Abstract
Aims/hypothesis
Raised maternal plasma total homocysteine (tHcy) concentrations predict small size at birth, which is a risk factor for type 2 diabetes mellitus. We studied the association between maternal vitamin B12, folate and tHcy status during pregnancy, and offspring adiposity and insulin resistance at 6 years.
Methods
In the Pune Maternal Nutrition Study we studied 700 consecutive eligible pregnant women in six villages. We measured maternal nutritional intake and circulating concentrations of folate, vitamin B12, tHcy and methylmalonic acid (MMA) at 18 and 28 weeks of gestation. These were correlated with offspring anthropometry, body composition (dual-energy X-ray absorptiometry scan) and insulin resistance (homeostatic model assessment of insulin resistance [HOMA-R]) at 6 years.
Results
Two-thirds of mothers had low vitamin B12 (<150 pmol/l), 90% had high MMA (>0.26 μmol/l) and 30% had raised tHcy concentrations (>10 μmol/l); only one had a low erythrocyte folate concentration. Although short and thin (BMI), the 6-year-old children were relatively adipose compared with the UK standards (skinfold thicknesses). Higher maternal erythrocyte folate concentrations at 28 weeks predicted higher offspring adiposity and higher HOMA-R (both p < 0.01). Low maternal vitamin B12 (18 weeks; p = 0.03) predicted higher HOMA-R in the children. The offspring of mothers with a combination of high folate and low vitamin B12 concentrations were the most insulin resistant.
Conclusions/interpretation
Low maternal vitamin B12 and high folate status may contribute to the epidemic of adiposity and type 2 diabetes in India.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-007-0793-y) contains supplementary material, which is available to authorised users.
Keywords: Adiposity, Folate, Insulin resistance, Maternal nutrition, Offspring, Pregnancy, Vitamin B12
Introduction
The ‘thrifty phenotype’ hypothesis [1] introduced a new paradigm in the aetiology of chronic disease and stimulated research into the relationship between maternal nutrition, fetal growth and offspring risk of type 2 diabetes mellitus. India has the largest number of diabetic patients in the world [2] and this may be related to the fact that Indian babies are amongst the smallest in the world [3]. We were the first to demonstrate that low birthweight of Indian babies predicts insulin resistance and adiposity in childhood [4]. Subsequently we demonstrated that the apparently small Indian newborn babies are relatively adipose, hyperinsulinaemic and hyperleptinaemic compared with white babies [5, 6]. These babies grow into shorter, thinner (low BMI) but more adipose (higher body fat per cent and higher central fat) adults compared with whites, and have a many times higher risk of type 2 diabetes [7]. Thus, in addition to the role of putative genetic and modern day lifestyle factors, the intrauterine environment may also influence the risk of type 2 diabetes mellitus. The Pune Maternal Nutrition Study (PMNS) is the first study in India to investigate the relationship between maternal nutrition and offspring risk of type 2 diabetes and cardiovascular disease. Data were collected on the mother’s size, diet, micronutrient status and physical workload during pregnancy [6, 8, 9], and the newborns were measured in detail at birth and every 6 months thereafter. At 6 years of age, the offspring were investigated for risk of type 2 diabetes and cardiovascular disease.
We have reported that a higher frequency of maternal intake of green leafy vegetables (GLVs), milk and fruit and higher erythrocyte folate concentrations are associated with larger newborn size [8]. In a small sub-sample we found that higher maternal circulating total homocysteine (tHcy) predicted low offspring birthweight [10]. Other studies in Pune have shown that vitamin B12 deficiency is common and contributes to hyperhomocysteinaemia, while folate deficiency is rare [11, 12].
We therefore hypothesised that vitamin B12 and folate deficiency in the mother during pregnancy would predict greater adiposity and insulin resistance in the offspring.
Methods
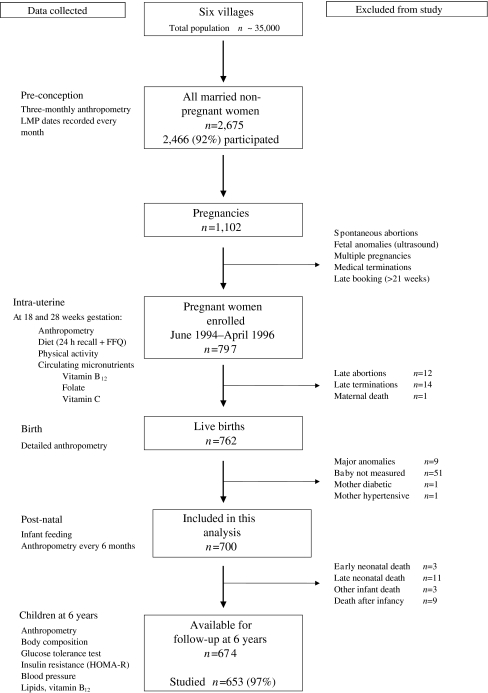
The recruitment of mothers and follow-up of the children in the PMNS (Fig. 1) has been described [6, 8, 9]. The study started in 1993. Non-pregnant married women living in six villages near Pune had detailed anthropometric measurements every 3 months and menstrual dates were recorded monthly. Women who became pregnant were assessed at 18 ± 2 and 28 ± 2 weeks of gestation for anthropometry, physical workload, dietary intake (24 h recall and food frequency questionnaire [FFQ]) and measurement of circulating nutrients. According to the National Nutritional Anemia Control Programme, women were given 100 tablets of iron (60 mg per tablet) and folic acid (500 μg per tablet) from 18 weeks of gestation. Newborn babies were measured within 72 h of birth (1994–1996), and the children were followed up post-natally with repeat anthropometry every 6 months.
Fig. 1.
The PMNS: a flow diagram to describe data collection and exclusions from the study. LMP Last menstrual period
At 6 years of age (December 2000–February 2003) the children were investigated for body size, body composition and risk factors for type 2 diabetes mellitus and cardiovascular disease. Families were instructed to continue their usual diet and activity prior to the study, and were transported to the Research Unit the previous evening. They were provided with a standard dinner and then water only until the next morning. A fasting blood sample was collected, followed by a 1.75 g/kg body weight oral anhydrous glucose load and a further blood sample 120 min later.
The family’s socioeconomic status (SES) was assessed using the Standard of Living Index devised by the National Family Health Survey [13]. Weight and height were measured using standardised protocols. Whole-body dual-energy X-ray absorptiometry (DEXA) scans (Lunar DPX-IQ 240; Lunar Corporation, Madison, WI, USA; paediatric software) were carried out to measure total, truncal and leg fat mass, percentage body fat and lean mass. Standard quality assurance tests were performed every day. The DEXA machine was installed 3 months after the study started, and therefore 59 children had DEXA measurements later than the other investigations.
Plasma glucose was measured using an Hitachi 911 automated analyser (Hitachi, Tokyo, Japan) by the glucose oxidase peroxidase method. Plasma insulin was measured using a Delfia technique (Victor 2; Wallac, Turku, Finland) [14]. Insulin resistance was calculated using the fasting insulin and glucose concentrations (homeostatic model assessment of insulin resistance [HOMA-R]) [15]. As previously described, maternal erythrocyte folate concentrations were measured at the time of the original study (1993–1996), taking all necessary precautions in the collection, transport and storage of samples [8]. Based on the findings of a preliminary study [10], we measured plasma vitamin B12, tHcy and methylmalonic acid (MMA) concentrations in all the stored maternal fasting samples (−80°C) in February 2004, as described [11].
Ethical permission for the study was granted by the KEM Hospital Ethical Committee, and by the local village leaders. Parents gave informed written consent. None of these volunteers were paid for participating in the study.
Statistics Skewed variables were transformed to normality using the following transformations: log to the base e (ln) (plasma vitamin B12 [18 weeks], tHcy and MMA and erythrocyte folate concentrations, maternal fat intake, and the child’s trunk and leg fat mass), square root (maternal energy, protein, and carbohydrate intakes, and the child’s total fat mass) and reciprocal of square root (maternal vitamin B12 concentrations at 28 weeks). Maternal pre-pregnant fat mass was calculated from the sum of four skinfold thickness measurements [16]. Maternal intakes of specific foods, based on the FFQ, were analysed as categorical variables. Low vitamin B12 and erythrocyte folate concentrations were defined as <150 pmol/l and <283 nmol/l, respectively. Elevated tHcy and MMA concentrations were defined as >10 and >0.26 μmol/l [17]. Correlations between different nutritional measures in the mothers were tested using Pearson correlation coefficients. Relationships between maternal nutritional variables and outcomes in the children were analysed using multiple linear regression.
As a final stage of the analysis, we summarised the inter-relationships between maternal factors and outcomes in the children using principal components analysis (PCA) and conditional independence analysis. We first performed a PCA to condense the information contained in large groups of variables into a small number of ‘component’ variables. These components are independent of each other and therefore can be used in regression analysis without the problem of colinearity [18]. The groups of variables were: (1) maternal pre-pregnant size (height, skinfold thicknesses, head circumference, mid-upper-arm circumference [MUAC] and waist and hip circumferences); (2) maternal macronutrient intakes (energy, protein, carbohydrate and fat intakes at 18 and 28 weeks); (3) maternal micronutrient-rich foods in pregnancy (frequency of intake of dairy products, GLV, fruit and non-vegetarian items [meat, fish and eggs] at 18 and 28 weeks of gestation); (4) maternal micronutrient status (vitamin B12, folate, tHcy and MMA concentrations at 18 and 28 weeks); (5) newborn size (birthweight, length, skinfold thicknesses, MUAC, head and abdominal circumferences); and (6) 6 year body composition (height and DEXA measurements of lean mass, and total, truncal and limb fat). Other key variables (maternal SES and physical workload score [mean of 18 and 28 week scores], and HOMA-R in the child at 6 years) remained as single variables. These, and the relevant principal components were included simultaneously in a conditional independence analysis, which is a method of displaying ‘pathways’ of association between a pre-specified set of variables (in this case, the groups of variables described above). The partial correlation (that is, the correlation while holding the remaining variables constant in the set) of each pair of variables was calculated [19]. A ‘path’ diagram was then drawn, connecting pairs of variables that were significantly correlated (p < 0.01). Two-tailed significance was calculated at 5% level. Analyses were performed using SPSS 11.0 for windows (SPSS, Chicago, IL, USA) and STATA 7.0 (STATA, College Station, TX, USA).
Results
Many of the maternal size and nutritional measurements and their relationship to neonatal size have been described before [8]. The age (median [25th, 75th centiles]) of the mothers when they became pregnant was 21 (19, 23) years. They were short (152.0 [148.5, 155.4] cm) and had a low pre-pregnant BMI (17.8 [16.7, 19.1] kg/m2) but a relatively high percentage body fat (20% [18, 24]). Their dietary intakes and micronutrient status during pregnancy are shown in Table 1. The women’s energy and protein intakes were low compared with the recommended dietary allowances of 10,565 kJ (2,525 kcal) and 65 g/day, respectively [20]. At 18 and 28 weeks, their energy intakes were 1.57 and 1.43 times their calculated basal metabolic rate [21]. One-third of women were lacto-vegetarian, and only 15% of women ate non-vegetarian foods more frequently than once every alternate day. The portion sizes of non-vegetarian foods were small (<120 g/day for chicken, fish and meat dishes and ~60 g/day for eggs).
Table 1.
Maternal nutrition data during pregnancy (median and 25th and 75th centiles, unless otherwise stated)
| Number | 18 weeks of gestation | Number | 28 weeks of gestation | |
|---|---|---|---|---|
| Macronutrient intakes | ||||
| Energy (kJ) | 692 | 7,293 (5,858, 8,774) | 670 | 6,803 (5,523, 8,268) |
| Energy (kcal) | 692 | 1,743 (1,400, 2,090) | 670 | 1,626 (1,320, 1,976) |
| Carbohydrate (g) | 692 | 313 (257, 376) | 670 | 296 (236, 360) |
| Protein (g) | 692 | 44.9 (35.4, 54.8) | 670 | 41.7 (33.8, 51.3) |
| Fat (g) | 692 | 32.7 (23.9, 42.9) | 670 | 29.6 (22.4, 39.2) |
| FFQ, n (%) | ||||
| GLVs | ||||
| Never | 692 | 15 (2) | 671 | 69 (10) |
| <1 per week | 692 | 139 (20) | 671 | 197 (29) |
| >1 per week | 692 | 277 (40) | 671 | 243 (36) |
| >Alternate day | 692 | 261 (38) | 671 | 162 (24) |
| Dairy products, n (%) | ||||
| Never | 692 | 107 (15) | 671 | 96 (14) |
| <1 per week | 692 | 141 (20) | 671 | 137 (20) |
| >1 per week | 692 | 129 (19) | 671 | 144 (21) |
| >Alternate day | 692 | 315 (46) | 671 | 294 (44) |
| Non-vegetarian foods, n (%) | ||||
| Never | 692 | 228 (33) | 671 | 254 (38) |
| <1 per week | 692 | 182 (26) | 671 | 181 (27) |
| >1 per week | 692 | 182 (26) | 671 | 149 (22) |
| >Alternate day | 692 | 100 (14) | 671 | 87 (13) |
| Circulating micronutrients | ||||
| Vitamin B12 (pmol/l) | 638 | 135 (103, 175) | 594 | 122 (94, 160) |
| <150 pmol/l, n (%) | 380 (60) | 423 (71) | ||
| Erythrocyte folate (nmol/l) | 618 | 874 (687, 1,106) | 562 | 961 (736, 1,269) |
| <283 nmol/l, n (%) | 1 (0.2) | 1 (0.2) | ||
| MMA (μmol/l) | 636 | 0.80 (0.50, 1.34) | 594 | 0.73 (0.44, 1.18) |
| >0.26 μmol/l, n (%) | 586 (94) | 533 (90) | ||
| tHcy (μmol/l) | 639 | 8.1 (6.8, 10.3) | 593 | 8.6 (6.7, 10.8) |
| >10 μmol/l, n (%) | 177 (28) | 193 (33) | ||
Dairy products refers to whole milk plus milk products (milk in tea and other beverages, yoghurt, buttermilk, ghee, ice cream and other milk-based preparations). Non-vegetarian foods are meat, fish and eggs
Maternal vitamin B12 and folate status Women in whom vitamin B12, folate and related measurements were not available had similar pre-pregnant weight, SES and weight gain at 28 weeks of pregnancy compared with those who were studied. The majority of women (>60%) had low plasma vitamin B12 concentrations (Table 1), over 90% had elevated MMA concentrations, and one-third were hyperhomocysteinaemic. On the other hand only one woman had a low erythrocyte folate concentration. Between 18 and 28 weeks of gestation, plasma vitamin B12, MMA and tHcy concentrations remained similar (p ~ 0.11, for all), while erythrocyte folate concentrations increased (p < 0.001). Plasma tHcy and MMA concentrations were inversely related to plasma vitamin B12 (p < 0.001 for both at 18 and 28 weeks of gestation). Low vitamin B12 concentration contributed 41 and 24% to the population attributable risk of hyperhomocysteinaemia, and 40 and 12% to high MMA at 18 and 28 weeks of gestation, respectively; the contribution of low folate concentration could not be calculated because of small numbers. Higher frequency of intake of dairy products and non-vegetarian foods was associated with higher plasma vitamin B12 concentrations (p = 0.005 and 0.04, respectively) and lower tHcy (p = 0.1 and p = 0.04) and MMA concentrations (p = 0.01 and p = 0.003). Plasma vitamin B12 concentrations were also related to protein intakes (28 weeks, p = 0.03) but not to energy intake. Higher frequency of GLV intake predicted higher erythrocyte folate concentrations (p = 0.001). All these associations were independent of the energy intake and SES.
Maternal nutrition during pregnancy and newborn size Maternal vitamin B12 and MMA concentrations were unrelated to neonatal measurements. As previously described [8], lower maternal folate concentrations were associated with smaller newborn weight, MUAC and abdominal circumference (p = 0.003, 0.008, 0.008, respectively). Higher tHcy concentrations at 18 weeks were associated with smaller newborn size (MUAC, p = 0.02; abdominal circumference, p = 0.02; and subscapular and triceps skinfold thicknesses, p = 0.01 and p = 0.007).
Maternal nutrition during pregnancy and offspring size, body composition and HOMA-R at 6 years At 6 years, the children were light, short and had a low BMI compared with an international (UK) reference [22] (Table 2); none were overweight or obese as defined by International Obesity Task Force [23] criteria. However, skinfold thickness measurements showed that the children were relatively truncally adipose; the mean SD score for subscapular skinfold thickness was −0.42 compared with the UK growth standards [24], in contrast with −2.23 for weight and −1.86 for BMI. Higher fat mass and higher body fat per cent were associated with higher fasting insulin concentrations, higher HOMA-R and higher 120 min plasma glucose concentrations (p < 0.05 for all).
Table 2.
Characteristics of the children at 6 years (n = 653)
| Median (IQR) | Mean SD scorea (SD) | |
|---|---|---|
| Age (years) | 6.1 (6.1, 6.2) | |
| Weight (kg) | 16.0 (14.8, 17.3) | −2.23 (1.00) |
| Height (cm) | 109.7 (106.7, 112.9) | −1.39 (0.89) |
| BMI (kg/m2) | 13.3 (12.8, 14.0) | −1.86 (0.90) |
| Subscapular skinfold thickness (mm) | 5.0 (4.4, 5.6) | −0.42 (0.68) |
| Triceps skinfold thickness (mm) | 6.2 (5.4, 7.1) | −1.41 (0.79) |
| Fat mass (kg) | 3.1 (2.4, 3.7) | |
| Fat (%) | 18.8 (15.5, 22.2) | |
| Leg fat mass (kg) | 1.3 (1.0, 1.5) | |
| Trunk fat mass (kg) | 0.9 (0.7, 1.2) | |
| Lean mass (kg) | 12.7 (11.7, 13.7) | |
| Insulin resistance (HOMA-R) | 0.70 (0.37, 1.06) | |
| 120 min glucose (mmol/l) | 5.49 (4.82, 6.21) | |
| Vitamin B12 (pmol/l) | 224 (167, 311) |
Higher frequency of maternal intake of GLVs and higher erythrocyte folate concentrations (28 weeks) were associated with higher fat mass and higher per cent body fat in the children (GLVs: p = 0.02, 0.08; and folate: p = 0.01, 0.002; Table 3). None of the maternal nutritional variables were related to lean mass in the children.
Table 3.
Outcomes in the children at 6 years (body composition and insulin resistance) according to maternal vitamin B12 concentrations at 18 weeks, erythrocyte folate at 28 weeks and GLV and dairy product intake at 28 weeks
| Number | Height (cm) | Fat mass (kg) | Lean mass (kg) | Insulin resistance (HOMA-R) | ||
|---|---|---|---|---|---|---|
| Vitamin B12 at 18 weeks (pmol/l) | ||||||
| <103 | 146 | 110.2 (4.3) | 3.3 (0.9) | 12.8 (1.6) | 0.78 (0.42, 1.34) | |
| 103–134 | 150 | 109.3 (4.6) | 3.0 (1.0) | 12.7 (1.5) | 0.68 (0.35, 0.99) | |
| 135–174 | 145 | 109.6 (4.9) | 3.1 (1.0) | 12.9 (1.7) | 0.69 (0.30, 0.95) | |
| ≥175 | 151 | 110.0 (4.9) | 3.2 (1.0) | 12.8 (1.7) | 0.61 (0.35, 1.00) | |
| p valuea | 0.8 | 0.5 | 0.3 | 0.03 | ||
| p valueb | 0.6 | 0.4 | 0.3 | 0.04 | ||
| Erythrocyte folate at 28 weeks (nmol/l) | ||||||
| <734 | 129 | 109.2 (4.6) | 3.0 (1.0) | 12.8 (1.7) | 0.52 (0.28, 0.81) | |
| 734–691 | 136 | 109.8 (4.5) | 3.1 (0.9) | 12.9 (1.6) | 0.65 (0.36, 0.92) | |
| 962–1,268 | 131 | 109.9 (4.7) | 3.2 (1.2) | 12.7 (1.8) | 0.71 (0.39, 1.12) | |
| ≥1,269 | 127 | 110.7 (4.8) | 3.4 (1.1) | 13.0 (1.6) | 0.85 (0.51, 1.27) | |
| p valuea | 0.02 | 0.001 | 0.5 | <0.001 | ||
| p valueb | 0.7 | 0.01 | 0.4 | <0.001 | ||
| GLVs at 28 weeks | ||||||
| Never | 63 | 109.1 (5.5) | 3.0 (0.9) | 12.5 (1.7) | 0.83 (0.25, 1.23) | |
| <Once per week | 183 | 109.5 (4.7) | 3.0 (1.0) | 12.7 (1.7) | 0.73 (0.36, 1.05) | |
| >Once per week | 231 | 109.8 (4.6) | 3.2 (1.0) | 12.8 (1.7) | 0.69 (0.34, 0.99) | |
| ≥Alternate days | 146 | 110.5 (4.1) | 3.3 (1.2) | 13.0 (1.6) | 0.66 (0.41, 1.00) | |
| p valuea | 0.002 | 0.001 | 0.01 | 0.99 | ||
| p valueb | 0.4 | 0.02 | 0.4 | 0.98 | ||
| Dairy products at 28 weeks | ||||||
| Never | 87 | 108.4 (4.9) | 2.9 (0.9) | 12.6 (1.8) | 0.60 (0.32, 0.95) | |
| <Once per week | 124 | 110.2 (4.1) | 3.2 (1.0) | 12.8 (1.6) | 0.62 (0.37, 0.99) | |
| >Once per week | 134 | 109.4 (4.6) | 3.2 (0.9) | 12.7 (1.7) | 0.73 (0.40, 1.06) | |
| ≥Alternate days | 278 | 110.3 (4.6) | 3.2 (1.2) | 12.9 (1.7) | 0.74 (0.37, 1.11) | |
| p valuea | 0.005 | 0.03 | 0.3 | 0.02 | ||
| p valueb | 0.2 | 0.2 | 0.8 | 0.006 | ||
Values are mean (SD) or median (interquartile range)
ap value adjusted for the child’s age and sex
bp value adjusted for the above, plus: SES, the mother’s pre-pregnant height and fat mass, the child’s birthweight, skinfold thicknesses and gestation at delivery and the mother’s protein intake at the time of measurement
Higher frequency of maternal intake of dairy products at 28 weeks, and lower plasma vitamin B12 (18 weeks) and higher erythrocyte folate concentrations (28 weeks) were associated with higher HOMA-R in the children (Table 3). The highest HOMA-R was in children whose mothers had the lowest vitamin B12 and highest folate concentrations (Fig. 2). There was no statistically significant interaction, however, between vitamin B12 and folate concentrations in relation to HOMA-R (Table 4). The associations remained significant after further adjustment for the child’s own fat mass and plasma vitamin B12 concentrations. None of the other maternal dietary or macronutrient variables were related to HOMA-R in the children.
Fig. 2.
Insulin resistance (HOMA-R) in the children at 6 years in relation to maternal vitamin B12 (18 weeks) and erythrocyte folate (28 weeks)
Table 4.
Multiple linear regression models for child’s insulin resistance (HOMA-R) at 6 years as the dependent variable
| Independent variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| β | p value | β | p value | β | p value | β | p value | |
| Plasma vitamin B12 at 18 weeks (pmol/l) | −0.16 | 0.03 | −0.16 | 0.04 | ||||
| Erythrocyte folate at 28 weeks (nmol/l) | 0.38 | <0.001 | 0.38 | <0.001 | ||||
| Interaction term (vitamin B12 × folate) | −0.08 | 0.7 | ||||||
The independent variable included maternal plasma vitamin B12 concentration at 18 weeks gestation, erythrocyte folate at 28 weeks gestation, child’s age, sex, fat mass and standard of living index. The models progressively included vitamin B12, erythrocyte folate, both, and an interaction term (models 1 to 4, respectively)
Maternal vitamin B12 concentrations were not related to the child’s insulinogenic index (a measure of beta cell function) but 28 week erythrocyte folate concentration was directly related (p < 0.01).
PCA and conditional independence analysis PCA and conditional independence analyses are summarised in Fig. 3 and the Electronic supplementary material (ESM) Table 1. Mothers of higher SES had higher intakes of the micronutrient-rich foods, except non-vegetarian foods (correlation A). Larger maternal body size (correlation B) and larger size of the offspring at birth (correlation C) were associated with larger 6 year size. None of the maternal nutritional variables were related to 6 year body composition in the children, but the contrast between folate and vitamin B12 concentrations (micronutrient status, factor 3: higher maternal folate and MMA and lower vitamin B12) was associated with higher HOMA-R in the child at 6 years (correlation D).
Fig. 3.
The groups of variables used in the PCAs are described in Statistical methods. The notation F1–F3 indicates the first to third principal components, or factors (F), derived from each group of variables. Lines connecting boxes indicate significant positive correlations (p < 0.01), and bold lines denote correlations significant at p < 0.001. Correlations labelled A–D are explained in the Results (PCA and conditional independence analysis). Veg Vegetables
Discussion
We have demonstrated for the first time in a purposeful, community-based prospective study an association between maternal nutritional measurements in pregnancy and two major risk factors for type 2 diabetes in the offspring. Among rural mothers in Pune, energy and protein intakes are lower than the recommended daily allowance, vitamin B12 status is poor but folate status is adequate. Maternal macronutrient intakes were unrelated to adiposity and insulin resistance in the offspring. However, higher maternal folate concentrations predicted greater adiposity (fat mass and body fat per cent) and higher insulin resistance, and lower vitamin B12 concentrations predicted higher insulin resistance. Children born to mothers with low vitamin B12 concentrations but high folate concentrations were the most insulin resistant. The 18 week vitamin B12 concentration was more strongly associated with insulin resistance than the 28 week value, while the reverse was true for erythrocyte folate. The lifespan of erythrocytes (4 months) means that 28 week erythrocyte folate reflects nutritional status earlier in pregnancy. Thus, early-mid pregnancy may be a critical period for the programming of adiposity and insulin resistance.
The PMNS children are short and thin but relatively adipose compared with white children, similar to the situation in Indian newborns [5, 6] and adults [25]. There were no excessively adipose or insulin-resistant children in this cohort, but adiposity measures were strongly related to metabolic risk factors (glycaemia and insulin resistance) in the normal range. Other studies have shown that higher levels of risk factors in the normal range in childhood predict an increased risk of disease in later life [26, 27].
We have demonstrated that the ‘thin-fat’ phenotype of Indians is associated with a higher risk of type 2 diabetes [7]. This phenotype reflects the simultaneous involvement of two major body compartments (less lean but more adipose), which contribute to the pathogenesis of type 2 diabetes [28]. Our study suggests that an intrauterine imbalance between two related micronutrients (vitamin B12 and folate) may be responsible. A related concept is that of sarcopenic obesity, which increases risk of metabolic and skeletal adverse outcomes [29].
We did not find an association between maternal vitamin B12 concentrations and size at birth, possibly because so many women had values in the deficient range. However, higher maternal tHcy concentrations (which were related to low vitamin B12 concentrations) predicted smaller newborn size. A study in Bangalore, South India, where mothers had higher vitamin B12 concentrations, found that low maternal vitamin B12 concentrations predicted fetal growth retardation [30]. These findings are consistent with the ‘thrifty phenotype hypothesis,’ which proposed that ‘poor’ maternal nutritional status increases the risk of type 2 diabetes in the offspring [1]. On the other hand, the positive associations of maternal folate and intakes of GLVs and dairy products with adiposity or insulin resistance in the children, are contrary to the hypothesis because they predicted larger offspring size at birth. Our results suggest a need for caution in designing nutritional strategies to improve fetal growth and future health based on relationships with birth size alone.
Previous studies linking maternal nutrition in pregnancy to cardiovascular risk factors in the offspring did not measure vitamin B12 or folate [31]. These two vitamins play a crucial role in nucleic acid synthesis and one-carbon metabolism. Low maternal folate status has been implicated in the aetiology of neural tube defects [32] and high tHcy levels have been associated with poor pregnancy outcomes [10, 33, 34]. This has led to a policy of supplementation with folic acid before and during pregnancy, and fortification of flour with folic acid in some countries. The Indian policy is to provide iron and folic acid (60 mg and 500 μg per day) to all pregnant mothers [35]. Despite the evidence of widespread deficiency [11] vitamin B12 supplementation is not a consideration in pregnant Indian women. Vegetarianism and low milk intakes contribute to low vitamin B12 status in Indians [36, 37]. Vegetarianism is multigenerational, and influenced by religious and socioeconomic factors. King Samrat Ashok (273 bc) first banned the killing of animals for food and this was institutionalised by three religions: Jainism, Hinduism and Buddhism. The role of socioeconomic factors is evident in the small portion sizes of non-vegetarian foods habitually eaten by our mothers. Adequate folate status in the PMNS indicates adequate dietary intake even before supplementation was started (18 weeks of gestation), which further increased the levels. In addition, it is our experience that many Indian obstetricians routinely prescribe high doses of folic acid (5 mg or more) in ‘early’ pregnancy with the intention of preventing neural tube defects, even though the majority of pregnant women approach the doctor after 12 weeks of gestation, when the neural tube is already closed. Thus religious and socioeconomic factors, and medical practices, contribute to create an imbalance in these two related vitamins. The association of higher maternal intake of dairy products with insulin resistance in the offspring requires further exploration. It is intriguing that higher milk consumption is associated with higher insulin resistance in European children [38].
We can only speculate about the possible mechanisms for our findings (Fig. 1). Vitamin B12 deficiency will trap folate as 5-methyltetrahydrofolate [39], prevent the generation of methionine from homocysteine and therefore reduce protein synthesis and lean tissue deposition. Elevated methylmalonyl-CoA could contribute to increased lipogenesis by inhibiting carnitine palmitoyltransferase [40] and thereby inhibit β-oxidation [41]. An analogous clinical situation is high-dose folic acid treatment of severely vitamin B12-deficient pernicious anaemia patients: anaemia improves but neurological damage worsens, possibly because of accumulation of odd-chain carbon fatty acids [42]. Epigenetic regulation, involving DNA methylation, may be another mechanism of nutritional programming, as demonstrated in animal models [43–45]. It would be interesting to study these aspects further.
The PMNS has many strengths including a population-based design and high participation and follow-up rates. The nutritional assessment methods were developed specifically for the population. The rural setting is representative of over 70% of the Indian population, in whom the prevalence of diabetes is rapidly rising [46]. The lacto-vegetarian food habits of our women are similar to ~40% of Indian households and ~10% of the world population [47]. Although vitamin B12 was measured in stored frozen samples, it is stable during long-term storage [48]. In the absence of accepted guidelines for interpreting vitamin B12 status in pregnancy [49], we cannot define the true extent of vitamin B12 deficiency, but high levels of MMA and tHcy suggested its presence in a majority of the women in our study [17]. Associations with folate and vitamin B12 concentrations but not with tHcy and MMA could be due to dependence of the latter two on non-nutritional factors that are altered in pregnancy (haemodilution and elevated glomerular filtration rate), although we did find an association between high maternal MMA and insulin resistance in the children in the conditional independence analysis. A limitation of the study is that it is observational, and therefore causality cannot be ascertained.
In conclusion, our data raise the important possibility that high folate intakes in vitamin B12-deficient mothers could increase the risk of type 2 diabetes in the offspring. This is the first report in humans to suggest that defects in one-carbon metabolism might be at the heart of intra-uterine programming of adult disease. There is a need for further studies to test our findings and to determine the correct management of low vitamin B12 concentrations in Indian mothers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1 Suggested metabolic mechanisms for adiposity, insulin resistance and altered gene expression in a situation of dietary vitamin B12 deficiency combined with adequate folate status (PDF 35.7 kb)
Unrotated factor loadings from the PCA (PDF 75.3 kb)
Acknowledgements
We are grateful to the community, in particular the children and their families, for taking part in this study. We thank the late B. Coyaji, Director of the KEM Hospital, Pune, and the late V. N. Rao, initiators 25 years ago, of the rural primary health care programme in the study area. We acknowledge major contributions by S. Hirve and P. Gupta. We thank A. Ganpule for DEXA measurements. We acknowledge the invaluable community work contributed by T. Deokar, S. Chaugule, A. Bhalerao and V. Solat. We thank S. Joshi and Jane Pearce for final editing and submission. We appreciate the help of O. Netland, H. Bergesen, E. Blomdal and B. Olsen with the biochemical analyses in Bergen, Norway, P. Wood, Endocrine Laboratory, Southampton General Hospital, UK, for assistance with insulin assays, and the Special Haematology Laboratory, Southampton General Hospital, UK, for folate assays. The study was funded by the Wellcome Trust and the Medical Research Council, UK, and the Advanced Research Programme of Norway. We acknowledge the support of SNEHA-INDIA.
Conflict of interest statement The authors declare that there is no duality of interest associated with this manuscript.
Abbreviations
- DEXA
dual-energy X-ray absorptiometry
- FFQ
food frequency questionnaire
- GLV
green leafy vegetable
- HOMA-R
homeostatic model assessment of insulin resistance
- MMA
methylmalonic acid
- MUAC
mid-upper-arm circumference
- NTD
neural tube defect
- PCA
principal components analysis
- PMNS
Pune Maternal Nutrition Study
- SES
socioeconomic status
- tHcy
total homocysteine
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-007-0793-y) contains supplementary material, which is available to authorised users.
References
- 1.Hales CN, Barker DJP (1992) Type 2 (non-insulin dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601 [DOI] [PubMed]
- 2.The International Diabetes Federation diabetes atlas. Available at www.eatlas.idf.org, accessed 4 March 2007
- 3.Gopalan C (1994) Low birthweight: significance and implications. In: Sachdev HPS, Chaudhary P (eds) Nutrition in children: developing country concerns. Imprint, New Delhi
- 4.Bavdekar A, Yajnik CS, Fall CHD et al (1999) Insulin resistance in 8 year old Indian children, small at birth, big at 8 years, or both? Diabetes 48:2422–2429 [DOI] [PubMed]
- 5.Yajnik CS, Lubree HG, Rege SS et al (2002) Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab 87:5575–5580 [DOI] [PubMed]
- 6.Yajnik CS, Fall CHD, Coyaji KJ et al (2003) Neonatal anthropometry: the thin-fat Indian baby; the Pune Maternal Nutrition Study. Int J Obes 27:173–180 [DOI] [PubMed]
- 7.Yajnik CS (2001) The insulin resistance epidemic in India: fetal origins, later lifestyle, or both? Nutr Rev 59:1–9 [DOI] [PubMed]
- 8.Rao S, Yajnik CS, Kanade A et al (2001) Maternal fat intakes and micronutrient status are related to fetal size at birth in rural India; the Pune Maternal Nutrition Study. J Nutr 131:1217–1224 [DOI] [PubMed]
- 9.Rao S, Kanade A, Margetts BM et al (2003) Maternal activity in relation to birth size in rural India; the Pune Maternal Nutrition Study. Eur J Clin Nutr 57:531–542 [DOI] [PMC free article] [PubMed]
- 10.Yajnik CS, Deshpande SS, Panchanadikar AV et al (2005) Higher maternal plasma homocysteine concentrations at 28 weeks gestation predicts smaller offspring size in rural India; a pilot study. Asia Pacific J Clin Nutr 14:179–181 [PubMed]
- 11.Refsum H, Yajnik CS, Gadkari M et al (2001) Hyperhomocysteinemia and elevated methylmalonic acid indicate high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr 74:233–241 [DOI] [PubMed]
- 12.Yajnik CS, Deshpande SS, Lubree HG et al (2006) Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Phys India 54:1–8 [PubMed]
- 13.International Institute for Population sciences (IIPS) and ORC Macro (2001) National Family Health survey (NFHS-2), India, 1998–99: Maharashtra. Mumbai, IIPS, pp 52–57
- 14.Toivonen E, Hemmila I, Marniemi J, Jorgensen PN, Zeuthen J, Lovgren T (1986) Two-site time-resolved immunofluorometric assay of human insulin. Clin Chem 32:637–640 [PubMed]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed]
- 16.Durnin JV, Womersley J (1974) Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32:77–97 [DOI] [PubMed]
- 17.Refsum H, Smith AD, Ueland PM et al (2004) Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 50:3–32 [DOI] [PubMed]
- 18.Joliffe IT, Morgan BJT (1992) Principal component analysis and exploratory factor analysis. Stat Methods Med Res 1:69–95 [DOI] [PubMed]
- 19.Whittaker J (1989) Graphical models in applied statistics. Wiley, Chichester
- 20.Gopalan C, Rama Sastri BV, Balasubramanian SC (2000) Nutritive value of Indian foods. Revised by Narasingha Rao BS, Deosthale YG, Pant KC. National Institute of Nutrition and Indian Council of Medical Research, Hyderabad, India
- 21.Energy and protein requirements (1985) Report of a Joint FAO/WHO/UNU Expert Consultation. Technical Report Series No. 724. World Health Organization, Geneva [PubMed]
- 22.Cole TJ, Freeman JV, Preece MA (1998) British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 17:407–429 [DOI] [PubMed]
- 23.Cole TJ, Bellizi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition of child overweight and obesity worldwide: international survey. BMJ 320:1240–1243 [DOI] [PMC free article] [PubMed]
- 24.Davies PS, Day JM, Cole TJ (1993) Converting Tanner–Whitehouse reference triceps and subscapular skinfold measurements to standard deviation scores. Eur J Clin Nutr 47:559–566 [PubMed]
- 25.Deurenberg-Yap M, Chew SK, Deurenberg P (2002) Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes Rev 3:209–215 [DOI] [PubMed]
- 26.Bhargava SK, Sachdev HPS, Fall CHD et al (2004) Relation of serial changes in childhood body mass index to impaired glucose tolerance in young adulthood. N Engl J Med 350:865–875 [DOI] [PMC free article] [PubMed]
- 27.Srinivasan SR, Myers L, Berenson GS (2002) Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood; the Bogalusa Heart Study. Diabetes 51:204–209 [DOI] [PubMed]
- 28.Vella A, Rizza RA (2003) Metabolic disturbances in diabetes mellitus. In: Pickup JC, Williams G (eds) Textbook of diabetes, vol. I. Blackwell, Oxford, pp 31.1–31.4
- 29.Roubenoff R (2004) Sarcopenic obesity: the confluence of two epidemics. Obes Res 12:887–888 [DOI] [PubMed]
- 30.Muthayya S, Kurpad AV, Duggan CP et al (2006) Low maternal vitamin B(12) status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr 60:791–801 [DOI] [PubMed]
- 31.Fall CHD, Yajnik CS, Rao S, Davies AA, Brown N, Farrant HJW (2003) Micronutrients and fetal growth. J Nutr 133:1747S–1756S [DOI] [PubMed]
- 32.Refsum H (2001) Folate, vitamin B12 and homocysteine in relation to birth defects and pregnancy outcome. Br J Nutr 85S:109–113 [PubMed]
- 33.Vollset SE, Refsum H, Irgens LM et al (2000) Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: The Hordaland Homocysteine Study. Am J Clin Nutr 71:962–968 [DOI] [PubMed]
- 34.Lindblad B, Zaman S, Malik A et al (2005) Folate, vitamin B12 and homocysteine levels in South Asian women with growth-retarded fetuses. Acta Obstet Gynecol 84:1055–1061 [DOI] [PubMed]
- 35.Indian Council of Medical Research (1989) Evaluation of the national nutritional anaemia prophylaxis programme, an ICMR task force study. Central Electric, New Delhi
- 36.Kumar S, Ghosh K, Das KC (1989) Serum vitamin B12 levels in an Indian population: an evaluation of three assay methods. Med Lab Sci 46:120–126 [PubMed]
- 37.Gomber S, Kumar S, Rusia U, Gupta P, Agarwal KN, Sharma S (1998) Prevalence and etiology of nutritional anaemias in early childhood in an urban slum. Indian J Med Res 107:269–273 [PubMed]
- 38.Hoppe C, Molgaard C, Vaag A, Barkholt V, Michaelsen KF (2005) High intake of milk, but not meat, increase s-insulin and insulin resistance in 8-year-old boys. Eur J Clin Nutr 59:393–398 [DOI] [PubMed]
- 39.Scott JM, Weir DG (1981) The methyl folate trap. A physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid induced exacerbation of sub-acute combined degeneration in pernicious anaemia. Lancet 2:337–340 [DOI] [PubMed]
- 40.Ruderman NB, Saha AK, Kraegen EW (2003) Minireview: Malonyl CoA, AMP-activated protein kinase and adiposity. Endocrinology 144:5166–5171 [DOI] [PubMed]
- 41.Ruderman NB, Saha AK, Vavvas D, Witters LA (1999) Malonyl-Co-A, fuel-sensing, and insulin resistance. Am J Physiol 276:E1–E18 [DOI] [PubMed]
- 42.Metz J (1992) Cobalamin deficiency and the pathogenesis of nervous system disease. Ann Rev Nutr 12:59–79 [DOI] [PubMed]
- 43.Cooney CA, Dave AA, Wolff GL (2002) Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 132(Suppl 8):2393S–2400S [DOI] [PubMed]
- 44.Waterland RA, Jirtle RL (2003) Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 23:5293–5300 [DOI] [PMC free article] [PubMed]
- 45.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC (2005) Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135:1382–1286 [DOI] [PubMed]
- 46.Gupta R, Misra A (2007) Type 2 diabetes in India: regional disparities. Br J Diabetes Vasc Dis 7:12–16
- 47.Delgado CL, Narrod CA, Tiongco MM (2003) Policy, technical, and environmental determinants and implications of the scaling-up of livestock production in four fast-growing developing Countries: A Synthesis. Submitted to the Food and Agricultural Organization of the United Nations by the International Food Policy Research Institute. Available at www.fao.org/WAIRDOCS/LEAD/x6170e/x6170e00.htm, accessed 10 April 2007
- 48.Ocke MC, Schrijver J, Obermann-de Boer GL, Bloemberg BP, Haenen GR, Kromhout D (1995) Stability of blood (pro)vitamins during four years of storage at −20 degrees C: consequences for epidemiologic research. J Clin Epidemiol 48:1077–1085 [DOI] [PubMed]
- 49.Snow CF (1999) Laboratory diagnosis of vitamin B12 and folate deficiency. A guide for the primary care physician. Arch Intern Med 159:1289–1298 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Figure S1 Suggested metabolic mechanisms for adiposity, insulin resistance and altered gene expression in a situation of dietary vitamin B12 deficiency combined with adequate folate status (PDF 35.7 kb)
Unrotated factor loadings from the PCA (PDF 75.3 kb)