Abstract
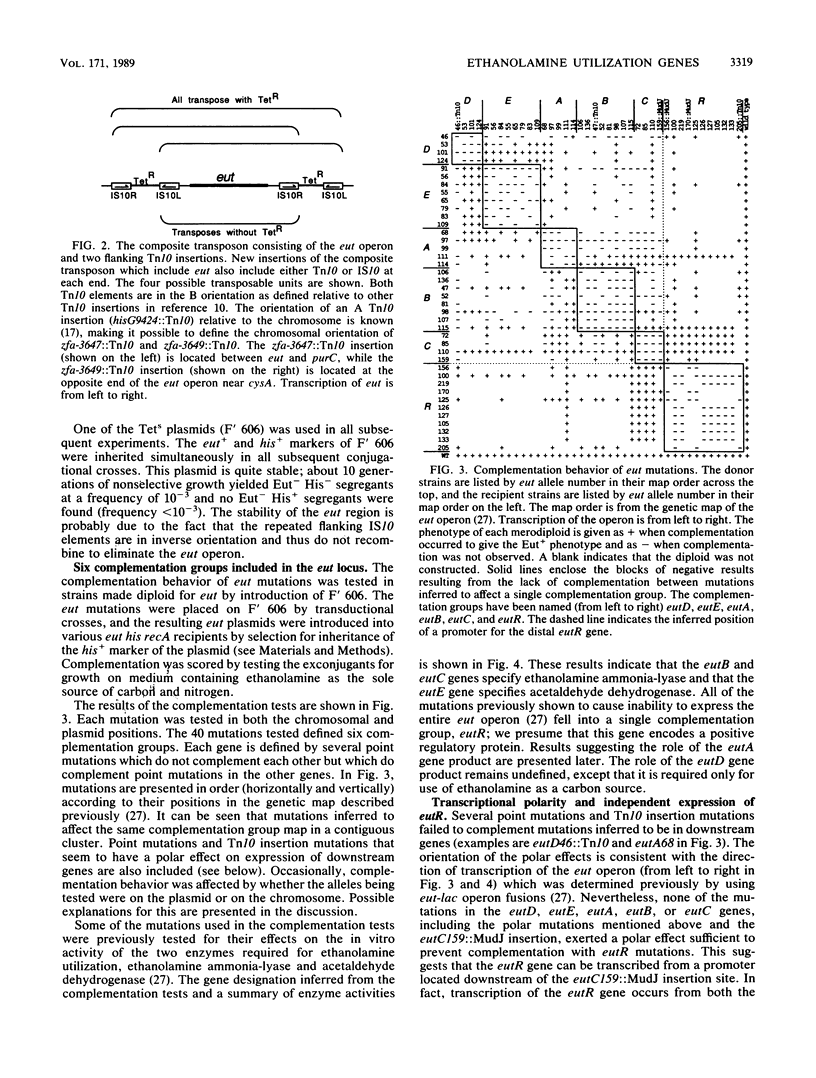
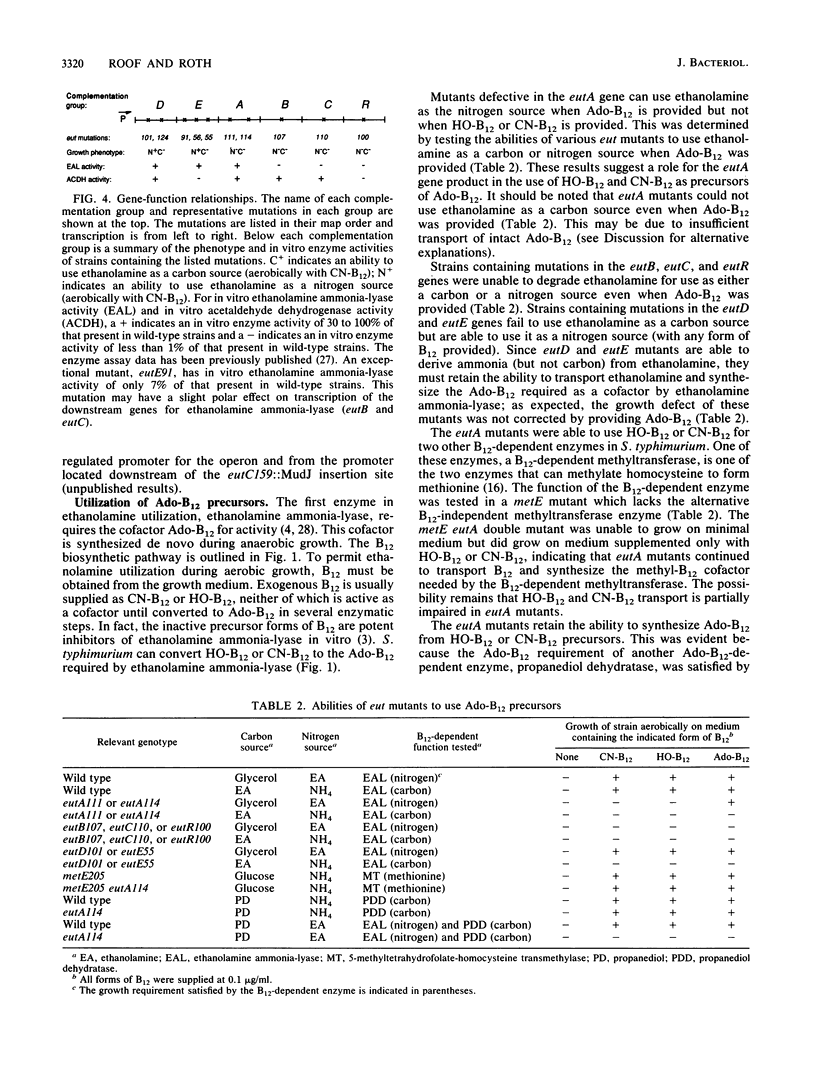
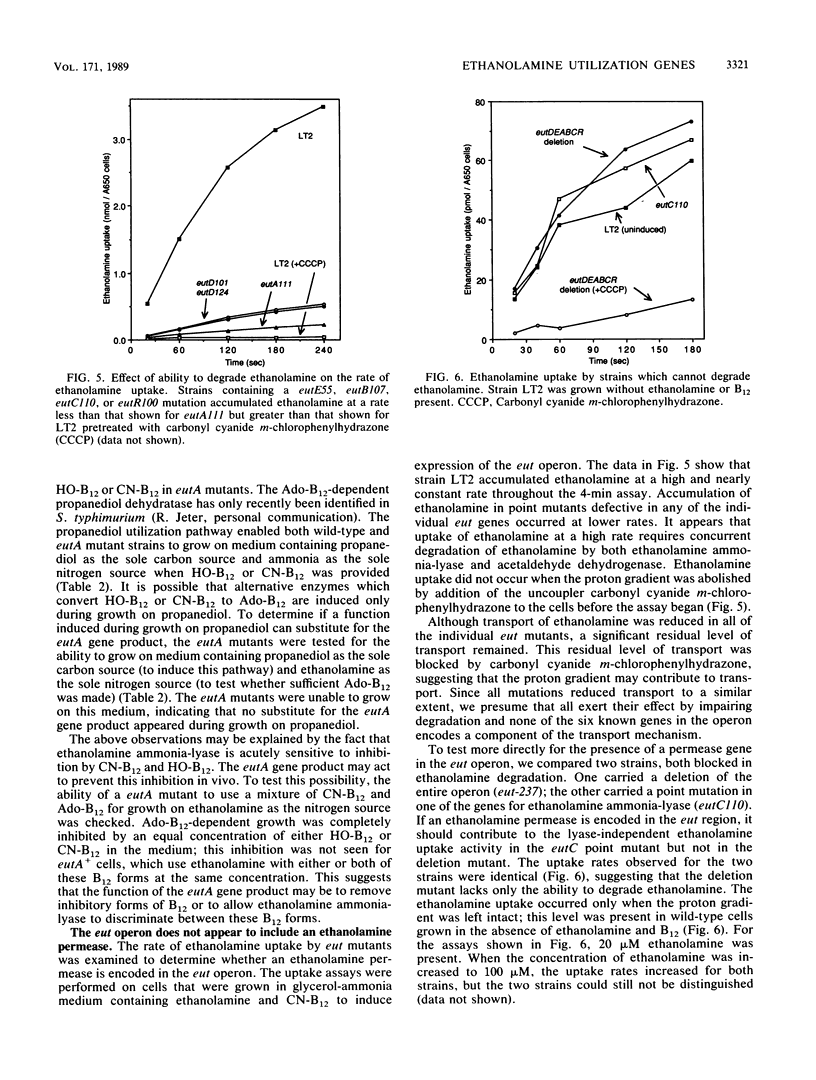
When B12 is available, Salmonella typhimurium can degrade ethanolamine to provide a source of carbon and nitrogen. B12 is essential since it is a cofactor for ethanolamine ammonia-lyase, the first enzyme in ethanolamine breakdown. S. typhimurium makes B12 only under anaerobic conditions; in the presence of oxygen, exogenous B12 must be provided to permit ethanolamine utilization. Genes required for ethanolamine utilization are encoded in the eut operon. For complementation testing, an F' plasmid containing the eut genes was constructed by transposition of the eut operon (flanked by two Tn10 elements) to an existing F plasmid. Complementation tests defined six genes in the eut operon. Three of these genes encode enzymes known to be involved in degradation of ethanolamine: ethanolamine ammonia-lyase (eutB and eutC) and acetaldehyde dehydrogenase (eutE). One gene (eutR) seems to encode a positive regulatory protein required for induction of transcription of eut. The function of one of the remaining two genes (eutA) was shown to be required for ethanolamine utilization only when cyano-B12 or hydroxy-B12 were the precursors of the adenosyl-B12 cofactor of ethanolamine ammonia-lyase; eutA mutants could use ethanolamine as the nitrogen source only when adenosyl-B12 was provided. No function has been assigned to the eutD gene, which is required for use of ethanolamine as a carbon source. Ethanolamine uptake assays of eut mutants suggest that no ethanolamine permease is encoded in the eut operon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADY R. O., CASTANERA E. G., BARKER H. A. The enzymatic synthesis of cobamide coenzymes. J Biol Chem. 1962 Jul;237:2325–2332. [PubMed] [Google Scholar]
- Babior B. M., Carty T. J., Abeles R. H. The mechanism of action of ethanolamine ammonia-lyase, a B12-dependent enzyme. The reversible formation of 5'-deoxyadenosine from adenosylcobalamin during the catalytic process. J Biol Chem. 1974 Mar 25;249(6):1689–1695. [PubMed] [Google Scholar]
- Baker J. J., van der Drift C., Stadtman T. C. Purification and properties of -lysine mutase, a pyridoxal phosphate and B 12 coenzyme dependent enzyme. Biochemistry. 1973 Mar 13;12(6):1054–1063. doi: 10.1021/bi00730a006. [DOI] [PubMed] [Google Scholar]
- Blackwell C. M., Turner J. M. Microbial metabolism of amino alcohols. Purification and properties of coenzyme B12-dependent ethanolamine ammonia-lyase of Escherichia coli. Biochem J. 1978 Nov 1;175(2):555–563. doi: 10.1042/bj1750555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Chang G. W., Chang J. T. Evidence for the B12-dependent enzyme ethanolamine deaminase in Salmonella. Nature. 1975 Mar 13;254(5496):150–151. doi: 10.1038/254150a0. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Roth J. R. Characterization of Tn10d-Cam: a transposition-defective Tn10 specifying chloramphenicol resistance. Mol Gen Genet. 1988 Aug;213(2-3):332–338. doi: 10.1007/BF00339599. [DOI] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Roth J. R. Regulation of cobalamin biosynthetic operons in Salmonella typhimurium. J Bacteriol. 1987 May;169(5):2251–2258. doi: 10.1128/jb.169.5.2251-2258.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M. A., Tejerina G., Guest J. R., Woods D. D. Two enzymic mechanisms for the methylation of homocysteine by extracts of Escherichia coli. Biochem J. 1964 Sep;92(3):476–488. doi: 10.1042/bj0920476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Davis M. A., Roberts D. E., Takeshita K., Kleckner N. Genetic organization of transposon Tn10. Cell. 1981 Jan;23(1):201–213. doi: 10.1016/0092-8674(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Frey B., McCloskey J., Kersten W., Kersten H. New function of vitamin B12: cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 May;170(5):2078–2082. doi: 10.1128/jb.170.5.2078-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. P., Isberg R. R., Syvanen M., Silhavy T. J. Conferral of transposable properties to a chromosomal gene in Escherichia coli. J Mol Biol. 1980 Aug 15;141(3):235–248. doi: 10.1016/0022-2836(80)90179-5. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter R. M., Olivera B. M., Roth J. R. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol. 1984 Jul;159(1):206–213. doi: 10.1128/jb.159.1.206-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. W., Turner J. M. Interrelationships between the enzymes of ethanolamine metabolism in Escherichia coli. J Gen Microbiol. 1984 Feb;130(2):299–308. doi: 10.1099/00221287-130-2-299. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Altered cobalamin metabolism in Escherichia coli btuR mutants affects btuB gene regulation. J Bacteriol. 1989 Jan;171(1):154–161. doi: 10.1128/jb.171.1.154-161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Roth J. Cluster of genes controlling proline degradation in Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):744–754. doi: 10.1128/jb.133.2.744-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof D. M., Roth J. R. Ethanolamine utilization in Salmonella typhimurium. J Bacteriol. 1988 Sep;170(9):3855–3863. doi: 10.1128/jb.170.9.3855-3863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett F. A., Turner J. M. Microbial metabolism of amino alcohols. Ethanolamine catabolism mediated by coenzyme B12-dependent ethanolamine ammonia-lyase in Escherichia coli and Klebsiella aerogenes. J Gen Microbiol. 1976 Jul;95(1):173–176. doi: 10.1099/00221287-95-1-173. [DOI] [PubMed] [Google Scholar]
- Schmieger H. A method for detection of phage mutants with altered transducing ability. Mol Gen Genet. 1971;110(4):378–381. doi: 10.1007/BF00438281. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vitols E., Walker G. A., Huennekens F. M. Enzymatic conversion of vitamin B-12s to a cobamide coenzyme, alpha-(5,6-dimethylbenzimidazolyl)deoxyadenosylcobamide (adenosyl-B-12). J Biol Chem. 1966 Apr 10;241(7):1455–1461. [PubMed] [Google Scholar]
- Walker G. A., Murphy S., Huennekens F. M. Enzymatic conversion of vitamin B 12a to adenosyl-B 12: evidence for the existence of two separate reducing systems. Arch Biochem Biophys. 1969 Oct;134(1):95–102. doi: 10.1016/0003-9861(69)90255-0. [DOI] [PubMed] [Google Scholar]
- Wallis O. C., Johnson A. W., Lappert M. F. Studies on the subunit structure of the adenosylcobalamin-dependent enzyme ethanolamine ammonia-lyase. FEBS Lett. 1979 Jan 1;97(1):196–199. doi: 10.1016/0014-5793(79)80083-6. [DOI] [PubMed] [Google Scholar]
- Whitfield C. D., Steers E. J., Jr, Weissbach H. Purification and properties of 5-methyltetrahydropteroyltriglutamate-homocysteine transmethylase. J Biol Chem. 1970 Jan 25;245(2):390–401. [PubMed] [Google Scholar]
- de Veaux L. C., Clevenson D. S., Bradbeer C., Kadner R. J. Identification of the btuCED polypeptides and evidence for their role in vitamin B12 transport in Escherichia coli. J Bacteriol. 1986 Sep;167(3):920–927. doi: 10.1128/jb.167.3.920-927.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


