Introduction
Severe comorbidities such as cardiovascular disease, type 2 diabetes, hypertension, certain cancers; and sleep apnea are associated with obesity (Poirier et al., 2006). Obesity rates and rates of medical comorbidities among patients with schizophrenia exceed those of population estimates (Allison et al., 1999; McEvoy et al., 2005). Among other factors such as poor dietary choices (Strassnig et al., 2003a) or a sedentary lifestyle (Brown et al., 1999), treatment with both classic and novel antipsychotics have been associated with the high prevalence of overweight and obesity observed among schizophrenia patients (Ganguli, 1999), and may, in part, be related to the excess morbidity and mortality reported in this population (Osby et al., 2000).
Over the past decade, use of antipsychotics, especially that of novel agents, has become more liberal and has substantially increased in the adolescent and young-adult age group (Daumit et al., 2003). The likelihood that first-episode psychosis patients will remain on antipsychotic medication for years is high. Yet, while they demonstrate higher rates of therapeutic response and symptomatic remission than chronic patients with multiple prior episodes (Lieberman et al., 1998), first-episode psychosis patients are also more susceptible to side-effects of both typical (McEvoy et al., 1991) and novel antipsychotics (Thakore, 2004), including higher risks of significant weight gain (Addington et al., 2003).
However, there is a striking paucity of pharmacological studies in first-episode psychosis patients which assess efficacy and side-effects of antipsychotics (Kelly et al., 2005). Most studies to date oversample chronically ill schizophrenia patients, who often have histories of multiple prior medication trials, high non-adherence rates (Ganguli et al., 2001), or multiple medical problems requiring concomitant medical pharmacotherapy (Meyer et al., 2005). Weight gain liabilities introduced through antipsychotic agents derived from such populations may thus be biased and give a less accurate representation than estimates derived from previously medication-naïve first-episode psychosis patients.
Given the significant health risks that are associated with being obese, it is important to quantify the actual magnitude of weight gain that these medications induce. To eliminate the bias introduced through inclusion of large proportions of chronic schizophrenia patients, medicationnaïve first-episode psychosis patients who receive their very first antipsychotic treatment and remain on it for a substantial time period may serve as the ideal basis to accurately assess weight gain liabilities of commonly prescribed antipsychotic medications. We thus examine the weight gain liabilities of various classic and novel antipsychotics in a large group of first-episode psychotic patients during their first year of treatment, compare results to age-, gender- and socioeconomic status comparable healthy controls, and identify those medications which induce clinically significant weight gain of 7 % or more, a value warranting clinical intervention (Marder et al., 2004).
Methods
Setting
The study was conducted at the Western Psychiatric Institute and Clinic Center for the Neuroscience of Mental Disorders (CNMD), a program specifically developed to treat and longitudinally follow first-episode psychosis patients. “First-episode psychosis” is broadly defined to encompass both schizophrenia-spectrum and mood-disorder associated psychotic presentations. Data presented here are a subset (time frame 1990-2006) of an ongoing large and comprehensive study on first-episode psychosis patients. Approval by the Institutional Review Board (IRB) of the University of Pittsburgh was obtained. Data was analyzed retrospectively at baseline and year one. Medication compliance was assured by prescription count.
Subjects
Subjects were outpatients, aged between 18 and 50, suffering from a first-episode psychotic episode; and age- and gender comparable mentally healthy controls. We excluded patients younger than 18 years of age to reduce the confounding effect of continuing growth on body weight and body mass index. Every participant including controls subjects received a SCID (First et al., 1996) to ascertain psychiatric diagnosis/comorbidity; psychiatric symptom rating scales were completed for SCID-diagnosed first episode psychosis patients only.
General exclusion criteria
pertinent to both first-episode patients and healthy controls encompassed a) DSM-IV mental retardation b) medical or neurological illness, head injury c) pregnancy d) co-morbidity for DSM-IV Psychoactive Substance Dependence within six months or Substance Abuse within the past 1 month preceding inclusion.
First-episode subjects
carried a DSM-IV psychosis-spectrum diagnosis. Prior treatment with continuous antipsychotic medications for over 2 weeks at any period during the lifetime or more than 3 doses of any oral antipsychotic drug during the month prior to study entry precluded inclusion.
Normal control subjects
could not have a history of Axis I psychiatric disorder or be treated with any neuroleptics at any time and other psychotropic medication 6 months prior to baseline assessment and were to be within the same age range as the patients.
Assessment
Patients and controls were assessed at the index assessment and at year one after the first assessment. Seven first-episode psychosis patients who refused antipsychotic medication but were clinically monitored served as patient controls. Height and weight were obtained and BMI (kg/m2) was calculated at the due time points. A SECA 700 ® Physician Balance Beam Scale, professionally calibrated, and a standard wall mounted stadiometer were used to measure weight and height, respectively. Patients were asked to remove shoes and outer layers of garments; assessments usually took place in the morning, after breakfast.
BMI change from baseline to year one and a dichotomized variable of “clinically significant weight gain”, defined as at least 7 % weight increase from baseline to year one, were the main outcome variables. The Scales for the Assessment of Positive and Negative Symptoms, respectively (SANS, Andreasen 1989; SAPS, Andreasen, 1990) and the Hamilton Depression Rating Scale (HAMD-24, Hamilton, 1960) were used to estimate levels of psychopathology. We employed the Hollingshead Four-Factor Index (Hollingshead, 1975) to ascertain parental socioeconomic status (SES); the Likert-style scale rates both paternal and maternal education- and occupation levels, respectively, from 1-7 and 1-9, low to high. Results, prior to averaging, are multiplied by 3 for the educational attainment, and by 5 for occupation level; thus the scale is weighted towards occupation levels as determinant of SES. Education level scores range from 3 to 21; occupation level scores range from 5 to 45. The total social status index ranges from 8 to 66.
Drug assignments were made per prescriber’s choice. No patient received a second antipsychotic during the observation time reported. Patients receiving thioridazine (n=1), loxapine (n=2), fluphenazine (n=1), aripiprazole (n=2) and quetiapine (n=1) were analyzed as part of the classic vs novel antipsychotic group comparison of weight gain liabilities only. Medication exposure time was calculated in days between the baseline- and year one assessments. Medication doses over this specified time period were averaged and include clinically due dose adjustments. Seventeen patients (18.7 % of the n=91 receiving antipsychotics) required a medication switch during the first year (information available upon request), which occurred on average after 56.1 days; since in all these cases the exposure to the 2nd medication within the first year was significantly longer (240.7 days, p<0.001), we used the 2nd medication in our analysis. Four patients were switched from haloperidol to either loxapine, thioridazine, or perphenazine (n=2) and seven patients were switched from risperidone to loxapine, haloperidol (n=2) or olanzapine (n=4), all secondary to efficacy and EPS concerns; two from olanzapine to ziprasidone (weight gain) and risperidone (efficacy); three from aripiprazole to risperidone and olanzapine (n=2) (efficacy and EPS) and one from ziprasidone to risperidone (efficacy). Psychotropic co-medication was grouped into sedatives (benzodiazepines, hypnotics, antihistamines), mood stabilizers (lithium, valproate, carbamazepine), antidepressants (tricyclics, dual agents, SSRI) and side-effect medication (propanolol, anticholinergics); and was incorporated in the analysis only if exposure time during antipsychotic therapy was longer than 4 weeks. Only one patient received sedatives for longer than 4 weeks; sedatives were thus not further analyzed. The National Institute of Health, National Heart, Lung, and Blood Institute (1998) Body Mass Index (BMI, kg/m2) cut-off points were used to (ie, ≤ 25, 25-30, ≥30) to delineate between healthy weight, overweight, and frank obesity.
Statistics
SPSS (for windows) software was used for data analysis. Descriptive analysis including mean, range and standard deviation for continuous variables was carried out to determine whether the variables were normally distributed and frequency counts for categorical data (for example gender, diagnosis, etc) were done to examine the proportions of various socio-demographic characteristics. Main outcome variables were the increase in BMI, and “clinically significant” weight gain (that is, an increase of >7% from baseline). These measures were examined for the patient group versus healthy controls, and for patient subgroups according to different antipsychotics. Students t-tests, chi-square tests, and where appropriate, analysis of variance (ANOVA) and covariance (ANCOVA) were employed to look for statistical differences between the means of 2 or more variables.
Results
The sample consisted of 98 first-episode psychosis patients and 30 healthy comparisons, all over 18 years of age (Table 1.). Compared to healthy controls (Table 2.), first-episode psychosis patients experienced significantly more weight gain during the 1-year observation period and their body mass index increased to a significantly greater extent (+2.21±3.1 vs. 0.37±1.6; t=3.103, df=126, p=0.002).
Table 1.
Baseline Characteristics
| First Episode Psychoses n=98 | Healthy Comparisons n=30 | ||
|---|---|---|---|
| Gender/male (%) | 67 (69.8%) | 17 (61.5%) | χ2=1.394 df=1 |
| p=0.168 | |||
| Age | 27.2 (±7.5)* | 21.3 (±3) | t=4.171 df=126 |
| p≤0.001 | |||
| Parental SES+ | 40.7 (±13.5) | 43.5 (±9.1) | t=-1.032 df=122 |
| p=0.304 | |||
| Smokers | 33 (33.6 %) | 3 (10 %) | χ2=5.5 df=3 |
| p=0.139 | |||
| BMI | 23.9 (±5) | 25.1 (±4.6) | t=-1.18 df=126 |
| p=0.467 |
According to Hollingshead and Redlich (1954)
Table 2.
One-Year Weight Increases+
| Groups | n | Dose/mg | Weight/lbs | BMI | % of Baseline Weight |
|---|---|---|---|---|---|
| Patients on Antipsychotics | |||||
| Haloperidol | 24 | 4 (±3.1) | 9 (±12.3) | 1.4 (±1.9) | 5.6 (±7.8) |
| Risperidone | 43 | 3.2 (±1.3) | 16.6 (±21.9) | 2.5 (±3.2) | 10.7 (±13.8) |
| Olanzapine | 11 | 12.7 (±7.5) | 37.3 (±27.7) | 5.5 (±4.3) | 22.4 (±17.6) |
| Perphenazine | 13 | 11.2 (±11.2) | 3.4 (±6.1) | 0.5 (±1.0) | 2.2 (±3.9) |
| Controls, and Patients not on Antipsychotics | |||||
| Healthy Controls | 30 | 2.4 (±11.4) | 0.4 (±1.6) | 1.2 (±6) | |
| Patients | 7 | 3.3 (±16.7) | 0.5 (±2.4) | 2.8 (±10.4) | |
| p≤0.001 | p≤0.001 | p≤0.001 |
ANCOVA; age accounted for as covariate
First-episode psychosis patients were diagnosed with schizophrenia or schizoaffective disorders (n=71) and mood disorders with psychotic features (n=27). Nine of the mood disorders patients were bipolar. Baseline body mass index (F=0.93, p=0.45), age at consent (F=0.620, p=0.685) and smoking status (F=0.466, p=0.8) were not different. Patients receiving perphenazine had a higher baseline SES (F=2.67, p=0.037). No differences in SANS and HAMD-24 scores were appreciated (F=1.51, p=0.21, F=0.96, p=0.43) but SAPS scores were different (F=3.68, p=0.008) secondary to lower positive symptom scores in the first-episode psychosis patients not receiving antipsychotics.
Mean duration of medication exposure was 303±115 days and was similar in all antipsychotic groups (F=0.267 p=0.849). More weight gain was observed in younger patients (r=-0.24, p=0.019), and patients with more negative symptoms at baseline (SANS global; r=0.22, p=0.031; compound average SANS item score r=0.24, p=0.02). Significant in-between group difference in weight gain remained after controlling for age (corrected model ANCOVA, F=8.418, p≤0.001). No associations between parental socioeconomic status (r=-0.07, p=0.53), tobacco use (r=0.09, p=0.38); severity of baseline positive (SAPS global, SAPS compound items r=0.68, p=0.41; r=0.02, p=0.85; respectively) and depressive symptoms (HAMD-24 compound scores, r=0.06, p=0.53) with BMI at year one were found.
Side-effect medication (n=39) and mood stabilizer (n=11) prescription was distributed unequally across antipsychotic groups (א2=17.5, p=0.00; א2=20.1, p=0.002, resp.), owing to more frequent use in patients receiving haloperidol and perphenazine. No group differences in the use of antidepressants (n=39, א2=7.79, p=0.1) was appreciated. Among concomitant medication, only a higher individual total co-medication count and antidepressant co-prescription (univariate ANOVA, F=6.43, p<0.001; F=2.63, p=0.040, resp.) were associated with weight gain independent of antipsychotics.
Fewer patients receiving typical (n=14 of 37; 38 %) than novel agents (n=32 of 54; 59 %) gained clinically significant amounts (>7 %) of weight (א2=7.093, p=0.008; Table 3.). Smoking status was not different (א2=2.11, p=0.55). No differences in psychopathology measures including SANS (t=-0.75, p=0.46), SAPS (t=-0.75, p=0.46) and HAMD-24 scores (t=-0.4, p=0.69) were evident.
Table 3.
Weight Gain with Classic and Novel Agents over 1 Year
| Classic n=37 | Novel n=54 | ||
|---|---|---|---|
| Sex, Males (%) | 22 (59.4%) | 39 (72.2 %) | χ2=1.618 |
| p=0.148 | |||
| Age | 26.8 (±7.3) | 27.9 (±7.9) | t=0.696 |
| p=0.488 | |||
| SES | 43.9 (±13.9) | 39.1 (±12.8) | t=1.678 |
| p=0.097 | |||
| Medication exposure/days | 314 (±112) | 295 (±118) | t=0.787 |
| p=0.433 | |||
| Weight Increase lbs | 8.2 11.8 | 20.8 24.5 | t=-2.912 |
| p=0.005 | |||
| BMI Increase | +1.3 (±1.8) | +3.1 (±3.6) | t=-2.862 |
| p=0.005 | |||
| >7% Weight Gain | 14 (38 %) | 32 (59 %) | t=-2.031 |
| p=0.045 |
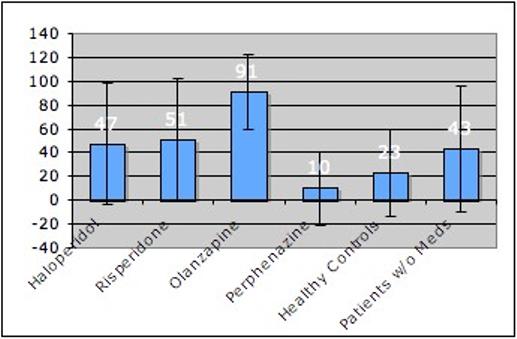
Figure 1. shows the proportions of patients and controls who gained clinically significant amounts of weight, defined as >7% increase from baseline. Patients receiving haloperidol (א2=3.04, p=0.081), risperidone =5.72, p=0.02), olanzapine (א2=15.14, p≤0.001), but not patients receiving perphenazine (א2=1.731, p=0.985) were individually more likely to have experienced clinically significant weight increases than healthy controls. There was no difference in clinically significant weight gain between healthy controls and first-episode patients not receiving medication (א2=1.1, p=0.3).
Figure 1.
Discussion
Our study demonstrates that first-episode psychosis patients treated with certain antipsychotics can gain much more weight than healthy controls and patients not receiving antipsychotics during a one-year observation period, placing them at high risk to develop obesity-associated medical comorbidities early on (Johnson et al., 2006). Olanzapine exposure for a year increases bodyweight in first-episode psychosis patients by almost a quarter over baseline weight (37 lbs) followed by risperidone (28 lbs) and haloperidol (9 lbs). Patients receiving perphenazine (3.4 lbs) or no antipsychotics (3.3 lbs), and healthy controls (2.4 lbs) experience minor body weight changes. Comparison between the groups of novel and classic agents reveals a considerable difference in their propensity to induce weight gain. While approximately two-thirds of patients treated with novel agents gain clinically significant amounts of weight, only one third of those treated with classic antipsychotics do. Younger patients and patients with more negative symptoms gain more weight when exposed to antipsychotics; also, a greater number of comedication per patient, and co-prescription of antidepressants independently worsen antipsychotic-associated weight gain.
Even higher rates of clinically significant weight gain in first-episode psychosis patients receiving olanzapine and haloperidol than we observed in our sample have recently been reported by Zipursky et al. (2005), who estimated that “virtually all patients” receiving olanzapine and approximately three-quarters of those receiving haloperidol gained over 7 % of their original weight after 1 year of treatment. Interestingly, in our sample, all patients treated with olanzapine were males, potentially reflecting greater concern about body image and weight gain in female patients (Strassnig et al, 2005); also because females appear to be at higher risk than males for antipsychotic-associated weight gain (Homel et al, 2002; Russell and Mackell, 2001). In a Chinese first-episode psychosis sample, Lieberman et al. (2003) reported mean absolute weight gains of 6.5 kg in patients receiving chlorpromazine vs. 9.9 kg in those on clozapine over a 52-week observation period. Shorter-term trials indicate that a large proportion of the antipsychotic-induced weight gain may occur early in treatment. Ratzoni et al. (2002) for example, have shown significant weight gain of first-episode psychosis patient treated with olanzapine (+7.2 kg), risperidone (+3.9 kg) but not for haloperidol (+1.1 kg) while in psychiatric inpatient treatment, thus keeping environmental influences such as differences in diet and physical exercise to a minimum. Evidence for the assumption that a large proportion of weight gain occurs early in treatment is provided by observations that the weight gain may, after various time periods, level off (Kinon et al., 2001; Henderson et al., 2000). Early interventions to control body weight may thus be especially important in patients who are being prescribed antipsychotic medications for the first time.
Among adjunct medications, only the total count of co-medication a patient was exposed to, and antidepressants played an important role in predicting weight gain. While it is known that certain mood stabilizers such as valproate and lithium have considerable weight gain propensity as well, our sample of patients receiving concomitant mood stabilizer therapy was probably too small to detect any significant effects. The relatively young average age in the sample also permitted us to look at patients and control subjects who had not yet developed medical comorbidities and were thus free from pharmacological medical treatment, thereby further limiting possible medication interactions and bias. There was a significant age difference at baseline between patients and controls; yet while younger age was an important predictor for more weight gain, highly significant differences in weight gain across groups remained even after controlling for age. Socioeconomic status differences, known to influence dietary quality and body weight (Ball and Crawford, 2005), were minor and arguably not accentuated enough to account for the differences in weight gain as observed in our sample. A small group of first-episode psychosis patients who were not receiving antipsychotics were also followed and served as “intrinsic” controls. While were not randomly assigned, it is nevertheless instructive to note that they gained slightly more, albeit not significantly more weight than healthy controls over the 1-year observation period. The observed weight gain amounted to a clinically significant increase in almost half of them and may put into context the clinically significant weight gain seen in patients receiving haloperidol and risperidone, who had similar rates of clinically significant weight gain (Figure 1). We also observed more pronounced weight gain in first-episode psychosis patients with higher baseline negative symptom scores. The exact reasons why this occurred are unknown, but it has been hypothesized that the sedative effects of neuroleptics - which may have an immobilizing and slowing-down effect - may be compounded by negative symptoms such as reduced motivation or social withdrawal (Ryan and Thakore, 2002), thus translating into decreased energy expenditure and more long-term weight gain. Preliminary evidence showing weight loss after improvement of negative symptoms (Nakayama, 2005) seem to support this interpretation.
We believe that our results fairly accurately depict the magnitude of weight gain introduced through antipsychotic medication in a first-episode psychosis population. The study is unique in examining weight gain effects of antipsychotics and concomitant psychotropic medications in a large sample of first episode-psychosis patients and no or little previous exposure to antipsychotic medications, contrasted with weight changes seen in psychotropic-free first episode patients and healthy control subjects over the same time period. The findings from our study are important for several reasons and may warrant proactive measures. First, while development of the metabolic syndrome is usually age-dependent, its adverse effects can already occur in adolescents when they are overweight enough (Weiss et al., 2004). Obesity alone without any other cardiovascular risk factor present has recently been shown to increase risk for mortality from coronary artery disease, cardiovascular disease, and diabetes in later life (Yan et al., 2006). Second, excess weight gain introduced through antipsychotic medication also carries several potentially adverse psychological effects including decreased self-esteem, decreased quality of life and high rates of subjective distress (Weiden and Mackell, 1999; Strassnig et al., 2003b). Third, weight gain negatively affects adherence rates, especially in younger patients (Van Mastrigt et al., 2004); which translates into higher rates of relapse (Robinson et al., 1999; Coldham et al., 2002) and may worsen long-term prognosis (Helgason, 1990).
Sensible choice of the antipsychotic agent appears warranted as a first step (Ganguli and Strassnig, 2006). It also seems important to lay out preventative strategies on behalf of first-episode psychosis patients to help them be prepared for the weight gain potential associated with most antipsychotic medications, and if necessary, take appropriate rather than unhealthy measures (Strassnig et al, 2005). Effective weight control improves all risk factors associated with the metabolic syndrome (National Task Force on the Prevention and Treatment of Obesity, 2000). Behavioural treatments have been successful at controlling weight in chronic schizophrenia patients (Brar et al., 2005), and may also represent appropriate interventions in first-episode psychosis patients. Such programs, possibly supplemented by pharmacological augmentation (Faulkner et al., 2003), initiated right from the onset of pharmacotherapy may represent the optimal approach in preventing antipsychotic-associated weight gain in first-episode psychosis patients (Ganguli and Brar, 2005). Special care seems warranted, since the initial treatment intervention in drug naïve first-episode psychosis patients is a critical step that has the potential to influence the course and outcome of what could become a lifelong illness (Lieberman, 1996).
In summary, first-episode psychosis patients treated with certain antipsychotics gained much more weight over the one-year observation period than healthy controls. A greater number of comedication and also antidepressant co-prescription were independently associated with weight gain; as were younger age and more negative psychotic symptoms at baseline. Our study is unique in examining the weight gain associated with several frequently-used antipsychotics in a large sample of first episode-psychosis patients and no previous exposure to antipsychotic medications, and very limited confounding burden introduced by adjunct medication. The weight gain propensity of various antipsychotics in this population is so considerable that we suggest measures such as behavioral weight control programs as preventative step right from the outset of pharmacotherapy.
Limitations
Our analysis was limited to those subjects older than 18 years, to minimize confounding effects of growth on body weight and body mass index. Controls were younger and may have lead us to overestimate the observed medication effects, as residual growth in the control group could potentially have narrowed the reported BMI differences. The study was carried out as an observational rather than randomized controlled study; and several thus uncontrolled factors such physician prescribing behavior may have influenced outcomes. For example, relatively fewer patients were treated with olanzapine than with risperidone - all of which were males - indicating potential prescriber selection bias, and limiting interpretability of olanzapine-induced weight gain data to male first-episode psychosis patients. Although our data does not indicate antipsychotic switching behavior was influenced by body weight gain in all but one patient, we acknowledge that approximately 15 years ago - when the study commenced - clinicians may not have been as well attuned to the perils of metabolic side effects of antipsychotics, and switching secondary to weight gain may not have been as great a concern, as for example, switching secondary to EPS or lack of efficacy. The study also was limited to those first-episode patients who were willing to participate in the assessments and may not be generalizable to the entire spectrum of first-episode psychosis patients including those receiving antipsychotic medication for other illnesses than early schizophrenia-spectrum and mood disorders with psychotic features. There was a small sample size in several subgroups, thus results should be generalized to early psychosis patients with caution. However, studies of first-episode psychosis patients avoid confounding issues such as those introduced through prior antipsychotic exposure and its duration and allow for assessment of parameters associated with treatment that would not be possible in non-naïve patients.
Acknowledgement
We thank Drs. Nina R. Schooler PhD, Cameron S. Carter MD, Raymond Cho, MD, Gretchen Haas PhD, and the clinical core staff of the Center for the Neuroscience of Mental Disorders (MH45156) for their assistance in diagnostic and psychopathological assessments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Addington J, Mansley C, Addington D. Weight gain in first-episode psychosis. Can. J. Psychiatry. 2003;48(4):272–276. doi: 10.1177/070674370304800412. [DOI] [PubMed] [Google Scholar]
- Allison DB, Fontaine KR, Heo M, Mentore JL, Cappelleri JC, Chandler LP, Weiden PJ, Cheskin LJ. The distribution of body mass index among individuals with and without schizophrenia. J. Clin. Psychiatry. 1999;60(4):215–220. doi: 10.4088/jcp.v60n0402. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry Suppl. 1989:49–58. [PubMed] [Google Scholar]
- Andreasen NC. Methods for assessing positive and negative symptoms. Mod. Probl. Pharmacopsychiatry. 1990;24:73–88. doi: 10.1159/000418013. [DOI] [PubMed] [Google Scholar]
- Ball K, Crawford D. Socioeconomic status and weight change in adults: a review. Soc. Sci. Med. 2005;60(9):1987–2010. doi: 10.1016/j.socscimed.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. J. Clin. Psychiatry. 2005;66(2):205–212. doi: 10.4088/jcp.v66n0208. [DOI] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol. Med. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults / The Evidence Report. Obesity. Res. 1998;6(suppl2):51S–209S. [PubMed] [Google Scholar]
- Coldham EL, Addington J, Addington D. Medication adherence of individuals with a first episode psychosis. Acta Psychiatr. Scand. 2002;106:286–290. doi: 10.1034/j.1600-0447.2002.02437.x. [DOI] [PubMed] [Google Scholar]
- Daumit GL, Crum RM, Guallar E, Powe NR, Primm AB, Steinwachs DM, Ford DE. Outpatient prescriptions for atypical antipsychotics for African Americans, Hispanics and Whites in the United States. Arch. Gen. Psychiatry. 2003;60:121–128. doi: 10.1001/archpsyc.60.2.121. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Soundy AA, Lloyd K. Schizophrenia and weight management: a systematic review of interventions to control weight. Acta Psychiatr. Scand. 2003;108:324–332. doi: 10.1034/j.1600-0447.2003.00218.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) American Psychiatric Press; Washington DC: 1996. [Google Scholar]
- Ganguli R, Strassnig M. Are older antipsychotic drugs obsolete? Br. Med. J. 2006;332:1346–1347. doi: 10.1136/bmj.332.7554.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli R. Weight Gain Associated With Antipsychotics. J. Clin. Psychiatry. 1999;60(suppl 21):20–24. [PubMed] [Google Scholar]
- Ganguli R, Brar JS, Ayrton Z. Weight gain over 4 months in schizophrenia patients: a comparison of olanzapine and risperidone. Schizophr. Res. 2001;49:261–267. doi: 10.1016/s0920-9964(00)00080-3. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Brar JS. Prevention of Weight gain, by behavioral interventions, in patients starting novel antipsychotics. Schizophr. Bull. 2005;31(2):561–562. [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason L. Twenty years' follow up of first psychiatric presentation for schizophrenia: what could have been prevented? Acta Psychiatr. Scand. 1990;81:231–235. doi: 10.1111/j.1600-0447.1990.tb06486.x. [DOI] [PubMed] [Google Scholar]
- Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, Goff DC. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: A five-year naturalistic study. Am. J. Psychiatry. 2000;157(6):975–981. doi: 10.1176/appi.ajp.157.6.975. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: 1975. pp. 1–22. [Google Scholar]
- Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr. Res. 2002;55(3):277–284. doi: 10.1016/s0920-9964(01)00256-0. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Conley RR, Carpenter WT. First-Episode Schizophrenia. A Focus on Pharmacological Treatment and Safety Considerations. Drugs. 2005;65(8):1113–1138. doi: 10.2165/00003495-200565080-00006. [DOI] [PubMed] [Google Scholar]
- Johnson DB, Gerstein DE, Evans AE, Woodward-Lopez G. Preventing obesity: a life cycle perspective. J. Am. Diet. Assoc. 2006;106(1):97–102. doi: 10.1016/j.jada.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Basson BR, Gilmore JA, Tollefson GD. Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J. Clin. Psychiatry. 2001;62(2):92–100. [PubMed] [Google Scholar]
- Lieberman JA. Atypical antipsychotic drugs as a first-line treatment of schizophrenia. J. Clin. Psychiatry. 1996;57(suppl 11):68–71. [PubMed] [Google Scholar]
- Lieberman JA, Sheitman B, Chakos M, Robinson D, Schooler N, Keith S. The development of tretment resistance in patients with schizophrenia: a clinical and pathophysiological perspective. J. Clin. Psychopharmacol. 1998;18:S20–S24. doi: 10.1097/00004714-199804001-00005. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Phillips M, Gu H, Stroup S, Zhang P, Kong L, Ji J, Koch G, Hamer RM. Atypical and conventional antipsychotic drugs in treatment -naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs. chlorpromazine. Neuropsychopharmacol. 2003;28(5):995–1003. doi: 10.1038/sj.npp.1300157. [DOI] [PubMed] [Google Scholar]
- Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis J, Kane JM, Lieberman JA, Schooler NR, Covell N, Stroup S, Weissman EM, Wirshing DA, Hall CS, Pogach L, Pi-Sunyer X, Bigger JT, Jr., Friedman A, Kleinberg D, Yevich SJ, Davis B, Shon S. Physical health monitoring of patient with schizophrenia. Am. J. Psychiatry. 2004;161(8):1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia. Arch. Gen. Psychiatry. 1991;48:739–745. doi: 10.1001/archpsyc.1991.01810320063009. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Stroup TS, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr. Res. 2005;80:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, Patel JK, Keefe RSE, Stroup TS, Lieberman JA. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial: Clinical comparison of subgroups with and without the metabolic syndrome. Schizophr. Res. 2005;80:9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Nakayama KM. Body weight induced by a newer antipsychotic agent reversed as negative symptoms improved. Acta Psychiatr. Scand. 2005;112:75–77. doi: 10.1111/j.1600-0447.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- National Task Force on the Prevention and Treatment of Obesity Overweight, Obesity and Health Risk, 2000. Arch. Intern. Med. 160(7):898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr. Res. 2000;45(12):21–28. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Arterioscler. Thromb. Vasc. Biol. 2006;26(5):968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- Ratzoni G, Gothelf D, Brand-Gothelf A, Reidman J, Kikinzon L, Gal G, Phillip M, Apter A, Weizman R. Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J. Am. Acad. Child. Adolesc. Psychiatry. 2002;41(3):337–343. doi: 10.1097/00004583-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Robinson D, Woerner MG, Alvir JM, Bilder RM, Hinrichsen GA, Lieberman JA. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch. Gen. Psychiatry. 1999;56:241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- Russell JM, Mackell JA. Bodyweight gain associated with atypical antipsychotics: epidemiology and therapeutic implications. CNS Drugs. 2001;15(7):537–551. doi: 10.2165/00023210-200115070-00004. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Thakore JH. Physical consequences of schizophrenia and its treatment: the metabolic syndrome. Life Sci. 2002;71(3):239–257. doi: 10.1016/s0024-3205(02)01646-6. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophr. Bull. 2003a;29(2):393–397. doi: 10.1093/oxfordjournals.schbul.a007013. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Body mass index and quality of life in community-dwelling patients with schizophrenia. Schizophr. Res. 2003b;62(12):73–76. doi: 10.1016/s0920-9964(02)00441-3. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Self-reported body weight perception and dieting practices in community-dwelling patients with schizophrenia. Schizophr. Res. 2005;75(23):425–432. doi: 10.1016/j.schres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Thakore JH. Metabolic disturbance in first-episode schizophrenia. Br. J. Psychiatry. 2004;184(suppl 47):s76–s79. doi: 10.1192/bjp.184.47.s76. [DOI] [PubMed] [Google Scholar]
- Van Mastrigt S, Addington J, Addington D. Substance misuse at presentation to an early psychosis program. Soc. Psychiatry Psychiatr. Epidemiol. 2004;39:69–72. doi: 10.1007/s00127-004-0713-0. [DOI] [PubMed] [Google Scholar]
- Weiden P, Mackell J. Differing side effect burden with newer antipsychotics. Eur Neuropsychopharmacol. 1999;9(suppl 5):S266. [Google Scholar]
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- Yan LL, Daviglus ML, Liu K, Stamler J, Wang R, Pirzada A, Garside DB, Dyer AR, Van Horn L, Liao Y, Fries JF, Greenland P. Midlife Body Mass Index And Hospitalization and Mortality in Older Age. JAMA. 2006;295:190–198. doi: 10.1001/jama.295.2.190. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Gu H, Green AI, Perkins DO, Tohen MF, McEvoy JP, Strakowski SM, Sharma T, Kahn RS, Gur RE, Tollefson GD, Lieberman JA. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br. J. Psychiatry. 2005;187:537–543. doi: 10.1192/bjp.187.6.537. [DOI] [PubMed] [Google Scholar]


