Abstract
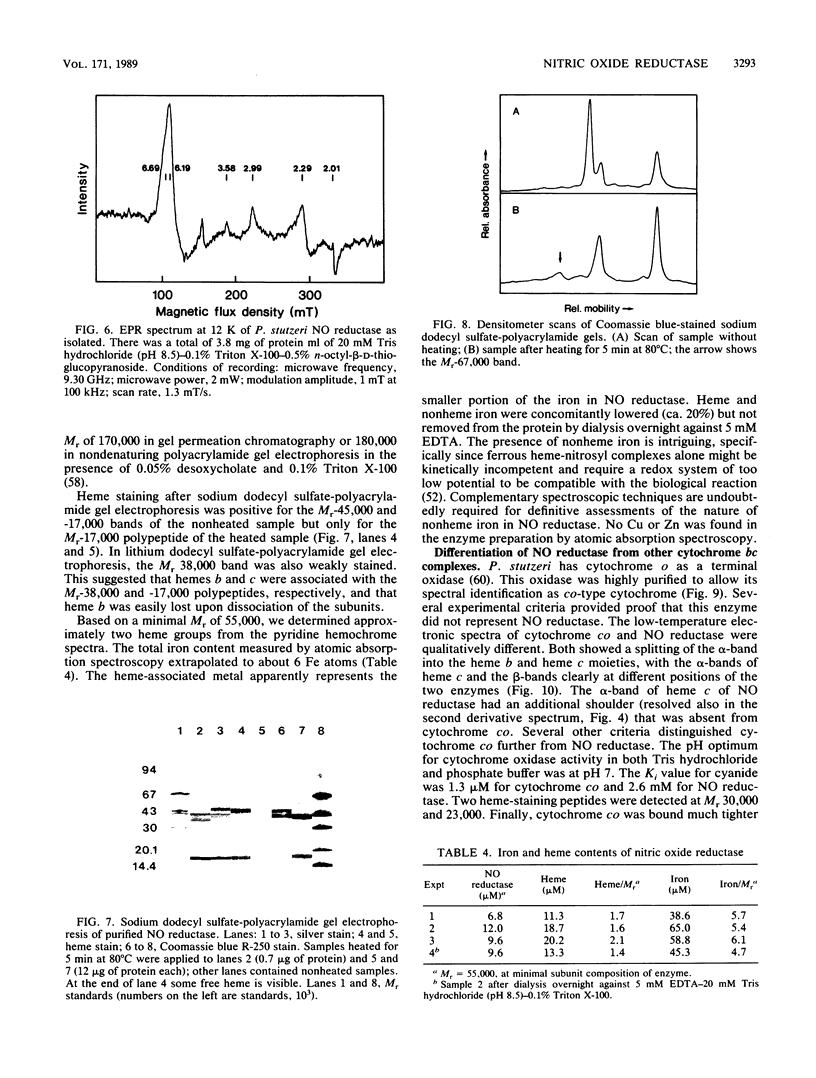
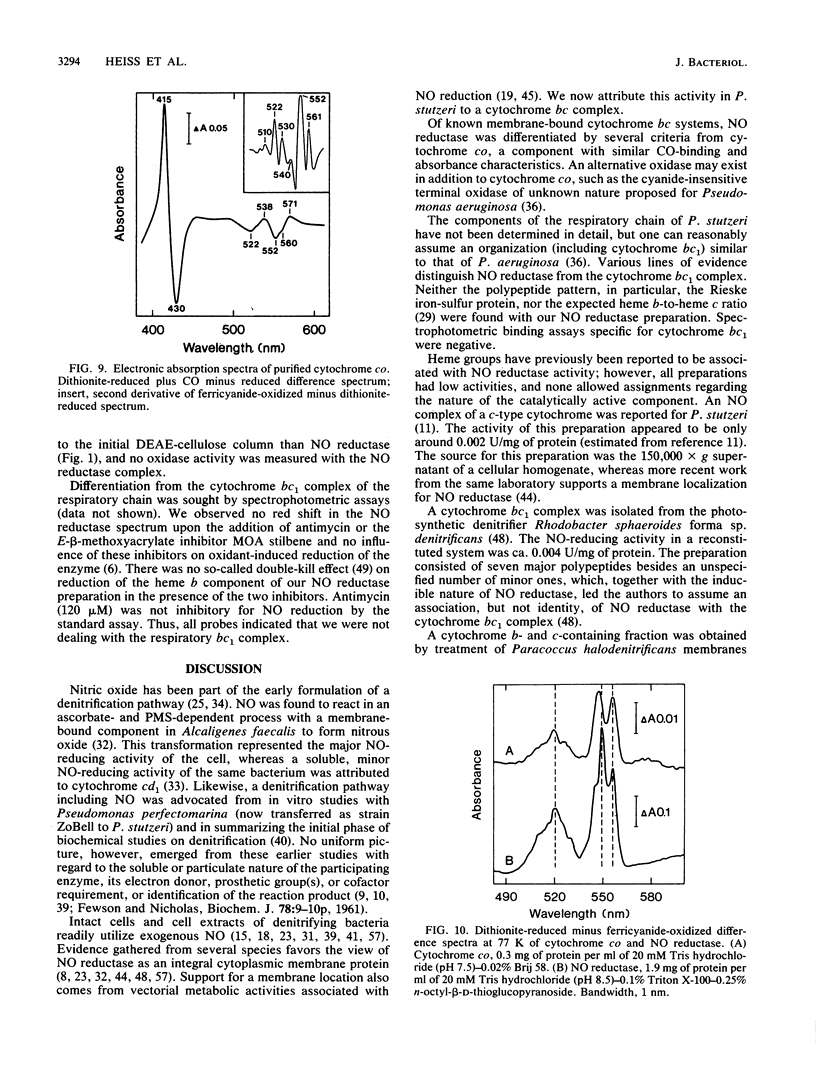
Nitric oxide (NO) reductase was solubilized by Triton X-100 from the membrane fraction of Pseudomonas stutzeri ZoBell and purified 100-fold to apparent electrophoretic homogeneity. The enzyme consisted of two polypeptides of Mr 38,000 and 17,000 associated with heme b and heme c, respectively. Absorption maxima of the reduced complex were at 420.5, 522.5, and 552.5 nm, with a shoulder at 560 nm. The electron paramagnetic resonance spectrum was characteristic of high- and low-spin ferric heme proteins; no signals typical for iron-sulfur proteins were found. Nitric oxide reductase stoichiometrically transformed NO to nitrous oxide in an ascorbate-phenazine methosulfate-dependent reaction with a specific activity of 11.8 mumols/min per mg of protein. The activity increased to 40 mumols upon the addition of soybean phospholipids, n-octyl-beta-D-glucopyranoside, or its thio derivative to the assay system. Apparent Km values for NO and phenazine methosulfate were 60 and 2 microM, respectively. The pH optimum of the reaction was at 4.8. Cytochrome co was purified from P. stutzeri to permit its distinction from NO reductase. Spectrophotometric binding assays and other criteria also differentiated NO reductase from the respiratory cytochrome bc1 complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashani Y., Catravas G. N. Highly reactive impurities in Triton X-100 and Brij 35: partial characterization and removal. Anal Biochem. 1980 Nov 15;109(1):55–62. doi: 10.1016/0003-2697(80)90009-3. [DOI] [PubMed] [Google Scholar]
- Averill B. A., Tiedje J. M. The chemical mechanism of microbial denitrification. FEBS Lett. 1982 Feb 8;138(1):8–12. doi: 10.1016/0014-5793(82)80383-9. [DOI] [PubMed] [Google Scholar]
- Berry E. A., Trumpower B. L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem. 1987 Feb 15;161(1):1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- Bessières P., Henry Y. Stoichiometry of nitrite reduction catalyzed by Pseudomonas aeruginosa nitrite-reductase. Biochimie. 1984 Apr;66(4):313–318. doi: 10.1016/0300-9084(84)90008-7. [DOI] [PubMed] [Google Scholar]
- Brandt U., Schägger H., von Jagow G. Characterisation of binding of the methoxyacrylate inhibitors to mitochondrial cytochrome c reductase. Eur J Biochem. 1988 May 2;173(3):499–506. doi: 10.1111/j.1432-1033.1988.tb14026.x. [DOI] [PubMed] [Google Scholar]
- CHUNG C. W., NAJJAR V. A. Cofactor requirements for enzymatic denitrification. II. Nitric oxide reductase. J Biol Chem. 1956 Feb;218(2):627–632. [PubMed] [Google Scholar]
- Carlson C. A., Ferguson L. P., Ingraham J. L. Properties of dissimilatory nitrate reductase purified from the denitrifier Pseudomonas aeruginosa. J Bacteriol. 1982 Jul;151(1):162–171. doi: 10.1128/jb.151.1.162-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Jr, Payne W. J., Dervartanian D. V. Electron paramagnetic resonance studies on the nature of hemoproteins in nitrite and nitric oxide reduction. Biochim Biophys Acta. 1971 Nov 2;253(1):290–294. doi: 10.1016/0005-2728(71)90256-8. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Jr, Payne W. J. Separation of soluble denitrifying enzymes and cytochromes from Pseudomonas perfectomarinus. Can J Microbiol. 1973 Jul;19(7):861–872. doi: 10.1139/m73-137. [DOI] [PubMed] [Google Scholar]
- Coyle C. L., Zumft W. G., Kroneck P. M., Körner H., Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina. Purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985 Dec 16;153(3):459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Firestone M. K., Firestone R. B., Tiedje J. M. Nitric oxide as an intermediate in denitrification: evidence from nitrogen-13 isotope exchange. Biochem Biophys Res Commun. 1979 Nov 14;91(1):10–16. doi: 10.1016/0006-291x(79)90575-8. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Castignetti D., Hollocher T. C. Proton translocation and proline uptake associated with reduction of nitric oxide by denitrifying Paracoccus denitrificans. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1504–1507. doi: 10.1016/s0006-291x(82)80169-1. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Hollocher T. C. 15N,18O tracer studies on the activation of nitrite by denitrifying bacteria. Nitrite/water-oxygen exchange and nitrosation reactions as indicators of electrophilic catalysis. J Biol Chem. 1982 Jul 25;257(14):8091–8097. [PubMed] [Google Scholar]
- Goretski J., Hollocher T. C. Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J Biol Chem. 1988 Feb 15;263(5):2316–2323. [PubMed] [Google Scholar]
- Görg A., Postel W., Westermeier R., Gianazza E., Righetti P. G. Gel gradient electrophoresis, isoelectric focusing and two-dimensional techniques in horizontal, ultrathin polyacrylamide layers. J Biochem Biophys Methods. 1980 Nov;3(5):273–284. doi: 10.1016/0165-022x(80)90008-1. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I., Tomlinson G. A. The enzymes associated with denitrification. Annu Rev Microbiol. 1988;42:231–261. doi: 10.1146/annurev.mi.42.100188.001311. [DOI] [PubMed] [Google Scholar]
- Kim C. H., Hollocher T. C. 15N tracer studies on the reduction of nitrite by the purified dissimilatory nitrite reductase of Pseudomonas aeruginosa. Evidence for direct production of N2O without free NO as an intermediate. J Biol Chem. 1983 Apr 25;258(8):4861–4863. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ljungdahl P. O., Pennoyer J. D., Robertson D. E., Trumpower B. L. Purification of highly active cytochrome bc1 complexes from phylogenetically diverse species by a single chromatographic procedure. Biochim Biophys Acta. 1987 May 6;891(3):227–241. doi: 10.1016/0005-2728(87)90218-0. [DOI] [PubMed] [Google Scholar]
- Mancinelli R. L., Cronin S., Hochstein L. I. The purification and properties of a cd-cytochrome nitrite reductase from Paracoccus halodenitrificans. Arch Microbiol. 1986;145:202–208. doi: 10.1007/BF00446781. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Iwasaki H. Enzymatic steps of dissimilatory nitrite reduction in Alcaligenes faecalis. J Biochem. 1971 May;69(5):859–868. doi: 10.1093/oxfordjournals.jbchem.a129537. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Iwasaki H. Nitric oxide-reducing activity of Alcaligenes faecalis cytochrome cd. J Biochem. 1972 Jul;72(1):57–64. doi: 10.1093/oxfordjournals.jbchem.a129897. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Mori T. Studies on denitrification. IX. Nitrous oxide, its production and reduction to nitrogen. J Biochem. 1968 Dec;64(6):863–871. doi: 10.1093/oxfordjournals.jbchem.a128968. [DOI] [PubMed] [Google Scholar]
- Matsubara T. Studies on denitrification. 8. Some properties of the N2O-anaerobically grown cell. J Biochem. 1971 Jun;69(6):991–1001. doi: 10.1093/oxfordjournals.jbchem.a129572. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Shinagawa E., Adachi O., Ameyama M. o-Type cytochrome oxidase in the membrane of aerobically grown Pseudomonas aeruginosa. FEBS Lett. 1982 Mar 22;139(2):255–258. doi: 10.1016/0014-5793(82)80864-8. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Yamada M., Shinagawa E., Adachi O., Ameyama M. Membrane-bound respiratory chain of Pseudomonas aeruginosa grown aerobically. A KCN-insensitive alternate oxidase chain and its energetics. J Biochem. 1983 Apr;93(4):1137–1144. doi: 10.1093/oxfordjournals.jbchem.a134239. [DOI] [PubMed] [Google Scholar]
- Miyata M., Matsubara T., Mori T. Studies on denitrification. XI. Some properties of nitric oxide reductase. J Biochem. 1969 Dec;66(6):759–765. doi: 10.1093/oxfordjournals.jbchem.a129205. [DOI] [PubMed] [Google Scholar]
- Miyata M. Studies on denitrification. XIV. The electron donating system in the reduction of nitric oxide and nitrate. J Biochem. 1971 Aug;70(2):205–213. doi: 10.1093/oxfordjournals.jbchem.a129632. [DOI] [PubMed] [Google Scholar]
- Saito S., Tsuchiya T. Characteristics of n-octyl beta-D-thioglucopyranoside, a new non-ionic detergent useful for membrane biochemistry. Biochem J. 1984 Sep 15;222(3):829–832. doi: 10.1042/bj2220829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapleigh J. P., Davies K. J., Payne W. J. Detergent inhibition of nitric-oxide reductase activity. Biochim Biophys Acta. 1987 Feb 25;911(3):334–340. doi: 10.1016/0167-4838(87)90074-4. [DOI] [PubMed] [Google Scholar]
- Shapleigh J. P., Payne W. J. Nitric oxide-dependent proton translocation in various denitrifiers. J Bacteriol. 1985 Sep;163(3):837–840. doi: 10.1128/jb.163.3.837-840.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer G., Kohl D. H. Nitrogen isotopic fractionation and 18O exchange in relation to the mechanism of denitrification of nitrite by Pseudomonas stutzeri. J Biol Chem. 1988 Sep 15;263(26):13231–13245. [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Wharton D. C., Weintraub S. T. Identification of nitric oxide and nitrous oxide as products of nitrite reduction by Pseudomonas cytochrome oxidase (nitrate reductase). Biochem Biophys Res Commun. 1980 Nov 17;97(1):236–242. doi: 10.1016/s0006-291x(80)80159-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Iwasaki H., Shidara S., Suzuki S., Nakahara A., Matsubara T. Nitric oxide complex of cytochrome c' in cells of denitrifying bacteria. J Biochem. 1988 Jun;103(6):1016–1019. doi: 10.1093/oxfordjournals.jbchem.a122372. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Döhler K., Körner H., Löchelt S., Viebrock A., Frunzke K. Defects in cytochrome cd1-dependent nitrite respiration of transposon Tn5-induced mutants from Pseudomonas stutzeri. Arch Microbiol. 1988;149(6):492–498. doi: 10.1007/BF00446750. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Frunzke K. Discrimination of ascorbate-dependent nonenzymatic and enzymatic, membrane-bound reduction of nitric oxide in denitrifying Pseudomonas perfectomarinus. Biochim Biophys Acta. 1982 Sep 15;681(3):459–468. doi: 10.1016/0005-2728(82)90188-8. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Sherr B. F., Payne W. J. A reappraisal of the nitric oxide-binding protein of denitrifying Pseudomonas. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1230–1236. doi: 10.1016/0006-291x(79)91111-2. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Vega J. M. Reduction of nitrite to nitrous oxide by a cytoplasmic membrane fraction from the marine denitrifier Pseudomonas perfectomarinus. Biochim Biophys Acta. 1979 Dec 6;548(3):484–499. doi: 10.1016/0005-2728(79)90060-4. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Engel W. D. Complete inhibition of electron transfer from ubiquinol to cytochrome b by the combined action of antimycin and myxothiazol. FEBS Lett. 1981 Dec 21;136(1):19–24. doi: 10.1016/0014-5793(81)81206-9. [DOI] [PubMed] [Google Scholar]