Abstract
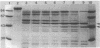
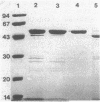
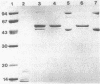
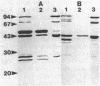
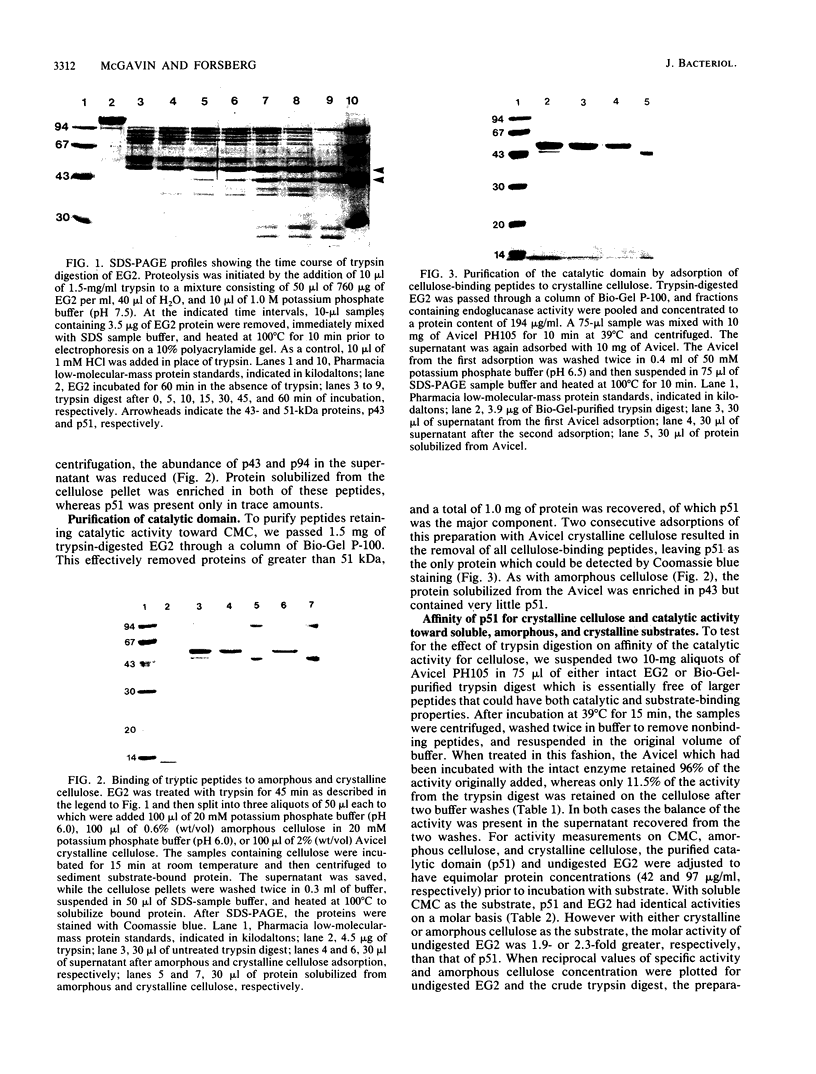
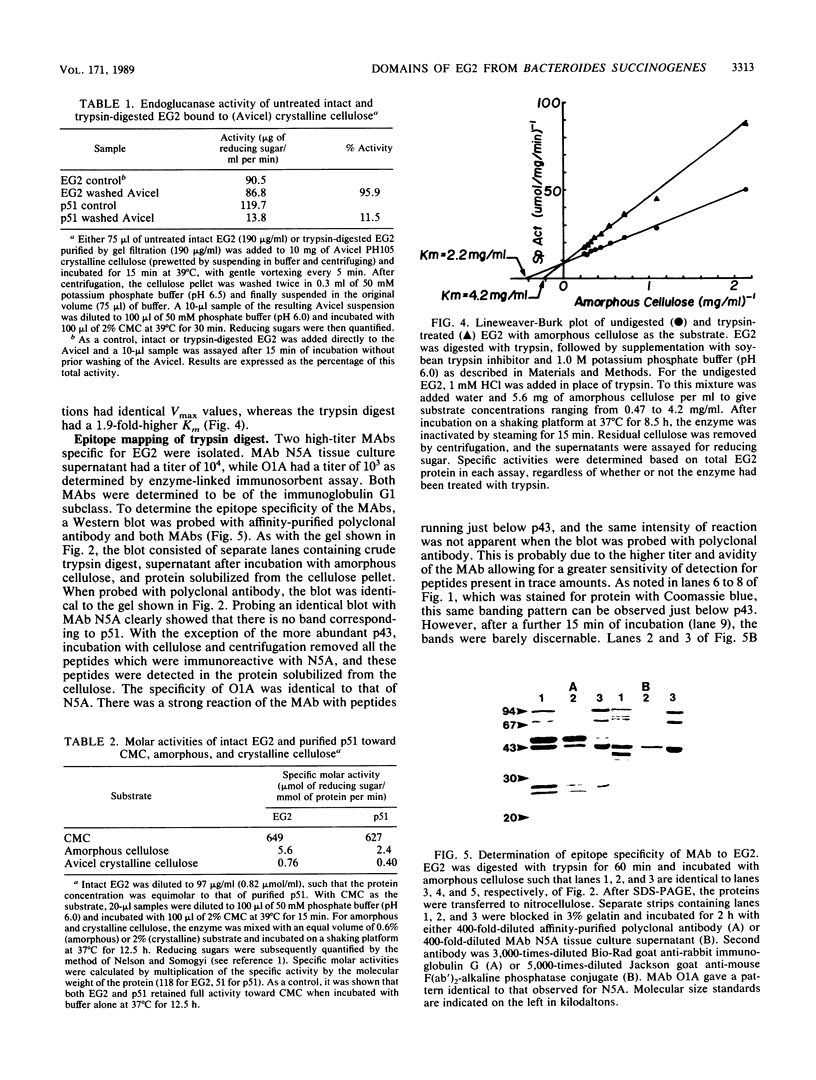
Endoglucanase 2 (EG2) of the cellulolytic ruminal anaerobe Bacteroides succinogenes is a 118-kilodalton (kDa) enzyme which binds to cellulose and produces cellotetraose as the end product of hydrolysis. The purified enzyme was treated with the protease trypsin in an attempt to isolate peptides which retained the ability to either hydrolyze soluble carboxymethyl cellulose or bind to insoluble cellulose. There was no loss in endoglucanase activity (carboxymethylcellulase) over a period of 2 h following the addition of trypsin. In comparison, there was a greater than eightfold reduction in the binding of carboxymethylcellulase activity to crystalline cellulose. A Lineweaver-Burk plot with amorphous cellulose as the substrate revealed that the trypsin-digested enzyme had an identical Vmax but a 1.9-fold-lower Km in comparison with the intact enzyme. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the trypsin-digested enzyme revealed two major peptides of 43 and 51 kDa (p43 and p51). The 43-kDa peptide was able to bind to both amorphous and crystalline cellulose, whereas p51 did not. Purified p51 had a molar activity toward carboxymethyl cellulose which was identical to that of the intact enzyme, but activity toward both amorphous and crystalline cellulose was reduced approximately twofold. Two high-titer monoclonal antibodies from mice immunized with the intact protein recognized p43 but not p51. The results are consistent with a bifunctional organization of EG2, in which the 118-kDa enzyme is composed of a 51-kDa catalytic domain and a highly antigenic 43-kDa substrate-binding domain. In terms of its domain structure and activity toward cellulose, EG2 is very similar to cellobiohydrolase II of Trichoderma reesei.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ghangas G. S., Wilson D. B. Cloning of the Thermomonospora fusca Endoglucanase E2 Gene in Streptomyces lividans: Affinity Purification and Functional Domains of the Cloned Gene Product. Appl Environ Microbiol. 1988 Oct;54(10):2521–2526. doi: 10.1128/aem.54.10.2521-2526.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes N. R., Warren R. A., Miller R. C., Jr, Kilburn D. G. Precise excision of the cellulose binding domains from two Cellulomonas fimi cellulases by a homologous protease and the effect on catalysis. J Biol Chem. 1988 Jul 25;263(21):10401–10407. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y., Duchesne L. G., Southam G. G. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 May;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M., Forsberg C. W. Isolation and characterization of endoglucanases 1 and 2 from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2914–2922. doi: 10.1128/jb.170.7.2914-2922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomme P., Van Tilbeurgh H., Pettersson G., Van Damme J., Vandekerckhove J., Knowles J., Teeri T., Claeyssens M. Studies of the cellulolytic system of Trichoderma reesei QM 9414. Analysis of domain function in two cellobiohydrolases by limited proteolysis. Eur J Biochem. 1988 Jan 4;170(3):575–581. doi: 10.1111/j.1432-1033.1988.tb13736.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]