Abstract
Humans exposed to excess levels of manganese (Mn2+) express psychiatric problems and deficits in attention and learning and memory. However, there is a paucity of knowledge on molecular mechanisms by which Mn2+ produces such effects. We now report that Mn2+ is a potent inhibitor of [3H]-MK-801 binding to the NMDA receptor channel in rat neuronal membrane preparations. The inhibition of [3H]-MK-801 to the NMDA receptor channel by Mn2+ was activity-dependent since Mn2+ was a more potent inhibitor in the presence of the NMDA receptor co-agonists glutamate and glycine (Ki= 35.9 ± 3.1 μM) than in their absence (Ki= 157.1 ± 6.5 μM). We also show that Mn2+ is a NMDA receptor channel blocker since its inhibition of [3H]-MK-801 binding to the NMDA receptor channel is competitive in nature. That is, Mn2+ significantly increased the affinity constant (Kd) with no significant effect on the maximal number of [3H]-MK-801 binding sites (Bmax). Under stimulating conditions, Mn2+ was equipotent in inhibiting [3H]-MK-801 binding to NMDA receptors expressed in neuronal membrane preparations from different brain regions. However, under basal, non-stimulated conditions, Mn2+ was more potent in inhibiting NMDA receptors in the cerebellum than other brain regions. We have previously shown that chronic Mn2+ exposure in non-human primates increases Cu2+, but not zinc or iron concentrations in the basal ganglia (Guilarte et al., Experimental Neurology 202: 381-390, 2006). Therefore, we also tested the inhibitory effects of Cu2+ on [3H]-MK-801 binding to the NMDA receptor channel. The data shows that Cu2+ in the presence of glutamate and glycine is a more potent inhibitor of the NMDA receptor than Mn2+. Our findings suggest that the inhibitory effect of Mn2+ and/or Cu2+ on the NMDA receptor may produce a deficit in glutamatergic transmission in the brain of individuals exposed to excess levels of Mn2+ and produce neurological dysfunction.
Keywords: manganese, copper, NMDA receptor, [3H]-MK-801, rat brain, learning and memory
INTRODUCTION
Manganese (Mn2+) neurotoxicity in humans has been described as a continuum with different clinical presentations classified by their distinct and predominant manifestations (Mergler et al., 1999). An early clinical aspect of Mn2+-induced neurological dysfunction is psychiatric symptoms and memory impairment (Donaldson, 1987). However, there is a paucity of knowledge on mechanisms by which exposure to elevated levels of Mn2+ affects psychiatric and cognitive domains. Recent studies indicate that Mn2+-exposed workers express neurological impairment, attention deficits and learning and memory problems (Bowler et al., 2003; Josephs et al., 2005; Klos et al., 2006; Mergler and Baldwin, 1997; Santos-Burgoa et al., 2001). Impairment in attention and learning has been noted as a sign of frontal cortex and subcortical dysfunction (Klos et al., 2006). Further, Mn2+-exposed workers have a higher incidence of neuropsychiatric symptoms than referents (Bouchard et al., 2006; Sjogren et al., 1990) and elevated levels of Mn2+ markedly increase neuropsychiatric symptoms associated with alcohol abuse (Sassine et al., 2002). Effects of Mn2+ on working memory points to deficits in frontal cortex function, a brain region known to be involved in psychiatric illnesses such as schizophrenia (Abi-Dargham et al., 2002; Goldman-Rakic, 1999). Other reports also indicate effects of Mn2+ exposure on the intellectual performance of children (Wasserman et al., 2006; Woolf et al., 2002).
Studies in non-human primates chronically exposed to Mn2+ indicate that while the globus pallidus accumulates the highest concentration of Mn2+, other brain regions also exhibit significant elevations in Mn2+ concentrations (Dorman et al., 2006; Guilarte et al., 2006). For example, Dorman et al., (2006) showed that at any of the Mn2+ exposure levels administered to monkeys via inhalation, there were significant elevations of Mn2+ not only in basal ganglia structures but also in the frontal cortex, olfactory cortex, cerebellum and white matter. Therefore, Mn2+ is likely to have neurotoxic effects in brain structures outside of the basal ganglia. Consistent with this notion, we have shown that Mn2+-exposed non-human primates exhibit decreased in vivo levels of the metabolite N-acetylaspartate (NAA) in the cerebral cortex (Guilarte et al., 2006a), a finding that may reflect neuronal loss and/or dysfunction. Further, these same Mn2+-exposed animals expressed subtle deficits in spatial working memory and increased frequency of stereotypic and compulsive-like behaviors (Schneider et al., 2006). NAA is a brain metabolite of the parent compound N-acetyl-aspartyl glutamate (NAAG). NAAG is the most abundant neuropeptide in the brain and it is important in glutamatergic neurotransmission (Coyle, 1997). NAAG is also known to interact with the N-methyl-d-aspartate (NMDA) receptor subtype of excitatory amino acid receptors (Bergeron et al., 2007) and these receptors have a divalent cation binding site that modulates their function. Since NMDA receptors are known to play an essential role in synaptic plasticity and in learning and memory function (Morris et al., 1986; Upchurch and Wehner, 1990), we examined whether Mn2+ directly interacts with the NMDA receptor in neuronal membrane preparations from rat brain. Our studies indicate that Mn2+ inhibits NMDA receptor function in an activity-dependent manner and its putative site of interaction is at the NMDA receptor associated ion channel.
MATERIALS AND METHODS
[3H]-MK-801 with a specific activity of 22.0 Ci/mmol was purchased from Perkin Elmer (Boston, MA). Non-radioactive (+) MK-801 hydrogen maleate, manganese sulfate, copper sulfate, glutamate, and glycine were all obtained from Sigma (St Louise, MO).
Rat Brain Membrane Preparation
Normal adult male Long-Evan rats (Charles River, Wilmington, MA, body weight 250-300 g) were euthanized by decapitation. The brains were harvested and dissected into different regions including cerebral cortex, striatum, hippocampus, and cerebellum. The preparation of rat brain neuronal membranes and the [3H]-MK-801 binding assay have been described (Hashemzadeh-Gargari and Guilarte, 1999). Briefly, rat brain tissue was homogenized in 10 volumes of 0.32 M sucrose at 4°C and centrifuged at 1000g for 10 min. The supernatant was centrifuged at 18,000g for 20 min and the resulting pellet was resuspended in 10 volumes of 5 mM Tris-HCl (pH 7.7) with a polytron (6 setting) and centrifuged at 8000g for 20 min. The supernatant and upper buffy coat were centrifuged at 40,910g for 20 min. The resulting pellet was resuspended with a polytron in 10 volumes of 5 mM Tris-HCl buffer and centrifuged at 40,910g for 20 min. This washing procedure was repeated three times and the final pellet was stored at −80°C overnight. The next day the pellet was thawed and resuspended in 10 volumes of Tris-HCl buffer with a polytron and centrifuged at 40,910g. The washing procedure was repeated four times and the pellet was stored at −80°C until use. The extensive washing and freeze-thaw cycles described in this preparation is to remove endogenous glutamate (Glu) and glycine (Gly). In some cases, such as the striatum and hippocampus, tissue from more than one animal was needed for the binding assay. Therefore, preparations from more than one animal were combined.
[3H]-MK-801 Binding Assay
On the day of the assay, the rat brain membrane pellet was thawed and resuspended with a polytron in 5 mM Tris-HCl assay buffer, pH 7.5 (sodium-free) and distributed into assay tubes to provide approximately 100 to 200 μg protein/tube. Protein concentration of tissue homogenates was determined by Bradford protein assay using BSA as a standard. The final assay volume was 1 ml for cerebral cortex and cerebellum and 0.5 ml for striatum and hippocampus. For inhibition studies, 13 different concentrations (nominal) of manganese sulfate (MnSO4) (from 0.1 μM to 1.5 mM final) or 19 different concentrations of copper sulfate (CuSO4) (from 0.1 μM to 1.5 mM final) were added to membrane preparation of different brain regions and 2.5 nM [3H]-MK-801 with or without 50 μM Glu and 20 μM Gly. Membrane suspensions were incubated for 1 h at 24°C in a shaking water bath in triplicate for MnSO4 studies or duplicate for CuSO4. The reaction was terminated by filtration through Whatman GF/B filter paper with a BRANDEL filtering system.
For saturation isotherm, cerebral cortex membrane preparation was incubated with 7 different concentrations of [3H]-MK-801 (0.5 to 20 nM) under activation conditions (50 μM Glu and 20 μM Gly) in the presence or absence of 100 μM MnSO4.Nonspecific binding was measured in the presence of 100 μM non-radioactive (+)-MK-801 hydrogen maleate. Assay tubes were incubated for 1 h at 24°C in a shaking water bath. The reaction was terminated by filtration through Whatman GF/B filter paper with a BRANDEL filtering system. Filters were washed three times with 5 ml of ice-cold assay buffer. Radioactivity trapped in the filters was measured by liquid scintillation spectrometry. Binding parameters were estimated using the EBDA/LIGAND in KELL version 6 program for Windows (Biosoft, Cambridge, U.K.). For most of inhibition studies, IC50 and Ki were determined using one-site model. For CuSO4 inhibition studies in the activated stages, IC50 and Ki for two sites were determined. For saturation isotherm, the maximal number of binding sites (Bmax) and affinity constant (Kd) were determined using EBDA/LIGAND.
Statistics
Student's t-test or one-way analysis of variance with Student-Newman-Keuls test for multiple means comparison was used when appropriate. Statistical significance was set at p < 0.05. Values provide are mean ± sem.
RESULTS
Mn2+ inhibits [3H]-MK-801 binding to the NMDA receptor channel
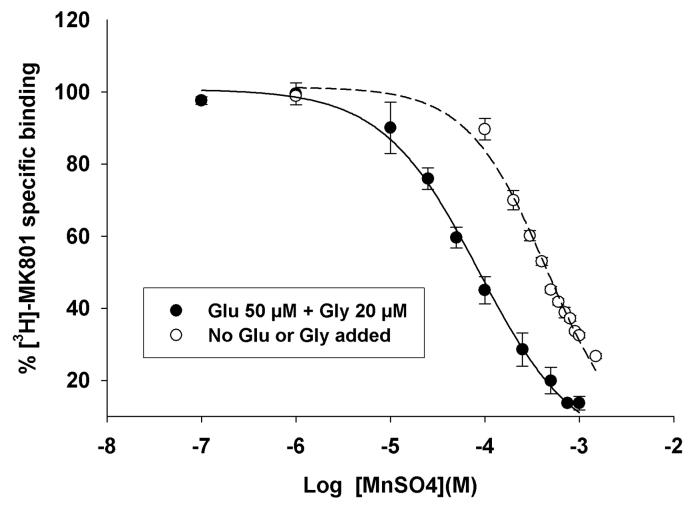
Radioligand-receptor binding assay was used to examine the effects of Mn2+ on [3H]-MK-801 binding to the NMDA receptor channel. [3H]-MK-801 is a well-characterized NMDA receptor channel antagonist and it is widely used in receptor binding studies (Wong et al., 1988). We used extensively washed neuronal membrane preparations from rat cerebral cortex and showed that the addition of exogenous Mn2+ inhibits [3H]-MK-801 binding to the NMDAR channel with an inhibitory constant (Ki) in the low μM range (Figure 1 and Table 1). Mn2+ was found to be a more potent inhibitor of [3H]-MK-801 binding in the presence of the co-agonists Glu and Gly than in their absence (Figure 1 and Table 1). Thus, Mn2+ behaves similar to the NMDA receptor channel blockers phencyclidine, ketamine, MK-801 and the endogenous divalent cation magnesium (Mg2+). The inhibitory constant (Ki) of NMDA receptor channel inhibition by Mn2+ was in 35.9 ± 3.1 μM, a value much lower than that for the endogenous channel blocker, Mg2+ (mM range).
Figure 1.
Inhibition of [3H]-MK-801 binding to the NMDA receptor channel in neuronal membranes from adult rat cerebral cortex by Mn2+. The data show that Mn2+ is a more potent inhibitory in the presence of the NMDA receptor activators Glu and Gly than in the absence. Each value is the mean ± sem of 3 different experiments, each done with a different neuronal membrane preparation.
Table 1.
Manganese inhibition of [3H]-MK-801 binding to native NMDA receptors in neuronal membrane preparations of adult rat cerebral cortex. Each value is the mean ± sem of 3 different experiments, each using a different neuronal membrane preparation.
| Ki (μM) | Bmax (fmol/mg protein) | |
|---|---|---|
| Basal | 157.1 ± 6.5 | 1683 ± 43 |
| Glutamate & Glycine | 35.9 ± 3.1 | 3731 ± 455 |
Effect of Mn2+ on [3H]-MK-801 binding parameters
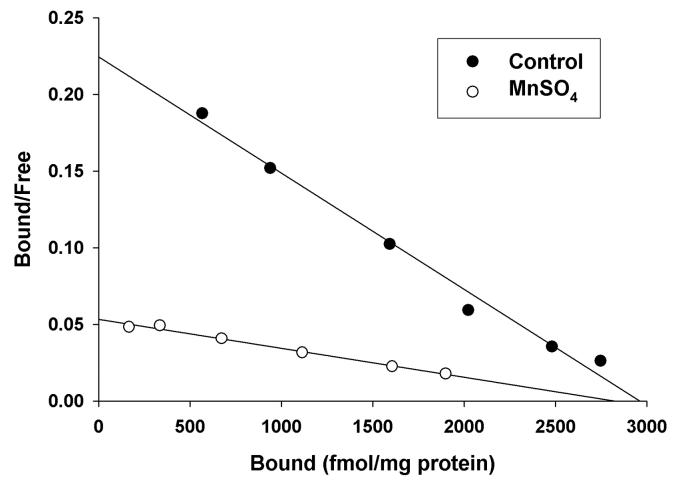
To study the nature of the inhibitory effect of Mn2+ on [3H]-MK-801 binding to the NMDA receptor channel, we performed [3H]-MK-801 saturation isotherms and Scatchard analysis using extensively washed rat cortical membranes. These assays were performed in the presence of activating concentrations of Glu and Gly with or without 100 μM Mn2+. Our results indicate that Mn2+ alters the affinity (Kd) of [3H]-MK-801 binding to the NMDA receptor channel with no significant effect on the maximal number of binding sites (Bmax) (See Figure 2 and Table 2). Therefore, the effect of Mn2+ on [3H]-MK-801 binding parameters is consistent with a competitive interaction with [3H]-MK-801 binding at the NMDA receptor channel.
Figure 2.
Representative graph of Mn2+ effects on [3H]-MK-801 binding parameters in rat cerebral cortex in the presence of Glu and Gly. Mn2+ produces a significant change in the affinity constant (Kd) represented by a change in the slope of the Scatchard plot with no change in the maximal number of binding sites (Bmax).
Table 2.
Effect of manganese on [3H]-MK-801 binding parameters to cerebral cortex NMDA receptors. Each value is the mean ± sem of 3 different experiments, each using a different neuronal membrane preparation.
| Kd (nM) | Bmax (fmol/mg protein) | |
|---|---|---|
| Control | 2.16 ± 0.20 | 3191 ± 174 |
| MnSO4 (100μM) | 8.15 ± 0.45 | 3198 ± 327 |
| t-test | p=0.003 | p=0.96 |
Effects of Mn2+ on NMDA receptors expressed in different regions of the rat brain
We examine the inhibitory effect of Mn2+ on the binding of [3H]-MK-801 to the NMDA receptor channel in neuronal membrane preparations from different regions of the rat brain. This was done to determine if native NMDA receptors expressed in different brain areas are differentially sensitive to Mn2+ inhibition. Analysis of variance showed a significant effect of brain region on the ability of Mn2+ to inhibit [3H]-MK-801 binding (F3,8 = 9.09, p = 0.0045) under basal conditions (no Glu or Gly added). That is, although Mn2+ was equally potent in inhibiting native NMDA receptors expressed in neuronal membranes from adult rat cerebral cortex, hippocampus and striatum, the inhibitory constant for Mn2+ was significantly different in neuronal membranes from cerebellum relative to all other brain regions (p < 0.05; Table 3). No significant differences amongst brain regions were noted for the inhibitory effects of Mn2+ under stimulating conditions (Glu and Gly added) (Table 3). Consistent with the levels of NMDA receptor sites in different brain regions, the Bmax of [3H]-MK-801 was highest in the cerebral cortex and hippocampus and lowest in the cerebellum (Table 3).
Table 3.
Manganese inhibition of [3H]-MK-801 binding to NMDA receptors in different brain regions. Each value is the mean ± sem of 3 different experiments, each using a different neuronal membrane preparation.
| Brain Region | Ki (μM) | Bmax (fmol/mg protein) | ||
|---|---|---|---|---|
| Basal | Glutamate & Glycine | Basal | Glutamate & Glycine | |
| Cerebral Cortex | 157.1 ± 6.5 | 35.9 ± 3.1 | 1683 ± 43 | 3731 ± 455 |
| Hippocampus | 146.3 ± 18.2 | 42.6 ± 1.1 | 2076 ± 27 | 3014 ± 104 |
| Striatum | 187.8 ± 8.6 | 34.5 ± 2.5 | 1097 ± 37 | 1958 ± 34 |
| Cerebellum | 68.1 ± 6.6 | 34.3 ± 2.7 | 174 ± 10 | 264 ± 12 |
Effects of Copper on [3H]-MK-801 binding to NMDA receptors in neuronal membrane preparations from rat brain
We have previously shown that chronic Mn2+ exposure in non-human primates increases the concentration of copper (Cu2+), but not zinc or iron, in basal ganglia structures (Guilarte et al., 2006). Since Cu2+, like Mn2+, is a divalent cation, we hypothesized that it may also interact with the NMDA receptor to inhibit its function. Our results indicate that the Ki of Cu2+ inhibition of [3H]-MK-801 binding to the NMDA receptor channel was slightly higher to that of Mn2+ in the absence of Glu and Gly, that is 195 ± 17 μM vs 157.1 ± 6.5 μM for Mn2+ (see Tables 3 and 4). However, in the presence of Glu and Gly, Cu2+ exhibited high and low affinity inhibitory sites with a Ki of 9.4 ± 1.7 μM and 248 ± 8.0 μM, respectively (Table 4). The high affinity site was predominant over the low affinity site with a Bmax of 3570 ± 116 and 910 ± 68 fmol/mg protein, respectively. Therefore, under physiological conditions where the NMDA receptor co-agonists Glu and Gly are present, Cu2+ is a more potent inhibitor of the NMDA receptor than Mn2+.
Table 4.
Copper inhibition of [3H]-MK-801 binding to NMDA receptors in rat cerebral cortex membrane preparations. Each value is the mean ± sem of 3 different experiments, each using a different neuronal membrane preparation.
| Ki (μM) | Bmax (fmol/mg protein) | |
|---|---|---|
| Basal | 195.0 ± 17.2 | 606 ± 51 |
| Glutamate & Glycine | 9.4 ± 1.7 & 248.4 ± 7.8 (site-1) (site-2) |
3570 ± 116 & 910 ± 68 (site-1) (site-2) |
DISCUSSION
The present study demonstrates that Mn2+ is a competitive antagonist of [3H]-MK-801 binding to the NMDA receptor channel. This is based on the finding that Mn2+ altered the Kd but not the Bmax of [3H]-MK-801 binding parameters. Further, the inhibitory effect of Mn2+ is activity-dependent since Mn2+ was a more potent inhibitor in the presence of the NMDA receptor co-agonists Glu and Gly than in their absence. Together, our studies indicate that Mn2+ is a NMDA receptor channel blocker.
A review of the literature supports our findings based on electrophysiological recordings from central neurons in culture. Mayer and Westbrook (1987) showed that in hippocampal neurons Mn2+ produces a strong voltage-dependent block of responses to NMDA. This is similar to the channel block produced by Mg2+ but different to the voltage-independent inhibition of the NMDA receptor by other physiologically relevant divalent cations such as Zn2+ (Christine and Choi, 1990) and Cu2+ (Vlachova et al., 1996) as well as the environmental neurotoxicant Pb2+ (Alkondon et al., 1990; Guilarte et al., 1995; Guilarte, 1997). Therefore, divalent cations are important regulators of NMDA receptor function and they interact at distinct and different sites on the NMDA receptor protein. Mayer and Westbrook (1987) also showed that unlike Mg2+, Mn2+ is able to permeate the channel. Therefore, Mn2+ has two different effects on the NMDA receptor, it is a channel blocker and it permeates the channel. The fact that Mn2+ is able to permeate the NMDA receptor channel provides a possible mechanism by which Mn2+ can be “transmitted” from neuron to neuron in the brain.
We also found that under stimulating conditions, Mn2+ was equally potent in inhibiting NMDA receptors expressed in different brain regions. However, in the absence of the activators Glu and Gly, Mn2+ was most potent in inhibiting NMDA receptors in the cerebellum (Table 3). It is known that cerebellar NMDA receptors have a different subunit composition than forebrain NMDA receptor and this may provide a potential explanation for the differences in inhibition by Mn2+. For the most part NMDA receptors in forebrain structures are composed of NR1 in combination with NR2A, NR2B or both NR2 subunits (Monyer et al., 1994). On the other hand, in the adult cerebellum the NR1/NR2C subtype predominates (Monyer et al., 1994). Mn2+ was equally potent in inhibiting the NMDA receptor in all brain regions in the presence of Glu and Gly (Table 3). NMDA receptors are widely distributed throughout the brain and the potential for Mn exposure to inhibit NMDA receptor function in vivo may not only depend on regional differences in the subunit composition of NMDA receptors but also in the regional brain Mn2+ concentrations.
What is the toxicological relevance of these findings? Previous studies have shown that Mn2+ is taken up and released from neurons in a calcium and impulse-dependent manner (Takeda et al., 2002). Further, Mn2+ is released with Glu from nerve terminals suggesting a function in glutamatergic synapses. Therefore, the effect of Mn2+ that we have identified on the NMDA receptor channel is of toxicological relevance. We propose that when Mn2+ concentrations in the brain are elevated to toxic levels as a result of chronic exposures, Mn2+ may accumulate in glutamatergic synapses and inhibit NMDAR function producing a hypofunctioning of the glutamatergic system.
It is also noteworthy that chronic Mn2+ exposure in non-human primates increases Cu2+, but not zinc or iron concentrations in basal ganglia structures (Guilarte et al., 2006). This effect of Mn2+ on brain Cu2+ concentrations appears to have some degree of regional brain selectivity as Cu2+ concentrations in the white matter of the same Mn2+-exposed non-human primates were not increased (Guilarte et al., 2006).
Cu2+ is also a potent NMDA receptor antagonist as defined in our present study and in previous electrophysiological studies (Vlachova et al., 1996; Trombley and Shepherd, 1996). Similar to Mn2+, Cu2+ is released from nerve terminals following depolarization (Kardos et al., 1989) and it is known to inhibit NMDA receptors (Vlachova et al., 1996) and long-term potentiation in hippocampal slices (Doreulee et al., 1997). Therefore, chronic exposure to elevated levels of Mn2+ may inhibit NMDA receptor dependent cellular function not only by increasing endogenous levels of Mn2+ but also of Cu2+. Further, Mn2+ and Cu2+ inhibit NMDA receptor function at different sites, thus they may act synergistically.
A remaining question is whether Mn2+ and/or Cu2+ concentrations in the brain of individuals exposed to environmental or occupational levels of Mn2+ are sufficiently high to produce an inhibitory effect on brain NMDA receptors in situ. Previous studies have estimated the physiological concentrations that may be achievable in the synaptic cleft after stimulated release of Cu2+ (Kardos et al., 1989) and Zn2+ (Assaf and Chung, 1984) to be as high as 250-300 μM. Clearly, if the accumulation of Mn2+ in neuronal terminals of exposed individuals are markedly increased above physiological levels, then it is highly likely that higher synaptic concentrations of Mn2+ and Cu2+ are achieved in the Mn2+-exposed brain and they would inhibit NMDA receptor mediated neurotransmission.
In summary, these studies suggest that under conditions of excess Mn2+ accumulation in the brain, as it may occur with chronic occupational or environmental exposures and in certain medical conditions, Mn2+ may be able to inhibit NMDA receptor function. The observation that Mn2+ acts as a NMDA receptor channel blocker has important implications to its known effects on cognitive function in adults and in children since NMDA receptor antagonists produce impairments in learning and memory (Newcomer and Krystal, 2001). Therefore, our current findings have significant implications for Mn2+-induced changes in IQ and learning documented in occupational workers (Bowler et al., 2006) and in children with environmental exposures (Wasserman et al., 2006; Woolf et al., 2002).
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institute of Environmental Health Sciences number ES10975 to TRG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang D-R, Keilp J, van Heertum R, Gorman JM, Laurelle M. Prefrontal dopamine D1 receptors and working memory in Schizophrenia. J. Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Costa ACS, Radhakrishnan V, Aronstam RS, Albuquerque EX. Selective blockade of NMDA-activated currents may be implicated in learning deficits caused by lead. Fed Eur Biochem Soc. 1990;261:124–130. doi: 10.1016/0014-5793(90)80652-y. [DOI] [PubMed] [Google Scholar]
- Assaf SY, Chung SH. Release of endogenous Zn 2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Imamura Y, Frangioni JV, Greene RW, Coyle JT. Endogenous N-acetylaspartylglutamate reduces NMDA receptor-dependent current neurotransmission in the CA1 area of the hippocampus. J Neurochem. 2007;100:346–357. doi: 10.1111/j.1471-4159.2006.04253.x. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Roels HA. Neuropsychiatric symptoms and past manganese exposure in a ferro-alloy plant. Neurotoxicol. doi: 10.1016/j.neuro.2006.08.002. doi:10.1016/j.neuro.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Booty A, Hartney C, Roels HA. Neuropsychological sequelae of exposure to welding fumes in a group of occupationally exposed men. Int. J. Hyg Env Hlth. 2003;206:517–529. doi: 10.1078/1438-4639-00249. [DOI] [PubMed] [Google Scholar]
- Christine CW, Choi DW. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990;10:108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis. 1997;4:231–238. doi: 10.1006/nbdi.1997.0153. [DOI] [PubMed] [Google Scholar]
- Donaldson J. The physiopathologic significance of manganese in brain: Its relation to schizophrenia and neurodegenerative disorders. Neurotoxicol. 1987;8:451–462. [PubMed] [Google Scholar]
- Doreulee N, Yanovsky Y, Haas HL. Suppression of long-term potentiation in hippocampal slices by copper. Hippocampus. 1997;7:666–669. doi: 10.1002/(SICI)1098-1063(1997)7:6<666::AID-HIPO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol Sci. 2006;92:201–210. doi: 10.1093/toxsci/kfj206. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psych. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen M-K, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen M-K, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the non-human primate brain following chronic manganese exposure: A 1H-MRS and MRI study. Toxicol. Sci. 2006a;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Hashemzadeh-Gargari H, Guilarte TR. Divalent cations modulate n-methyl-d-aspartate receptor function at the glycine site. J Pharm Exp Ther. 1999;290:1356–1362. [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manisfestations in welders with pallidal MRI T1hyperintensity. Neurol. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kardos J, Kovacs I, Hajos F, Kalman M, Simonyi M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci Lett. 1989;103:139–144. doi: 10.1016/0304-3940(89)90565-x. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Chandler M, Kumar N, Ahiskog JE, Josephs KA. Neuropsychological profiles of manganese neurotoxicity. Eur J Neurol. 2006;13:1139–1141. doi: 10.1111/j.1468-1331.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurons. J. Physiol. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: An update. Env Res. 1997;73:90–104. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, Panisset M, Edwards R, de Geoffroy A, Sassine M-P, Hudnell K. Manganese neurotoxicity, a continuum of dysfunction: Results from a community based study. Neurotoxicol. 1999;20:327–342. [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate antagonist, AP5. Nature. 1986;329:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior. Hippocampus. 2001;11:529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- Santos-Burgoa C, Rios C, Mercado LA, Arechiga-Serrano R, Cano-Valle F, Eden-Wynter RA, Texcalac-Sangrador JL, Villa-Barragan JP, Rodriguez-Agudelo Y, Montes S. Exposure to manganese: Health effects on the general population, a pilot study in central Mexico. Env Res. 2001;85:90–104. doi: 10.1006/enrs.2000.4108. [DOI] [PubMed] [Google Scholar]
- Sassine M-P, Mergler D, Bowler R, Hudnell HK. Manganese accentuates adverse mental health effects associated with alcohol use disorders. Biol Psych. 2002;51:909–921. doi: 10.1016/s0006-3223(01)01350-6. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effect of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren B, Gustavsson P, Hogstedt C. Neuropsychiatric symptoms among welders exposed to neurotoxic metals. Brit J Inds Med. 1990;47:704–707. doi: 10.1136/oem.47.10.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Sotogaku N, Oku N. Manganese influences the levels of neurotransmitters in synapses in rat brain. Neurosci. 2002;114:669–674. doi: 10.1016/s0306-4522(02)00353-6. [DOI] [PubMed] [Google Scholar]
- Upchurch M, Wehner JM. Effects of N-methyl-D-aspartate antagonism on spatial learning in mice. Psychopharmacol. 1990;100:209–214. doi: 10.1007/BF02244408. [DOI] [PubMed] [Google Scholar]
- Vlachova V, Zemkova H, Vyklicky L. Copper modulation of NMDA responses in mouse and rat cultured hippocampal neurons. Eur J Neurosci. 1996;8:2257–2264. doi: 10.1111/j.1460-9568.1996.tb01189.x. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Green A, Slavkovich V, Lolacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Env Hlth Persp. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Env Hlth Persp. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EHF, Knight AR, Woodruff GN. [3H]-MK-801 labels a site on the N-methyl-d-aspartate receptor channel complex in rat brain membranes. J. Neurochem. 1988;50:274–281. doi: 10.1111/j.1471-4159.1988.tb13260.x. [DOI] [PubMed] [Google Scholar]