphin as well as enkephalin. 3) EA at P5-P6 activates ARC glutamatergic neurons, some of which contain opioids.
2. Results
2.1. Influence of EA on blood pressure and heart rate
After bilateral barodenervation and vagotomy, the baseline of mean BP (MAP, 146–154 mmHg) and HR (172–180 b.p.m.) were similar among all cats. A slight decrease in MAP (5–10 mmHg) was observed in three of six cats during EA stimulation at P5-P6. No changes in MBP were noted in the control animals (n=5) after acupuncture needles were placed at P5-P6 without electrical stimulation. HR was not changed during EA stimulation or control in any cat from either group.
2.2. c-Fos immunohistochemical labeling in ARC
Fos immunoreactivity was noted to be bilaterally distributed throughout the rostro-caudal extension of the ARC. Compared to the control animals, Fos-labeled neurons were found easily at multiple-levels throughout the ARC in the EA-treated cats. Photomicrographs in Fig. 1 show the distribution of c-Fos positive cells in the ARC from a cat in the sham-operated control and EA-treated groups, respectively. Abundant c-Fos immunoreactivity in the ARC was associated with EA stimulation in this figure.
Figure 1.
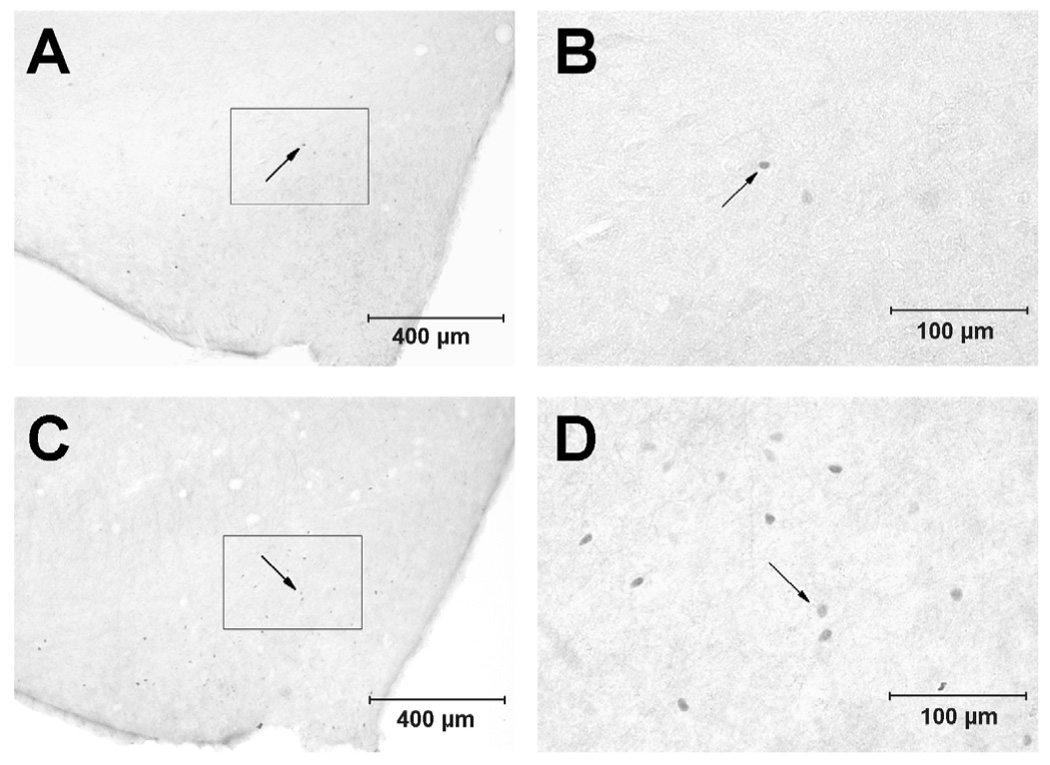
Photomicrographs showing Fos-like immunoreactive cells in the arcuate nucleus (A 11.0, Berman’s atlas) from a control animal (A and B, top) and a cat treated with electroacupuncture (C and D, bottom). A and C: low-power photomicrograph. B and D: magnified regions shown within boxes in the A and C, respectively. Arrows indicate dots representing c-Fos labeled cells. Scale bars in A and C, or B and D represent 400 and 100 µm, respectively. V3, third ventricle.
We observed similar patterns of c-Fos distribution in the ARC in single c-Fos labeled sections, double-stained sections containing c-Fos and β-endorphin or enkephalin and sections labeled with VGLUT3, c-Fos and β-endorphin. Therefore, we quantitatively evaluated c-Fos immunoreactivity in the double- and triple-labeled sections. We found that the numbers of c-Fos positive neurons were significantly increased in cats treated with EA (n=6; P<0.05, Table 1, Table 2 and Table 3), compared to controls (n=5).
Table 1.
C-Fos immunoreactivity and co-location with neurons labeled with β-endorphin in ARC
| Fos cells # | β-Endo cells # | β-Endo+Fos cells # | β-Endo+Fos cells β-Endo cells (%) | β-Endo+Fos cells Fos cells %) | |
|---|---|---|---|---|---|
| Control (n=5) | 28 ± 10 | 92 ± 8 | 1 ± 0 | 1 ± 0 | 2 ± 1 |
| EA-treated (n=6) | 108 ± 23* | 111 ± 15 | 11 ± 3** | 11 ± 3** | 10 ± 1** |
Means ± SE. Average total number (#) of c-Fos positive, neurons containing β-endorphin and cells co-localized with both stains per section in ARC. ARC, arcuate nucleus; Fos, c-Fos; β-Endo, β-endorphin; EA, electroacupuncture stimulation.
P<0.05
P<0.01; EA-treated group vs. control group.
Table 2.
C-Fos immunoreactivity and co-location with enkephalin-labeled neurons in ARC
| Fos cells # | Enk cells # | Enk+Fos cells # | Enk+Fos cells Enk cells (%) | Enk+Fos cells Fos cells (%) | |
|---|---|---|---|---|---|
| Control (n=5) | 30 ± 10 | 32 ± 5 | 1 ± 0 | 1 ± 1 | 1 ± 1 |
| EA-treated (n=6) | 100 ± 25* | 34 ± 8 | 9 ± 3** | 24 ± 5** | 8 ± 1** |
Means ± SE. Average total number (#) of c-Fos positive, enkephalin-containing neurons and cells co-localized with both stains per section in ARC. ARC, arcuate nucleus; Fos, c-Fos; Enk, enkephalin; EA, electroacupuncture stimulation.
P<0.05
P<0.01; EA-treated group vs. control group.
Table 3.
C-Fos immunoreactivity and co-location with VGLUT3-labeled neurons in ARC
| Fos cells # | VGLUT3 cells # | VGLUT3+Fos cells # | VGLUT3+Fos cells VGLUT3 cells (%) | VGLUT3+Fos cells Fos cells (%) | |
|---|---|---|---|---|---|
| Control (n=5) | 26 ± 8 | 117 ± 8 | 9 ± 4 | 7 ± 2 | 33 ± 3 |
| EA-treated (n=6) | 90 ± 14* | 123 ± 5 | 44 ± 7** | 36 ± 5** | 51 ± 2* |
Means ± SE. Average total number (#) of c-Fos positive, VGLUT3-containing neurons and cells co-localized with both stains per section in ARC. ARC, arcuate nucleus; Fos, c-Fos; VGLUT3, Vesicular glutamate transporter 3; EA, electroacupuncture stimulation.
P<0.05
P<0.01; EA-treated group vs. control group.
2.3. Immunohistochemical labeling with c-Fos + β-endorphin in ARC
We observed a robust population of cell bodies stained with β-endorphin in the ARC after application of colchicine, which were more numerous than those in cats not treated with colchicine in our preliminary study. Neurons containing β-endorphin were located bilaterally in the ARC. This observation was consistent with earlier findings (Bloom et al., 1978; Finley et al., 1981). We did not detect a difference in the average number of neurons containing β-endorphin in the ARC between EA-treated and the control groups (Table 1).
As mentioned above, c-Fos immunoreactivity was detected more frequently in the ARC of EA-treated cats compared to the control animals. Neurons co-labeled with β-endorphin and c-Fos were identified in the ARC of EA-treated cats and rarely in control animals. In comparison with the control group (n=5), more neurons were double-labeled with β-endorphin and c-Fos in the intervention group (n=6) when they were evaluated alone as well as in relation to the total population of β-endorphin or c-Fos positive cells (Table 1, Fig. 2). Photomicrographs in Fig. 3 provide an example of co-localization of Fos-like immunoreactive nuclei with perikarya containing β-endorphin in the ARC of an EA-treated cat.
Figure 2.
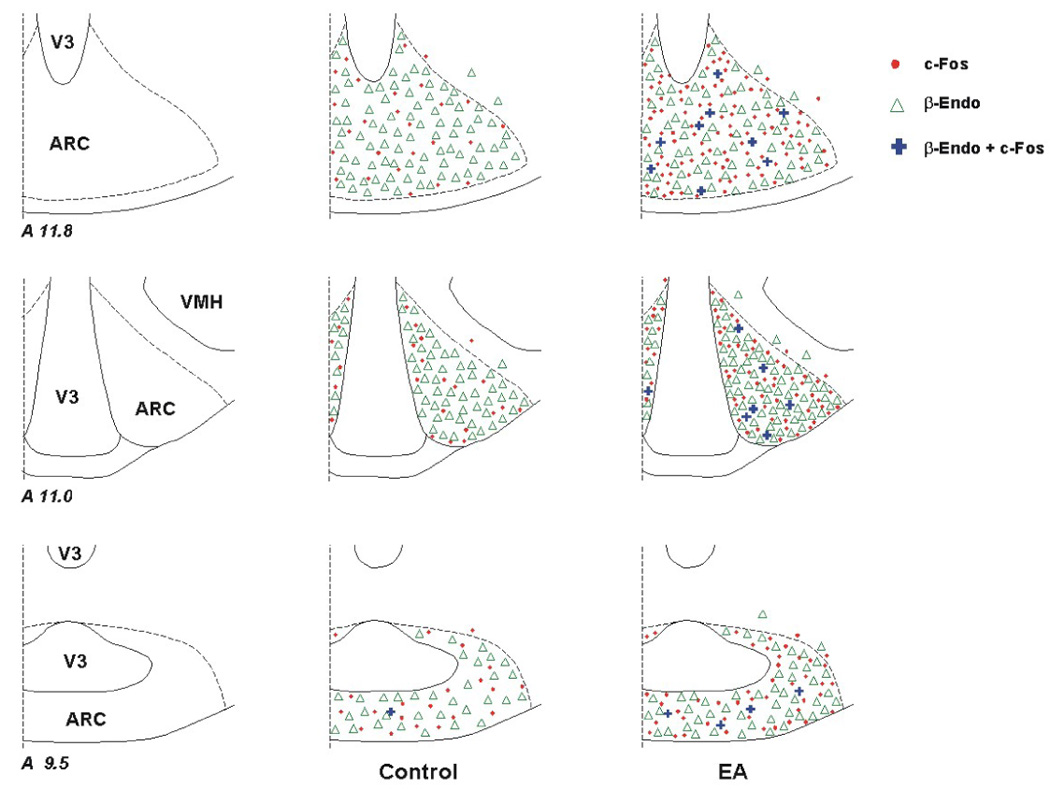
Distribution of β-endorphin (β-Endo) labeled cells and c-Fos immunoreactivity in the arcuate nucleus (ARC) following electroacupuncture (EA) and in a sham-operated control. Three coronal sections (Berman’s atlas) were selected from one animal in each experimental group. Each symbol, ●, △, or + represents one labeled cell with c-Fos, β-endorphin or c-Fos + β-endorphin, respectively.
Figure 3.
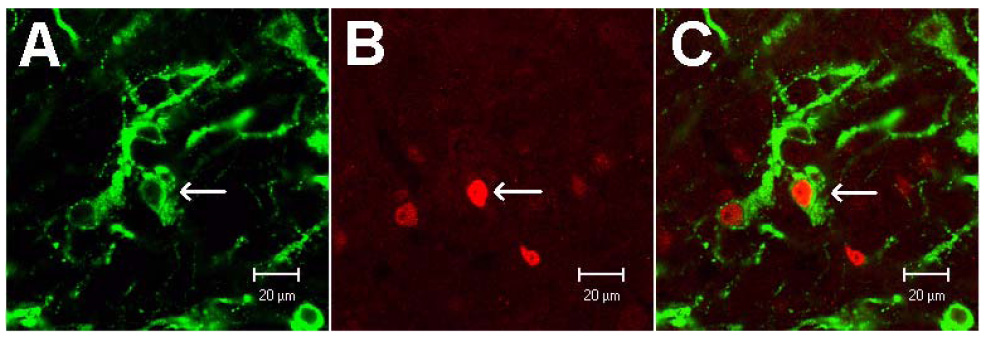
Confocal microscopic images of neurons double-labeled with β-endorphin and c-Fos in the arcuate nucleus at the level of A 11.0 (Berman’s atlas) following the stimulation with electrical acupuncture. Arrows in A, B and C indicate a neuron stained with β-endorphin, c-Fos positive nucleus and co-localization of β-endorphin with c-Fos immunoreactivity, respectively. Scale bars in A-C represent 20 µm.
2.4. Immunohistochemical labeling with c-Fos + enkephalin in ARC
Perikarya containing enkephalin were distributed bilaterally in the ARC following application of colchicine. This was not observed in cats without colchicine treatment in our preliminary study. Perikarya containing enkephalin in the ARC were less numerous than those containing β-endorphin (Table 1 and Table 2). There was no significant difference in distribution of neurons labeled with enkephalin in the ARC of colchicine-treated cats comparing EA and control groups (Table 2).
Similar to the increase in ARC c-Fos immunoreactivity following EA, we observed that neurons double-labeled with c-Fos and enkephalin were increased (P<0.05) in the cats treated with EA (n=6; Table 2, Fig. 4) compared to the control groups (n=5). Also, more cells in the EA group showed co-localization of c-Fos with enkephalin, when the numbers were expressed relative to the total population of enkephalin or c-Fos positive cells (Table 2). Fig. 5 shows examples of confocal images containing neurons double-labeled with c-Fos and enkephalin in the ARC of a cat from the EA-treated group.
Figure 4.
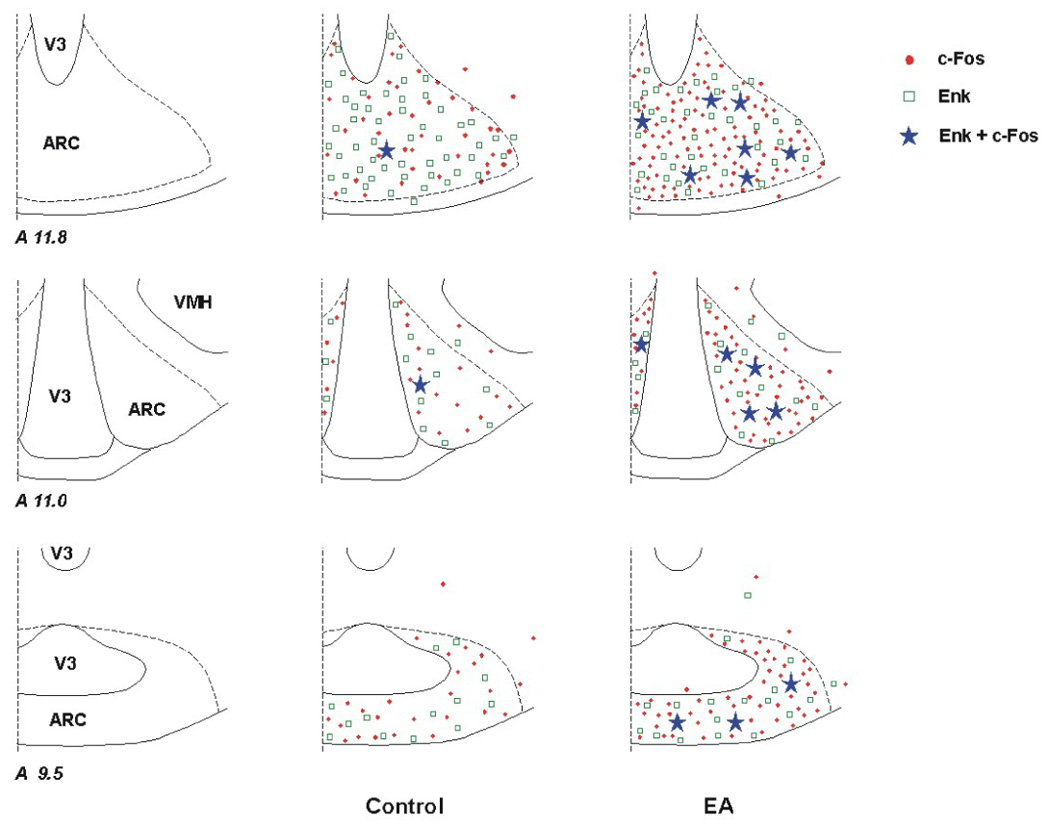
Distribution of enkephalin (Enk) labeled cells and c-Fos reactivity in the arcuate nucleus (ARC) of a control animal and a cat subjected to electroacupuncture (EA). Three coronal sections (Berman’s atlas) were selected from each animal. Each symbol, ●, □, or ★ represents one labeled cell with c-Fos, enkephalin or c-Fos + enkephalin, respectively.
Figure 5.
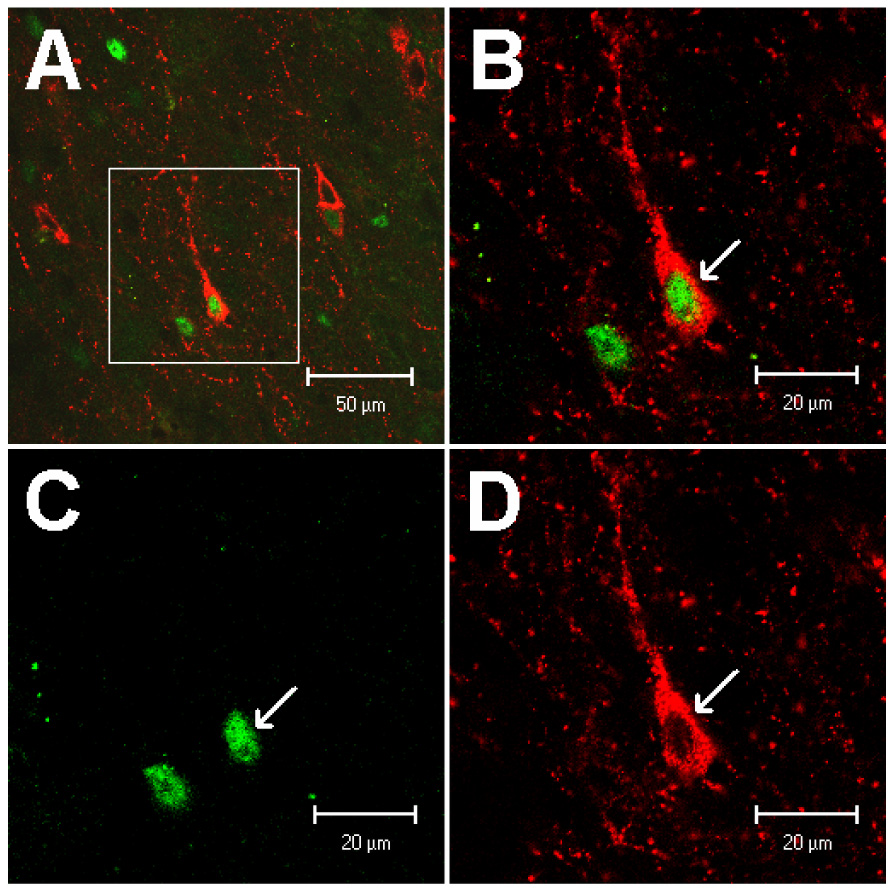
Confocal microscopic images of neurons double-labeled with enkephalin and c-Fos in the arcuate nucleus at the level of A 11.8 (Berman’s atlas) following the stimulation with electrical acupuncture. A: low-power photomicrograph; B: magnified region shown within box in A. Arrow indicates an example of co-localization of an enkephalin-labeled neuron with a c-Fos immunoreactive nucleus. B is a merged image from C and D. Arrows in C and D respectively indicate c-Fos positive nucleus and cytoplasm of a neuron stained with enkephalin. Scale bars in A and B-D represent 50 and 20 µm, respectively.
2.5. Immunohistochemical labeling with c-Fos + VGLUT3 + β-endorphin in ARC
Many perikaryia and neuronal processes labeled with VGLUT3 were observed in both right and left regions of the ARC in colchicine-treated cats. We did not find that the number of cell bodies containing VGLUT3 in the ARC was markedly altered after treatment with colchicine in the cat. We also noted no significant difference in distribution and density of VGLUT3 labeling in the ARC comparing controls and EA-treated cats (Table 3).
We noted that the numbers of cells labeled with c-Fos and/or β-endorphin in the ARC in sections subjected to the triple-labeling were similar to those shown in Table 1, which displays data from sections containing these two labels from the same animals. C-Fos and c-Fos + β-endorphin, but not β-endorphin was markedly increased in the ARC of cats treated with EA, compared to controls. Importantly, in the triple-labeled sections, we found more neurons double-labeled with c-Fos and VGLUT3 in this region of the ventral hypothalamus in EA-treated vs. control cats (P<0.01, Table 3). Moreover, we observed for the first time neurons (6 ± 1 cells per section) triple-labeled with c-Fos, VGLUT3 and β-endorphin in the ARC following EA. Triple-labeled neurons were not found in the ARC of control animals. Photomicrographs in Fig. 7 display an example of a neuron triple-labeled with c-Fos, VGLUT3 and β-endorphin in the ARC of a cat following EA treatment.
Figure 7.
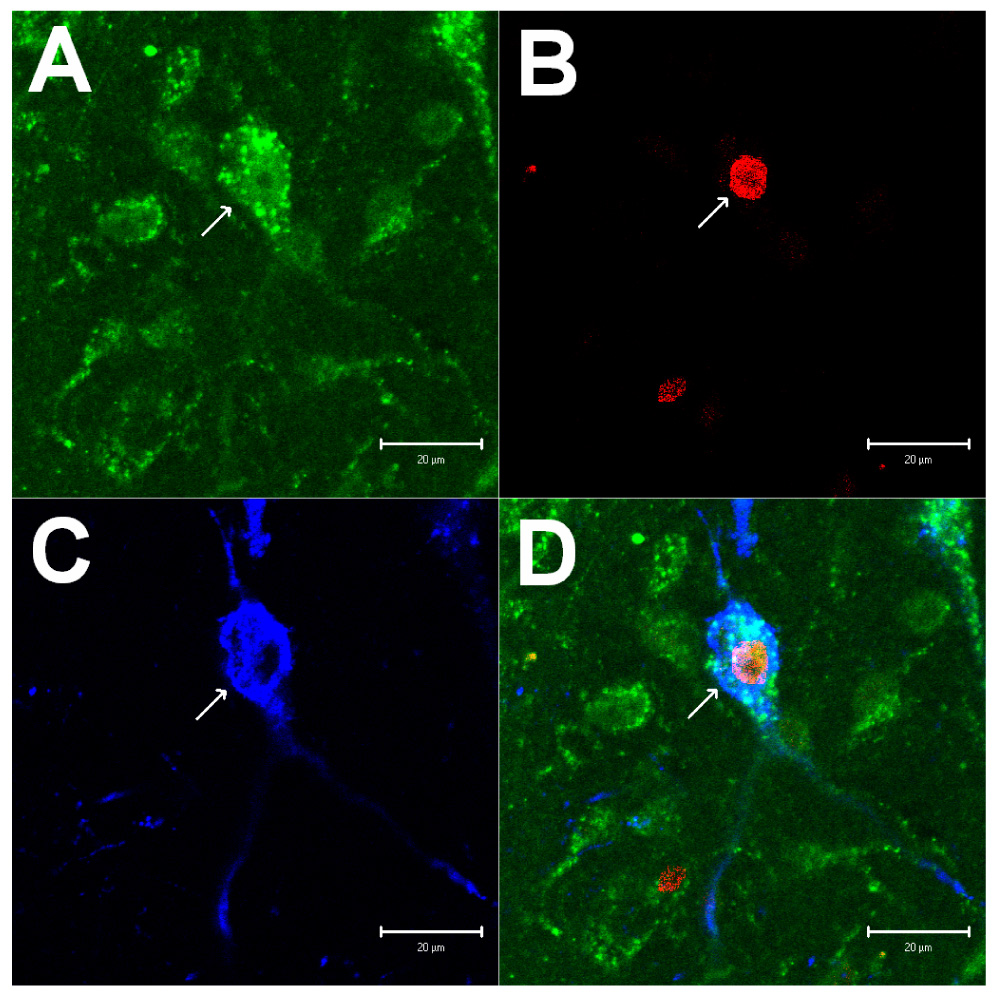
Confocal microscopic images displaying labelings of VGLUT3, c-Fos and β-endorphin in the arcuate nucleus at the level of A 11.0 (Berman’s atlas) from a cat treated with electrical acupuncture. Panels A-C show immunostaining for VGLUT3 (green), c-Fos (red) and β-endorphin (blue), respectively. Panel D demonstrates merged images from Panels A-C. Arrows in Panels A-D indicate a neuron containing VGLUT3, c-Fos, β-endorphin and VGLUT3 + c-Fos + β-endorphin, respectively. Scale bar = 20 µm.
3. Discussion
EA at the P5-P6 acupoints is applied commonly to treat cardiovascular disorders (Ho et al., 1999; Li et al., 1998; Lin et al., 2001). Our group has shown that stimulation of these acupoints attenuates sympathoexcitatory pressor responses induced by stimulation of visceral organs in animals through modulation of sympathetic activity (Tjen-A-Looi et al., 2004; Li et al., 2001 and 2002). The ARC is considered to be an important area that regulates a number of physiological and pathophysiological processes including emotion, autonomic activity, and pain, etc. (Chronwall, 1985; Finley et al., 1981; Li et al., 1996). Our recent physiological studies have shown that EA at P5-P6 increases neuronal activity in the ARC, which contributes to modulation of visceral-cardiovascular responses (Li et al., 2006). The present study provides further anatomical data on the extent of neuronal activation during acupuncture through mapping c-Fos expression in this region. Moreover, in this study, we demonstrated neurons that responded to EA co-localized with opioid peptides as well as with VGLUT3, a marker for glutamate (Fremeau et al., 2002; Gritti et al., 2006; Guo et al., 2005).
C-Fos, an immediate early gene, is rapidly but transiently expressed following cellular stimulation. It serves as a valuable marker for identification of neuronal activation to map functionally responsive neuronal pathways in the central nervous system to peripheral sensory neuronal activation (Morgen et al., 1987). In this respect, we and other investigators have used Fos immunohistochemical staining to identify specific brain areas and spinal cord that respond to acupuncture at various acupoints, for instance, P5-P6, Zusanli (S36, stomach meridian) acupoints (Guo et al., 1996; Guo et al., 2004). Like our previous studies (Guo and Longhurst, 2003; Guo et al., 2004), care was taken to minimize c-Fos expression in the ARC as a result of non-specific stimuli, including treatment with colchicine, anesthesia, surgical procedures and input from baroreceptors resulting from changes in blood pressure. As described previously (Guo et al., 2004), the smallest possible dose of colchicine was used in the present study to minimize expression of c-Fos induced by colchicine in this region (Gillen and Briski, 1996). The only difference in treatment between sham-operated controls and EA was electrical stimulation of acupuncture needles at P5-P6 in the latter group. Thus, the pattern of increased c-Fos in the EA-treated cat was induced solely by electrical stimulation of needles placed in the P5-P6 acupoints.
We observed a slight decrease in blood pressure (5–10 mmHg) during EA in three of the six cats. Stimulation of myelinated somatic afferents can elicit depressor responses (Johansson, 1962; Koizumi et al., 1970; Lee and Beitz, 1993). Since stimulation of median nerves with low intensity and frequency EA activates more myelinated than unmyelinated fibers (Li et al., 1998), a slight decrease in blood pressure may be expected to occur. However, the barodenervation eliminated any secondary baroreflex influence on c-Fos expression in the ARC during EA.
The ARC contains neurons that synthesize and release a variety of neurotransmitters and modulatory neuropeptides (Lantos et al., 1995). It serves a number of functions linking pain, emotion, homoeostatic and autonomic responses, among others, through the pituitary and brainstem (Chronwall, 1985; Li and Dampney, 1994). Previous studies have suggested participation of this nucleus in the analgesic effect of acupuncture at Zusanli acupoint (S36, overlying the deep peroneal nerve; Pan et al., 1996). Recent data from our laboratory suggested that ARC also plays a role in EA-modulated sympathoexcitatory cardiovascular responses (Li et al., 2006). Our observations of increases in c-Fos expression in the ARC induced by EA are similar to our previous observations in the rVLM and PAG (Guo et al., 2004). These data support our previous electrophysiological studies, indicating that the ARC receives somatic input from P5-P6 stimulation (Li et al., 2006).
The β-endorphin and enkephalin are two important opioid peptides present in the brain. Separate neurons contain each of these peptides (Bloom et al., 1978). Pan et al (1996) suggested that activated neurons containing β-endorphin in the ARC may be involved in analgesia induced by EA at Zusanli acupoints. However, it is not clear if acupoints used more frequently to treat cardiovascular disturbances, such as P5-P6, are capable of activating neurons containing β-endorphin as well as enkephalin in this region. In preliminary experiments, we observed only fibers containing enkephalin in the ARC. However, after treatment with colchicine, cell bodies containing enkephalin were observed in this region. In addition, more perikarya stained with β-endorphin were found in the ARC as compared to no treatment. These observations were consistent with previous studies (Ceccatelli et al., 1989; Cirriello and Caverson, 1989; Finley et al., 1981; Lantos et al., 1995). The method of using colchicine to enhance opioid immunolabeling thus allowed us to test our hypothesis that EA induces c-Fos expression in opioidergic neurons. In the present study, we found increased co-localization of c-Fos immunoreactive nuclei with perikarya containing β-endorphin as well as with cell bodies labeled with enkephalin in the ARC following EA at P5-P6. These data indicate that ARC neurons activated during EA at these acupoints synthesize β-endorphin or enkephalin. As mentioned above, our previous studies have shown a close anatomical relationship between EA-activated neurons and cells containing opioids in the rVLM and PAG (Guo et al., 2004). Taken together, our findings strongly suggested a role for the opioid system in several regions concerned with the long-loop pathway that is triggered by EA in its regulation of cardiovascular function.
Interestingly, in this study, we found that while approximately one fifth of c-Fos postive neurons contain either enkephalin or β-endorphin, many more or almost half of neurons that express c-Fos co-localize with VGLUT3 in the ARC following EA at P5-P6. VGLUT3 is considered to be a marker for neurons that contain glutamate (Fremeau et al., 2002; Gritti et al., 2006; Guo et al., 2005). In this respect, Gritti et al have shown that almost all (96–100%) VGLUT3-labeled neurons are positively stained for phosphate-activated glutaminase (pGlu), an enzyme that synthesizes glutamate from glutamine, and that approximately 70% of pGlu-containing neurons are co-labeled with VGLUT3. As such, our data imply that the glutamatergic neurons, responsive to somatic nerve stimulation during EA, are particularly important in the ARC. These results confirm other studies suggesting that glutamate functions as a major excitatory neurotransmitter in the ARC, including induction of release of β-endorphin and hormones (Bach and Yaksh, 1995; Kiss et al., 1997; MacDonald et al., 1993). The present study also supports our previous physiological findings that EA excites ARC neurons and that activation of the ARC by excitatory amino acid DL-homocysteic acid lead to inhibition of visceral sympathoexcitatory reflexes. Conversely, non-specific inhibition of glutamate receptors with kainic acid in the ARC prevents the EA-induced inhibition of visceral-cardiovascular pressor reflexes (Li et al., 2006). Thus, the ARC receives excitatory input from somatic afferents and plays an important role in EA-related inhibition of cardiovascular reflex responses.
We noted that co-localization of VGLUT3 and β-endorphin exists in some neurons activated by EA, identified by their expressions of c-Fos. These triple-labeled neurons represent about 67% of activated neurons containing β-endorphin and approximately 17% of activated neurons labeled with VGLUT3. These results suggest that a relationship exists between glutamate and opioids in processing information in the ARC during EA activation of somatic afferents. Glutamate and opioids may be co-released from the same neurons and thus could interact with each other during EA.
In addition, there is evidence showing that VGLUT3 also is present in some neurons that contain GABA, acetylcholine or serotonin (Gritti et al., 2006; Stornetta et al., 2005). Thus, it is possible that VGLUT3-labled neurons activated during EA also may contain other neurotransmitters that contribute to processing somatic information through the ARC (Chronwall, 1985; Kiss et al., 2005).
In summary, the present study for the first time provides initial anatomical evidence showing significant activation of ARC neurons in response to EA at the P5-P6 acupoints. Many neurons contain opioids and/or glutamate, which may interact during processing of neural input in response to EA. These data complement our past electrophysiological findings. Taken together, our results suggest that the ARC is an important site where acupuncture affects the opioid as well as excitatory neurotransmitter activity to subsequently modulate autonomic nerve activity, including cardiovascular responses.
4. Experimental Procedure
4.1. General Surgical Preparation
Surgical and experimental protocols of this study were approved by the animal use and care committee at the University of California, Irvine. All procedures were carried out in accordance with the US Society for Neuroscience and the National Institutes of Health guidelines. The minimum possible numbers of adult cats (n=11, 2.82 to 4.12 kg) of both sexes were used to obtain reproducible and statistically significant results. Throughout the study, steps were taken to minimize discomfort and suffering of the animals.
In a preliminary study, we observed neuronal fibers labeled with enkephalin and perikarya containing β-endorphin in the ARC. To clearly and comprehensively demonstrate perikarya containing either enkephalin or β-endorphin in this region, we used colchicine to inhibit microtubular transport of these opioids. We and others have shown that this technique enhances the number of neurons containing opioids (Ciriello and Caverson, 1989; Finely et al., 1981; Guo et al., 2004; Lantos et al., 1995).
Sterile surgical procedures were performed for administration of colchicine in the surgical operating room of the vivarium at the University of California, Irvine. Cats were pre-anesthetized with ketamine (25 mg/kg, im) and valium (5 mg/kg, im). Anesthesia was maintained with isoflurane (1–2%, inhalation). Each cat was placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) and the head was flexed approximately 30° forward in the frame. For microinjection, a one-inch midline incision was made from the external occipital protuberance located at the base of the skull. After exposing the foramen magnum near the brainstem, a 27×1.25-gauge hypodermic needle attached to a 1.0 ml syringe was inserted into the subarachnoid space through the atlanto-occipital membrane overlying the fourth ventricle. Colchicine (90–100 µg/kg, Sigma, St. Louis, MO, USA) in 0.08~0.13 ml of solution (3000 µg/ml) dissolved in 0.9% normal saline was injected. The dose of colchicine used in this study was based on our previous studies and Cirello and Caverson’s report (Cirello and Caverson, 1989; Guo et al., 2004). After injection of the colchicine, the incision was closed and the cats were allowed to recover.
Following the 22–24 hour post-operative period after administration of colchicine, cats were re-anesthetized with ketamine (40–50 mg/kg, im) and subsequently maintained with α-chloralose (50–60 mg/kg, iv). Supplemental α-chloralose (5–10 mg/kg, iv) was administered to maintain an adequate depth of anesthesia as judged by stability of blood pressure and respiration, and the lack of a withdrawal response to toe pinch. A femoral vein and artery were cannulated for administration of drugs and fluids, and measurement of arterial blood pressure (BP, Statham P 23 ID, Oxnard, CA, USA). Heart rate (HR) was derived from the arterial pressure pulse with a biotach (Gould Instrument, Cleveland, OH, USA). A cuffed endotracheal tube was inserted to artificially ventilate the animal. Arterial blood gases and pH were monitored with a blood gas analyzer (Radiometer, Inc., Model ABL-3, Westlake, OH, USA). They were kept within normal limits (PO2, 100–150 mmHg; PCO2, 28–35 mmHg; pH, 7.35–7.45) by enriching the inspired O2 supply and adjusting the ventilatory rate and/or volume, and as necessary, by administration of 1 M NaHCO3. Body temperature was maintained at 36–38°C by a water heating pad and a heat lamp.
Cardiovascular hemodynamic changes can cause secondary baro- and cardiopulmonary reflex activation, which can lead to c-Fos expression in the brain (Guo and Longhurst, 2003; Potts et al., 1997). To control for the input from this secondary activation of the neural pathways consequent to stimulation of the P5-P6 acupoints (Johansson, 1962; Koizumi et al., 1970; Li et al., 1998), bilateral sino-aortic denervation and cervical vagotomy were conducted. The carotid sinus nerves and cervical vagus were isolated and transected from the internal and common carotid artery, respectively. Barodenervation was verified by the absence of a normal decrease of heart rate in response to an increase in arterial blood pressure induced by administration of phenylephrine (10 µg/kg, iv; Gensia Sicor Pharmaceuticals, Irvine, CA, USA).
Cats were allowed to stabilize for 4 hours after surgical preparation. Similar to our previous studies (Guo et al., 2004), approximately 28–30 hours after administration of colchicine, pairs of stainless steel, 32 ga acupuncture needles were inserted bilaterally at the P5-P6 acupoints, overlying the median nerves (Guo et al., 2004; Li et al., 2006). Previous studies have documented that the P5-P6 acupoints on both forelimbs of small animals are analogous to those in humans (Hua, 1994). The needles were connected to a constant current stimulator with a stimulus isolation unit (Li et al., 1998 and 2006).
4.2. Experimental Protocols
Animals were divided randomly into an EA stimulation group (n=6; male 3, female 3) and a sham-operated group (n=5; male 3, female 2). Acupuncture typically is performed for 20–40 min. Our studies in anesthetized cats have demonstrated modulation of rVLM premotor sympathetic neuronal and reflex sympathetic responses for up to 60 min following 30 min of EA (Li et al., 1998 and 2001; Tjen-A-Looi et al., 2003). Low frequency EA attenuates sympathetic activity, at least in part, through the endogenous opiate system (Li et al., 2001; Cheng and Pomeranz, 1979). Thus, in the present study, low frequency EA (0.5 ms pulses, 2 Hz, 2–5 V, 1–4 mA) was carried out for 30 min. This stimulation was sufficient to produce moderate, repeated paw flexion on each forelimb. Each set of electrodes was stimulated separately so that current did not flow from one location to the contralateral side. In control animals, acupuncture needles were put into the P5-P6 acupoints for 30 min but were not stimulated electrically.
4.3. Histochemistry and Immunohistochemistry
4.3.1. Brain tissue preparations
As described in our previous studies (Guo and Longhurst, 2003; Guo et al., 2004), 90 min following termination of EA stimulation or control procedures, another larger dose of α-chloralose (100 mg/kg, iv) was administered to induce deep anesthesia. Subsequently, animals were perfused transcardially with 0.9% saline and cold 4% paraformaldehyde in phosphate buffer (PB, pH 7.2). The diencephalon was harvested and stored in 4% paraformaldehyde for 2 hours, then transferred to 30% sucrose for 48 hours to prevent ice crystallization.
Coronal sections of the brain (30 µm) were made with a cryostat microtome (Leica CM1850 Heidelberger Strasse, Nussloch, Germany) and collected serially in cold cryoprotectant solution (Chan and Sawchenko, 1994) in sets of five. Each set of sections were used for conducting four kinds of immunohistochemical labels described in the following section, or stained with Nissl to reveal the cellular architecture (Guo and Longhurst, 2003). Free-floating sections were used for labeling in this study.
4.3.2. C-Fos immunohistochemical labeling
The avidin-biotin-peroxidase complex (ABC) method was used for staining c-Fos protein (Guo and Longhurst, 2003). Briefly, brain sections were washed three times, 10 min for each wash, in 0.1 M PB (pH 7.2) containing 0.3% Triton X-100 (PBT), and then placed in 0.5% hydrogen peroxide for 10 min to quench endogenous peroxidase activity. After placing the sections in 1% normal goat serum (Vector ABC Kit, Vector Laboratories, Burlingame, CA, USA) for 20 min, they were incubated with a primary polyclonal rabbit anti-Fos antibody (1:20,000 dilution, Oncogene research product, Calbiochem, San Dieago, CA, USA) at 4°C for 48 hours. This antibody was raised specifically against amino acid 4–17 of human Fos. After a 48-hour period of incubation, sections were rinsed in 0.1 M PBT three times, then incubated in biotinylated goat anti-rabbit IgG (Vector Kit, 1:200) for 60 min. Following three washes in 0.1 M PBT, brain sections were placed in ABC solution (Vector Kit, 1:50) for 30 min. Sections were washed twice, each for 10 min, in 0.1 M PB and were incubated in a solution containing hydrogen peroxide and 3,3’-diaminobenzidine (DAB; DAB substrate kit for peroxidase, Vector laboratory) for 5–8 min. DAB is reduced by hydrogen peroxide in the ABC complex, and deposited in the tissue as a brown reaction product. The DAB reaction was stopped by washing sections in distilled water. Brain sections were mounted on slides in 0.1 M PB. Slides were allowed to air-dry overnight, cleared in alcohol and xylene baths, then covered by glass slips with permount (Fisher Scientific, Fair Lawn, New Jersey, USA). The c-Fos protein was visualized by the DAB reaction product as dark-brown staining. In an immunohistochemical control study, no labeling was detected when the primary antibody was omitted.
4.3.3. Double-fluorescent immunohistochemical labeling for c-Fos + β-endorphin or enkephalin
After washing three times (10 min each) with phosphate buffered saline containing 0.3% Triton X-100 (PBST, pH=7.4), brain sections were placed in 1% normal donkey serum (Jackson immunoresearch laboratories, Inc., West Grove, PA, USA) for 1 hour and incubated with primary antibodies at 4°C for 48 hours. PBST solution containing the two primary antibodies included a mouse monoclonal anti-c-Fos (1:2,000 dilution, Santa Cruz Biotechnology) and a rabbit polyclonal anti-β-endorphin (1:400, Chemicon International, Inc., Temecula, CA, USA), or a rabbit polyclonal anti-c-Fos (1:2,000 dilution, Oncogene research product) and a mouse monoclonal anti-enkephalin, a specific anti-body against met- and leu-enkephalin (1:200, Chemicon International, Inc.). Sections then were incubated with rhodamine-conjugated donkey anti-mouse antibody and fluorescein-conjugated donkey anti-rabbit antibody (both 1:100; Jackson immunoresearch laboratories, Inc.) in PBST for 24 hours at 4°C. These secondary antibodies raised in the donkey are made for multiple labels. They have minimal cross-reactivity to other nonspecific species (Catalog, specializing in second antibodies, Jackson immunoresearch laboratories, Inc., 2006). After rinsing the sections in phosphate buffered saline (PBS, pH=7.4) for 30 min (10 min × 3 times), they were mounted on slides and air dried. The slides were coverslippped using mounting medium (Vector Laboratories). Immunohistochemical control studies were performed by omission of the primary or secondary antibodies and by preabsorption with an excess (10 µg/ml) of the respective opioid peptide, i.e., β-endorphin or met- and leu-enkephalin (Bachem Peninsula Labs. San Carlos, CA). No labeling was detected under these conditions.
4.3.4. Triple-fluorescent immunohistochemical labels for c-Fos, glutamate, and β-endorphin
Currently, vesicular glutamate transporter 3 (VGLUT3), present in perikarya as well as neuronal processes, has been employed to identify neurons that use glutamate as a neurotransmitter (Fremeau et al., 2002; Gritti et al., 2006; Guo et al., 2005). Thus, we stained VGLUT3 to detect glutamatergic neurons. The staining procedures were similar to those used for double-fluorescent immunohistochemical labeling described above. Briefly, after treating with PBST and 1% normal donkey serum, brain tissues were incubated with three primary antibodies, i.e., a guinea-pig polyclonal anti-VGLUT3 (1:1000 dilution), a rabbit polyclonal anti-β-endorphin (1:400, both purchased from Chemicon International, Inc.) and mouse monoclonal anti-c-Fos (1:2,000 dilution, Santa Cruz Biotechnology), for 48 hours at 4°C. Sections then were incubated with fluorescein-conjugated donkey anti-guinea-pig antibody, coumarin-conjugated donkey anti-rabbit antibody and rhodamine-conjugated donkey anti-mouse antibody (all 1:100; Jackson immunoresearch laboratories, Inc.) at 4°C for 24 hours. The sections were mounted on slides and coverslippped with mounting medium (Vector Laboratories). In immunohistochemical control studies, no staining was detected when the corresponding primary or secondary antibody was omitted.
4.4. Data Analysis
Brain sections were scanned and examined with a standard light and fluorescent microscope (Nikon, E400, Melville, NY, USA). Cells labeled with c-Fos appeared as a round dot of about 7–12 µm in diameter and were distinguishable from background staining at 40x magnification. Three epi-fluorescence filters (B-2A, G-2A, or UV-2A) equipped in a fluorescent microscope were used to identify single stains appearing as green (fluorescein), red (rhodamine), or blue (coumarin) in brain sections. Two or three single fluorescent images were captured with a Spot digital camera (RT color v3.0, Spot Diagnostic Instruments, Inc., Sterling Heights, MI, USA) from the same site of the brain section. Using the software provided with the Spot digital camera, images were merged to identify double- or triple-labeled markers (Guo et al., 2005). We selected six sections that most closely match three standard stereotaxic planes (A9.5, A11.0 and A11.8) of Berman’s atlas for the cat (Berman, 1982). Two sections in each plane displaying the ARC were evaluated. The numbers of single-, double- or triple-stained cells in the same section were counted bilaterally in each animal. The average number of labeled cells in the three representative levels taken from the rostro-caudal extension of the ARC was calculated by dividing the total number of cells by six, thus representing the number of sections used for cell counting (Guo and Longhurst, 2003; Guo et al., 2004).
Confocal microscopy was used to confirm co-localization of two or three labels in the same cell. Thus, after examination with a fluorescent microscope, selected sections were evaluated with a laser scanning confocal microscope (Zeiss LSM 510, Meta system, Thornwood, NY, USA). This apparatus was equipped with Argon and HeNe lasers and allowed operation of multiple channels. Lasers of 488 and 543 nm wavelengths were used to excite fluorescein (green) and rhodamine (red), respectively. A 790 nm laser was applied for two-photon excitation of coumarin (blue). Digital images of the immunoreactive structures were captured and analyzed with software (Zeiss LSM) provided with the confocal microscope. Images in two or three colors in the same plane were merged to reveal the relationship between two or three immunoreactive elements (Fig. 3, Fig. 5 and Fig. 7). Single-, double- and triple-labeled neurons were analyzed.
Statistical Analysis
Data are expressed as means ± SE. We used the Kolmogorov-Smirnoff test to determine if the data were normally distributed. Comparisons between two groups were statistically analyzed with the Student’s t-test or Mann-Whitney Rank Sum Test. Values were considered to be significantly different when P<0.05. All statistical calculations were performed with a statistical software package (SigmaStat, Version 3.0, Jandel Scientific Software, San Rafael, CA, USA).
Figure 6.
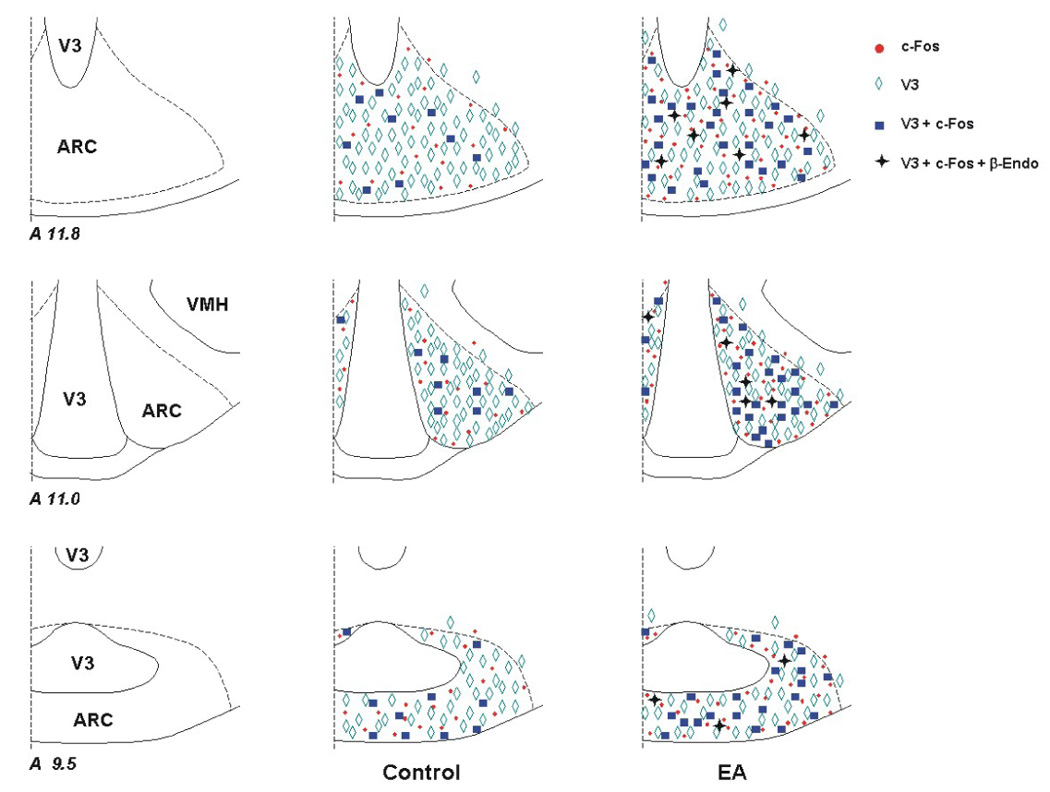
Distribution of VGLUT3, c-Fos and β-endorphin immunoreactivity in the arcuate nucleus (ARC) following control and electroacupuncture (EA) treatment. Three representative coronal sections were selected from one animal in each experimental group. Each symbol, ◊, ●, ■, or + represents a labeled cell with VGLUT3, c-Fos, VGLUT3 + c-Fos or VGLUT3 + c-Fos + β-endorphin (β-Endo), respectively. The levels of the sections are consistent with those shown in Berman’s atlas [Berman, 1982].
Acknowledgments
We are gratefully thankful to Ali R. Moazzami, B.S. and Teodora Agoncillo, B.S. for their technical assistance.
This study was supported by National Heart, Lung, and Blood Institute Grant, HL-072125, HL-66217 and HL-63313, the Larry K. Dodge and Susan-Samueli Endowed Chairs (JC Longhurst), and the American Heart Association, Western Affiliate Grant, 0365064Y (Z-L Guo).
Abbreviations
- ARC
Arcuate nucleus
- BP
Blood pressure
- ABC
the avidin-biotin-peroxidase complex
- DAB
3', 3' –diaminobenzidine
- EA
Electroacupuncture
- HR
Heart rate
- P5-P6
Neiguan-Jianshi acupoints
- PAG
Periaqueductal gray
- vlPAG
ventrolateral PAG
- PB
Phosphate buffer
- PBS
Phosphate buffered saline
- PBST
Phosphate buffered saline containing Triton X-100
- PBT
Phosphate buffer containing Triton X-100
- pGlu
Phosphate-activated glutaminase
- rVLM
Rostral ventrolateral medulla
- V3
Third ventricle
- VGLUT3
Vesicular glutamate transporter 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bach FW, Yaksh TL. Release of beta-endorphin immunoreactivity from brain by activation of a hypothalamic N-methyl-D-aspartate receptor. Neuroscience. 1995;65:775–783. doi: 10.1016/0306-4522(94)00528-d. [DOI] [PubMed] [Google Scholar]
- 2.Berman AL. Madison, WI: University of Wisconsin Press; 1982. The thalamus and basal telencephalon of the cat. [Google Scholar]
- 3.Bloom F, Battenberg E, Rossier J, Ling N, Guillemin R. Neurons containing β-Endorphin in rat brain exist separately from those containing enkephalin: Immunocytochemical studies. Prac. Natl. Acad. Sci. U.S.A. 1978;75:1591–1595. doi: 10.1073/pnas.75.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceccatelli S, Millhorn DE, Hokfelt T, Goldstein M. Evidence for the occurrence of an enkephalin-like peptide in adrenaline and noradrenaline neurons of the rat medulla oblongata. Exp. Brain Res. 1989;74:631–640. doi: 10.1007/BF00247366. [DOI] [PubMed] [Google Scholar]
- 5.Chan RKW, Sawchenko PE. Spatially and temporally differentiated patterns of c-Fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J. Comp. Neurol. 1994;348:433–460. doi: 10.1002/cne.903480309. [DOI] [PubMed] [Google Scholar]
- 6.Cheng RS, Pomeranz B. Electroacupuncture analgesia could be mediated by at least two pain-relieving mechanisms; endorphin and non-endorphin systems. Life Sci. 1979;25:1957–1962. doi: 10.1016/0024-3205(79)90598-8. [DOI] [PubMed] [Google Scholar]
- 7.Chronwall B. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6 Suppl 2:1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- 8.Ciriello J, Caverson MM. Relation of enkephalin-like immunoreactive neurons to other neuropeptide and monoamine-containing neurons in the ventrolateral medulla. Prog. Brain Res. 1989;81:3–15. doi: 10.1016/s0079-6123(08)61996-2. [DOI] [PubMed] [Google Scholar]
- 9.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 10.Finley JC, Lindstreom P, Petrusz P. Immunocytochemical localization of beta-endorphin-containing neurons in the rat brain. Neuroendocrinology. 1981;33:28–42. doi: 10.1159/000123197. [DOI] [PubMed] [Google Scholar]
- 11.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl. Acad. Sci. USA. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillen E, Briski KP. Expression of Fos-like proteins in the preoptic area and hypothalamus of the rat brain following intracerebral or peripheral administration of colchicine. Neurochem. Res. 1997;22:549–554. doi: 10.1023/a:1022424200948. [DOI] [PubMed] [Google Scholar]
- 13.Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience. 2006;143:1051–1064. doi: 10.1016/j.neuroscience.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo HF, Tian J, Wang X, Fang Y, Hou Y, Han J. Brain substrates activated by electroacupuncture of different frequencies (I): Comparative study on the expression of oncogene c-fos and genes coding for three opioid peptides. Brain Res. Mol. Brain Res. 1996:43157–43166. doi: 10.1016/s0169-328x(96)00170-2. [DOI] [PubMed] [Google Scholar]
- 15.Guo ZL, Longhurst JC. Activation of nitric oxide-producing neurons in the brain stem during cardiac sympathoexcitatory reflexes in the cat. Neuroscience. 2003;116:167–178. doi: 10.1016/s0306-4522(02)00707-8. [DOI] [PubMed] [Google Scholar]
- 16.Guo ZL, Moazzami AR, Longhurst JC. Electroacupuncture induces c-Fos expression in rostral ventrolateral medulla and periaqueductal gray in cats: relationship to opioid-containing neurons. Brain Res. 2004;1030:103–115. doi: 10.1016/j.brainres.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 17.Guo ZL, Moazzami AR, Longhurst JC. Stimulation of cardiac sympathetic afferents activates glutamatergic neurons in the parabrachial nucleus: relation to neurons containing nNOS. Brain Res. 2005;1053:97–107. doi: 10.1016/j.brainres.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 18.Ho FM, Huang PJ, Lo HM, Lee FK, Chern TH, Chiu TW, Liau CS. Effect of acupuncture at Nei-Kuan on left ventricular function in patients with coronary artery disease. Am. J. Chin. Med. 1999;27:149–156. doi: 10.1142/S0192415X99000197. [DOI] [PubMed] [Google Scholar]
- 19.Hua XB. Experimental acupuncture. Shanghai, China: Shanghai Science and Technology Publisher; 1994. Acupuncture manual for small animals; pp. 269–290. [Google Scholar]
- 20.Johansson B. Circulatory responses to stimulation of somatic afferents with special reference to depressor effects from muscle nerves. Acta. Physiol. Scand. 1962;57 Suppl 198:1–91. [PubMed] [Google Scholar]
- 21.Kiss J, Csaba Z, Csaki A, Halasz B. Glutamatergic innervation of neuropeptide Y and pro-opiomelanocortin-containing neurons in the hypothalamic arcuate nucleus of the rat. Eur. J. Neurosci. 2005;21:2111–2119. doi: 10.1111/j.1460-9568.2005.04012.x. [DOI] [PubMed] [Google Scholar]
- 22.Kiss J, Kocsis K, Csaki A, Gorcs TJ, Halasz B. Metabotropic glutamate receptor in GHRH and beta-endorphin neurones of the hypothalamic arcuate nucleus. Neuroreport. 1997;8:3703–3707. doi: 10.1097/00001756-199712010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Koizumi K, Collin R, Kaufman A, Brooks CM. Contribution of unmyelinated afferent excitation to sympathetic reflexes. Brain Res. 1970;20:99–106. doi: 10.1016/0006-8993(70)90158-7. [DOI] [PubMed] [Google Scholar]
- 24.Lantos TA, Gorcs TJ, Palkovits M. Immunohistochemical mapping of neuropeptides in the premamillary region of the hypothalamus in rats. Brain Res. Rev. 1995;20:209–249. doi: 10.1016/0165-0173(94)00013-f. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Beitz AJ. The distribution of brain-stem and spinal cord nuclei associated with different frequencies of electroacupuncture analgesia. Pain. 1993;52:11–28. doi: 10.1016/0304-3959(93)90109-3. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Pitsillides KF, Rendig SV, Pan HL, Longhurst JC. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation. 1998;97:1186–1194. doi: 10.1161/01.cir.97.12.1186. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Rowshan K, Tjen-A-Looi SC, Longhurst JC. Effect of electroacupuncture on pressor reflex during gastric distension. Am. J. Physiol. 2002;283:R1335–R1345. doi: 10.1152/ajpregu.00192.2002. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Tjen-A-Looi SC, Longhurst JC. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Autonomic Neuroscience: Basic & Clinical. 2001;89:38–47. doi: 10.1016/S1566-0702(01)00247-8. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Tjen-A-Looi SC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am. J. Physiol. 2006;290:H2535–H2542. doi: 10.1152/ajpheart.00972.2005. [DOI] [PubMed] [Google Scholar]
- 30.Li SJ, Scanlon MN, Jarai Z, Varga K, Gantenberg N, Lazar-Wesley E, Kunos G. Alpha-2-Adrenergic activation of proopiomelancocortin-containing neurons in the arcuate nucleus causes opioid-mediated hypotension and bradycardia. neuroendocrinology. 1996;63:275–283. doi: 10.1159/000126966. [DOI] [PubMed] [Google Scholar]
- 31.Li YW, Dampney RA. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience. 1994;61:613–634. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- 32.Lin MC, Nahin R, Gershwin ME, Longhurst JC, Wu KK. State of complementary & alternative medicine in cardiovascular, lung and blood. Circulation. 2001;103:2038–2041. doi: 10.1161/01.cir.103.16.2038. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald MC, Robertson HA, Wilkinson M. Age- and dose-related NMDA induction of Fos-like immunoreactivity and c-fos mRNA in the arcuate nucleus of immature female rats. Brain Res. Dev. Brain Res. 1993;73:193–198. doi: 10.1016/0165-3806(93)90138-z. [DOI] [PubMed] [Google Scholar]
- 34.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 35.Pan B, Castro-Lopes JM, Coimbra A. Activation of anterior lobe corticotrophs by electroacupuncture or noxious stimulation in the anaesthetized rat, as shown by colocalization of Fos protein with ACTH and beta-endorphin and increased hormone release. Brain Res. Bull. 1996;40:175–182. doi: 10.1016/0361-9230(96)00011-1. [DOI] [PubMed] [Google Scholar]
- 36.Potts PD, Polson JW, Hirooka Y, Dampney RA. Effects of sinoaortic denervation on Fos expression in the brain evoked by hypertension and hypotension in conscious rabbits. Neuroscience. 1997;77:503–520. doi: 10.1016/s0306-4522(96)00459-9. [DOI] [PubMed] [Google Scholar]
- 37.Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and gamma-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J. Comp. Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- 38.Tjen-A-Looi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neurons by electroacupuncture in cats. Auton. Neurosci. 2003;106:119–131. doi: 10.1016/S1566-0702(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 39.Tjen-A-Looi SC, Li P, Longhurst JC. Medullary substrate and differential cardiovascular responses duringstimulation of specific acupoints. Am. J. Physiol. 2004;287:R852–R862. doi: 10.1152/ajpregu.00262.2004. [DOI] [PubMed] [Google Scholar]


