Abstract
The transcription factor FNR (fumarate nitrate reduction) requires the presence of an iron-sulfur (Fe-S) cluster for its function as a global transcription regulator in Escherichia coli when oxygen becomes scarce. To define the oxidation state and type of Fe-S cluster present in the active form of FNR, we have studied anaerobically purified FNR with Mössbauer spectroscopy. Our data showed that this form of FNR contained a [4Fe-4S]2+ cluster (δ = 0.45 mm/s; ΔEQ = 1.22 mm/s) and that the [4Fe-4S]2+ cluster was rapidly destroyed on exposure of FNR to air. Under these conditions, the yellow–green active form of FNR turned deep red; analysis of sulfide indicated that 70% of the labile sulfide was still present, suggesting that the Fe-S cluster had been converted into a different form. Little [3Fe-4S] cluster was, however, detected by EPR. According to Mössbauer spectroscopy, the [4Fe-4S]2+ cluster was converted in about 60% yield to a [2Fe-2S]2+ cluster (δ = 0.28 mm/s; ΔEQ = 0.58 mm/s) following 17 min of exposure to air. The [2Fe-2S]2+ cluster form of FNR was much more stable to oxygen, but was unable to sustain biological activity (e.g., DNA binding). However, DNA binding and the absorption spectrum characteristic of the [4Fe-4S]2+ cluster could be largely restored from the [2Fe-2S]2+ form when Cys, Fe, DTT, and the NifS protein were added. It has yet to be determined whether the form of FNR containing the [2Fe-2S]2+ cluster has any biological significance, e.g., as an in vivo intermediate that is more rapidly converted to the active form than the apoprotein.
Keywords: transcriptional control, anaerobic metabolism, labile sulfide, oxygen sensing, Mössbauer spectra
The ability to adapt to changes in oxygen concentrations in the environment is common to many organisms. In the facultative anaerobe, Escherichia coli, the transcription factor FNR (fumarate nitrate reduction) regulates a network of genes that facilitates adaptation to oxygen deprivation by providing alternative pathways for energy generation (1). Recent data suggest that FNR contains a [4Fe-4S] cluster (2–4) and that this cluster apparently mediates the sensitivity of this transcription factor to oxygen, thus limiting FNR activity to anaerobic conditions. The stoichiometry of iron and labile sulfide relative to the number of cysteine ligands of anaerobically purified FNR is most compatible with the presence of a [4Fe-4S] cluster (3). Iron and sulfide analyses and CD spectra of FNR preparations derived from reconstitution of a cluster into apoprotein also supports this cluster assignment (4). The presence of the Fe-S cluster in the anaerobically purified form of FNR is correlated with an increase in dimerization and specific DNA binding (3), compared with the aerobically purified form that lacks an Fe-S cluster (2, 5, 6). Furthermore, the Fe-S cluster is disrupted by oxygen, and this is correlated with the conversion of FNR into an inactive monomeric protein (3). The loss of this cluster as well as the loss of specific DNA binding upon exposure of FNR to oxygen suggested that the [4Fe-4S] cluster, through its intrinsic instability, serves as an oxygen sensor.
This paper reports observations on the path of disassembly of the [4Fe-4S] cluster of FNR in vitro. Little is known about the cluster disassembly mechanism of proteins in the absence of chelators, detergents, chemical oxidants, and other nonphysiological agents. The rapid destruction of the Fe-S cluster of FNR, simply upon exposure of a purified protein solution to air, offers an opportunity to obtain information on the progress and mode of the disassembly of the [4Fe-4S] cluster of this protein. We have used chemical analysis for iron and sulfide as well as electronic, EPR, and Mössbauer spectroscopies to monitor the disassembly process. We report here the unexpected observation that the [4Fe-4S] cluster of FNR is converted in less than 5 min to a [2Fe-2S] cluster in about 60% yield as judged from Mössbauer spectra and this conversion results in a reduction in DNA-binding ability. The [4Fe-4S]2+ cluster can be regenerated from the [2Fe-2S] cluster or its components by reduction with dithionite.
MATERIALS AND METHODS
Cell Growth Conditions and Protein Purification.
FNR was purified from an E. coli B derivative, PK22 (crp-bs990 and Δfnr), carrying fnr under control of the inducible T7 promoter on the plasmid pPK823 (6). For purification of the 57Fe-containing form of FNR for Mössbauer spectroscopy, the inoculum was prepared by centrifuging 10 ml of overnight culture, grown in Luria broth (7), containing 0.2% glucose and 50 μg/ml ampicillin, at 5,000 × g for 5 min. The cell pellet was then added to 1 liter of the 57Fe-enriched M9 minimal medium (7) prepared as follows: The M9 salts solution was passed through a Chelex 100 (200–400 mesh) column (40 g of resin per 1 liter of medium), which eliminated naturally abundant 56Fe from the medium as shown by inductively coupled plasma atomic emission spectroscopy. 57Fe (95.9% abundance) was added to the medium to a final concentration of 20 μM as ferrous ethylenediammonium sulfate (brought to pH ≈3 with NaOH). The following reagent grade compounds were also added at the indicated final concentrations: 0.1 mM CaCl2, 1.0 mM MgSO4, 0.2% casamino acid, 0.2% glucose, 4 μg/ml vitamin B1, and 50 μg/ml ampicillin. When cultures enriched in 57Fe or naturally abundant Fe reached an OD600 of 0.35–0.60, FNR synthesis was induced as described (3). Following induction of FNR synthesis, cell cultures were sparged with argon at 4°C for 14 hr, which appeared to improve recovery of FNR as compared with our previous results. Purification of FNR from cell extracts was carried out under anaerobic conditions as described (3) except that DTT was added to the purification buffer to a final concentration of 1 mM. To minimize any exposure to oxygen, samples were manipulated either under a stream of copper scrubbed-argon gas or in an anaerobic chamber (Coy), which had an atmosphere of 5% CO2/5% H2/90% N2.
FNR prepared by this method contained Fe and S2− as previously observed (3). For FNR prepared with naturally abundant iron, a maximal [4Fe-4S] cluster content of 0.50–0.58 mol per mol of FNR monomer was obtained, whereas the samples labeled with 57Fe contained 0.76 and 0.59 mol [4Fe-4S] cluster per mol of FNR monomer. The Fe/S2− ratios were between 1.1 and 1.2, indicating the presence of some noncluster iron. Inductively coupled plasma atomic emission spectroscopy showed that no other metals were present to any significant extent in these FNR preparations.
Iron, Sulfide, and Protein Determinations.
Protein concentrations were determined as in ref. 2. In conjunction with Fe and S2− determinations, protein was determined by a biuret procedure, preceded by a trichloroacetic acid precipitation when interfering substances were present. In both cases, the protein determinations were standardized by amino acid analysis. Iron, sulfide, and S0 were determined as described in refs. 8–10, respectively. In general, the finding of sulfide together with iron is a reasonable indicator for the presence of protein-bound Fe-S clusters, since sulfide is an unlikely contaminant. This is, however, not the case when a reaction can occur in which “free” iron sulfides (Fe-S) are formed; this has been observed before on rare occasions (11). Moreover, these iron sulfides exist in a colloidal state and are not readily separated from protein, e.g., by passage through a Sephadex column. This will be a consideration in experiments to be reported below.
Spectroscopy.
Dithionite was removed from protein samples and UV-visible and EPR spectra were recorded as described (2, 3). Cuvettes were filled in an anaerobic chamber and sealed with Teflon caps. Photoreduction of EPR samples was carried out anaerobically with a Xenon arc in the presence of deazaflavin and oxalate (12).
DNA-Binding Assay.
Site-specific DNA binding of FNR was measured under anaerobic conditions using a gel-retardation assay as described (3) except that the DNA-binding reaction mixture also included 1 mM DTT (Sigma). The fraction of radioactively labeled DNA bound over a range of protein concentrations was quantified by use of a PhosphorImager (Molecular Dynamics). The amount of protein required to bind 50% of the maximally bound target DNA, termed S0.5, was calculated as described (2).
Oxygen-Induced Degradation of the Fe-S Cluster.
Typically, a 1-ml sample of anaerobically prepared FNR (5.0 mg/ml) was injected from a gas-tight syringe into a flat 1-ml dish and stirred in air at room temperature. Samples were withdrawn at desired times and frozen in liquid N2 for EPR or, for the DNA-binding assays, injected into an anaerobically sealed vial, the head space of which was additionally evacuated and flushed with argon. It was ascertained that samples so prepared did not further change their DNA-binding ability for up to 4 h. Samples for sulfide determination were rapidly quenched with zinc acetate/NaOH (9) and then further processed.
Reconstitution of the [4Fe-4S] Cluster After Oxidation of FNR.
FNR was oxidized by exposure of a 250 μl protein sample (5 mg/ml) to air for 15 min and the absorption spectrum of this sample was recorded. The sample was then made anaerobic and placed in a sealed cuvette containing 750 μl of 50 mM Tris⋅HCl buffer (pH 7.5), 1 mM cysteine, 2 mM ferrous ammonium sulfate, and 2.5 mM DTT. Reconstitution was initiated by addition of 1.0 μl of NifS protein (4.5 mg/ml). NifS protein from Azotobacter vinelandii (13) was kindly provided by Dennis R. Dean (Virginia Polytechnic Institute and State University, Blacksburg). For DNA-binding assays, reconstituted FNR was separated from the reaction mixture by passage through a spun column (Sephadex G-25) under anaerobic conditions. Alternatively, regeneration of the [4Fe-4S]2+ cluster was observed by Mössbauer spectroscopy after reducing the air-oxidized sample (17 min) under anaerobic conditions with dithionite (pH 9.0), in the presence of methylviologen.
RESULTS
Spectroscopic Observations on Anaerobically Purified FNR.
EPR spectroscopy. Previous determinations of Fe and S2− stoichiometries of anaerobically purified FNR (3) relative to the number of cysteine residues have suggested that the most likely type of cluster present in anaerobic preparations of FNR is a [4Fe-4S]2+ cluster. Since the [4Fe-4S]2+ cluster is diamagnetic, EPR can only be used for its 1 e− reduced state or when a [3Fe-4S]+ cluster is obtained from the [4Fe-4S]2+ cluster by oxidation. In photoreduced samples, a broad signal appeared centered around g ≈ 5 at high microwave power and liquid helium temperature indicating the presence of a [4Fe-4S]+ cluster in the S = 3/2 state. The [4Fe-4S]2+ cluster of FNR was not reduced by dithionite, even at pH 9 and in the presence of methylviologen, indicating a very low redox potential.
Mössbauer spectroscopy.
The 4.2 K Mössbauer spectrum of FNR, recorded in the absence of an applied magnetic field (Fig. 1A) exhibits a doublet with quadrupole splitting ΔEQ = 1.22(2) mm/s, and isomer shift, δ = 0.45(1) mm/s. These parameters are typical for [4Fe-4S]2+ clusters. The 8.0 T spectrum of the same sample (not shown) exhibited magnetic splittings that could be attributed solely to the applied field. Thus, the electronic ground state of the system is diamagnetic. Among the commonly occurring Fe-S clusters with diamagnetic ground states are cysteine-ligated clusters with [4Fe-4S]2+ and [2Fe-2S]2+ core oxidation states; the former typically exhibit, to within ± 0.03 mm/s, δ values around 0.45 mm/s, whereas the latter have δ ≈ 0.27 mm/s (14). Thus, our data show that anaerobically purified FNR contains a [4Fe-4S]2+ cluster.
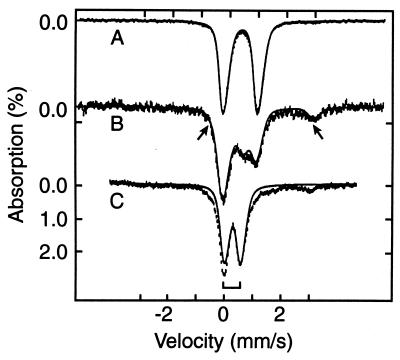
Figure 1.
The 4.2 K Mössbauer spectra of anaerobically isolated FNR (A), and FNR exposed to oxygen for 2 min (B) and 17 min (C). The solid line in A is a doublet for [4Fe-4S]2+ simulated with ΔEQ = 1.22 mm/s and δ = 0.45 mm/s. The solid line in C is a simulated spectrum using one doublet with ΔEQ = 0.58 mm/s and δ = 0.28 mm/s for the [2Fe-2S]2+ cluster (bracket). The solid line drawn through the data in B is a superposition of the theoretical curves for the [4Fe-4S]2+ cluster and the [2Fe-2S]2+ cluster, plus a contribution (arrows) from the Fe2+ component. The relative proportions of the various species are listed in Table 1.
Oxygen-Induced Degradation of the Fe-S Cluster in FNR.
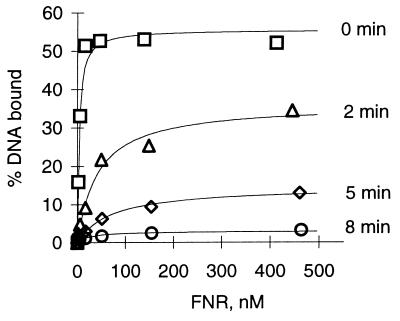
DNA binding. Previously, we observed that brief exposure of anaerobic FNR preparations to air caused a decrease in both specific DNA binding and the absorption spectrum characteristic of a [4Fe-4S] cluster (3). To provide further evidence that the decrease in specific DNA binding is a consequence of a loss of the [4Fe-4S] cluster, a time course of the oxygen-induced inactivation of FNR was first established by measuring the amount of specific DNA binding as a function of time of FNR incubation in air (Fig. 2). For anaerobic preparations of FNR, the amount of protein required to bind 50% of the maximally bound DNA, (S0.5), was estimated to be 4–8 nM. The maximal amount of target DNA that was specifically bound by anaerobically purified FNR using these solution conditions was typically between 50 and 70%; higher protein concentrations led to the formation of nonspecific protein–DNA complexes (15), which were not quantified in this study. DNA binding by FNR decreased significantly within the first 2 min of exposure to air, such that only 36% of DNA was bound with an apparent affinity (S0.5) of 41 nM. Exposure of FNR to air for 8 min nearly abolished site-specific DNA binding.
Figure 2.
Effect of oxygen on DNA binding by FNR. Assays for DNA binding were carried out under anaerobic conditions and quantified by measuring the percentage of the 48-bp DNA fragment bound by FNR as a function of protein concentration. Anaerobic protein (0 min) was exposed to air for 2, 5, and 8 min as described, prior to evacuating and flushing with argon gas.
Spectroscopy.
To correlate DNA-binding ability with the amount of the Fe-S cluster present in FNR, we examined changes in the Fe-S cluster content after exposure of the protein to air by UV-visible, EPR, and Mössbauer spectroscopies and by analyses for Fe, inorganic sulfide, and protein.
UV-visible spectroscopy.
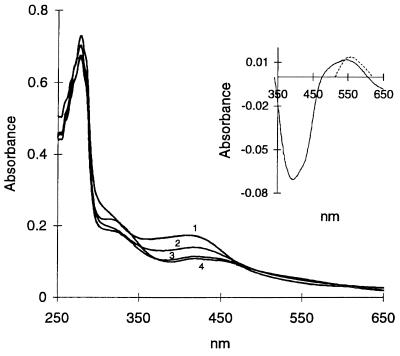
As described previously (3), the absorption spectrum of anaerobically purified FNR showed a maximum at 278 nm, a shoulder at 315 nm, and a broad maximum at 409 nm. After only 1 min of reaction of FNR with air, the A409 decreased by approximately 25% (Fig. 3). By 15 min, the A409 decreased to ≈50% of the original intensity with no further change observed for up to at least 1 h (Fig. 3). Since the maximum at 409 nm is typical of [4Fe-4S]2+ containing proteins, the decrease in the absorption at this wavelength indicated a loss in this form of the cluster. In addition to the decrease in A409 in the air-treated protein, two new maxima were observed at 423 nm and 550 nm (Fig. 3), imparting a distinct red color to the samples and suggesting formation of a new cluster species. These two maxima were not detected in our previous study, presumably due to the lower amounts of cluster analyzed (3).
Figure 3.
Absorption spectra of wild-type FNR before and after exposure to air. The spectrum of FNR (1.0 mg/ml) was recorded under anaerobic conditions (1) and after uncapping and mixing the cuvette contents with air for 1 min (2), 10 min (3), and 60 min (4). (Inset) Shows the difference spectrum of oxygen-exposed FNR (60 min) minus anaerobic FNR protein (solid line) and the difference spectrum of a [2Fe-2S] model cluster minus that of a [4Fe-4S] model cluster (broken line) replotted from Fig. 2 of ref. 16 in arbitrary units.
EPR spectroscopy.
The EPR spectra of FNR after exposure to air (1, 3, and 10 min, data not shown) revealed that about 5% of the [4Fe-4S]2+ clusters had been converted to [3Fe-4S] clusters. On adding stoichiometric and then increasing quantities of ferricyanide, the amount of the 3Fe form rose to only 8% of the total cluster content of the sample before oxidation and then dropped to zero upon prolonged exposure of FNR to air or on addition of excess amounts of ferricyanide. However, in protein preparations exposed to air for at least 24 h at room temperature, inorganic sulfide was still present and the typical red color persisted and then slowly declined. This suggested that the [4Fe-4S]2+ cluster in wild-type FNR had been converted to a considerable extent to a different form. When samples of wild-type FNR were exposed to air, then made anaerobic and reduced by photoreduction as above, no EPR signals were detected.
Mössbauer spectroscopy.
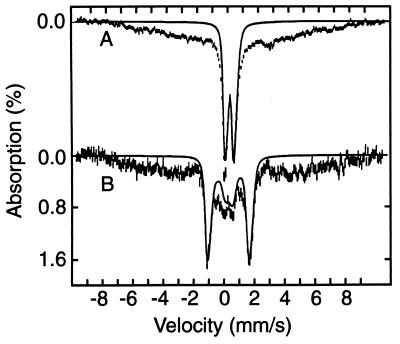
A sample of anaerobically isolated FNR was exposed to air for 2 min and studied with Mössbauer spectroscopy (Fig. 1B). Subsequently, the sample was thawed and exposed to air, under stirring, for an additional 15 min (Fig. 1C). Comparison of Fig. 1 A and C shows that the doublet assigned to the [4Fe-4S]2+ cluster has largely disappeared in the 17-min oxygen-exposed sample. In its place, two new spectral components appeared at 4.2 K (Fig. 4A): a broad unresolved magnetic component ranging from −8 mm/s to +8 mm/s Doppler velocity and a well-defined doublet. The broad magnetic features suggest a heterogeneous mixture of species, originating from Fe3+ released from the [4Fe-4S]2+ cluster. At 100 K, the major fraction of the iron of this spectral component contributes a doublet with ΔEQ ≈ 0.7 mm/s and δ ≈ 0.5 mm/s, indicating octahedral high-spin Fe3+ with nitrogen/oxygen coordination. However, we cannot rule out the possibility that this mixture of species contains some iron sulfide complexes.
Figure 4.
Mössbauer spectra of FNR exposed for 17 min to oxygen, recorded at 4.2 K in zero field (A) and a field of 8.0 T applied parallel to the observed γ radiation. The solid line in A is a doublet outlining the contribution of the [2Fe-2S]2+ cluster. The solid line in B is a theoretical spectrum computed with values of ΔEQ and δ obtained in zero field with the assumption that both (equivalent) Fe sites of the cluster reside in a diamagnetic environment. Theoretical curves are drawn to emphasize hyperfine splittings; they do not represent correct spectral areas.
The second component in the spectrum of Fig. 4A consists of a well-defined doublet (indicated by the bracket in Fig. 1C) with ΔEQ = 0.58(2) mm/s and δ = 0.28(1) mm/s. The 8.0 T spectrum of Fig. 4B shows (solid line) that this component is diamagnetic. The values of ΔEQ and δ are indicative of a high-spin ferric site with a (distorted) tetrahedral thiolate coordination. Such sites are observed for Fe3+ rubredoxin, (oxidized) ferredoxins containing a [2Fe-2S]2+ cluster, and for proteins containing an (oxidized) [3Fe-4S]1+ cluster. However, because the iron sites of monomeric Fe3+S4 and of [3Fe-4S]1+ clusters are paramagnetic, their spectra would exhibit substantial magnetic hyperfine interactions under the conditions of Fig. 4B, in contrast with our experimental evidence. This suggests that the diamagnetic spectral component belongs to a [2Fe-2S]2+ cluster formed during the exposure of FNR to oxygen. This is supported by the values of ΔEQ and δ, which match those observed for protein-bound [2Fe-2S]2+ clusters (14) and is in agreement with our observations with UV/visible spectroscopy, where the oxidized protein showed characteristics of [2Fe-2S] clusters (Fig. 3 Inset, ref. 16). The Mössbauer spectra of the native protein and of a 20-min air-exposed sample, prepared from a different batch of FNR, were identical with those shown here.¶
Fig. 1B shows a spectrum of FNR that had been exposed to air for 2 min. This spectrum can be represented quite well as a superposition of three spectral components, namely a contribution from the native [4Fe-4S]2+ cluster, a contribution from [2Fe-2S]2+, and one (or two) high-spin Fe2+ species with ΔEQ ≈ 3.2–3.4 mm/s and δ ≈ 1.3 mm/s. The latter accounts for ≈10% of total Fe and has parameters typical of octahedral complexes with nitrogen/oxygen coordination; most probably it represents Fe2+ released by cluster destruction. The theoretical curve in Fig. 1B was generated by adding the theoretical curves of Fig. 1 A (38%) and C (31%) together with a simulation of the Fe2+ species (10%). The amounts of these various Fe-containing species are summarized in Table 1 and demonstrate that the decrease in DNA binding observed upon exposure of FNR to air (Fig. 2) is correlated very well with the decline in the [4Fe-4S]2+ cluster.
Table 1.
Percentage of total Fe assigned to observed species
| Sample | [2Fe-2S]2+ | [4Fe-4S]2+ | Fe2+ | Fe3+ |
|---|---|---|---|---|
| As isolated | 0 | 100 | 0 | 0 |
| 2 min | 31 (3) | 38 (3) | 10 (2) | ≈20 |
| 17 min | 33 (2) | 5* | 4 | ≈60 |
Because the Fe3+ compounds exhibit broad and unresolved spectra at 4.2 K, the fractions of Fe associated with these compounds are difficult to determine. However, the relative percentages of the other three species can be determined reasonably well from the spectra in Fig. 1. Numbers in parentheses give the estimated uncertainties.
The spectral features suggest, but do not prove, the presence of a small amount of [4Fe-4S]2+ cluster.
Chemical analysis.
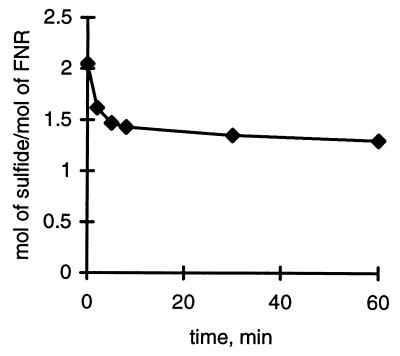
The data in Fig. 5 show that ≈70% of the labile S2− was still present after 5 min of exposure to air, whereas no further decrease in sulfide was observed for the next 30 min. The Mössbauer data, however, indicate that a [2Fe-2S]2+ cluster has been formed in 60–65% yield, when percentages are expressed in terms of the total cluster concentration in the sample before oxidation. Thus, when we consider the total cluster concentration indicated by Mössbauer spectroscopy at the 2-min point as 100%, 62% are already 2Fe and 38% 4Fe clusters indicating that no cluster has been lost. Because the 2Fe cluster has only two S2− per cluster instead of the four found in the 4Fe form, this amounts to a loss of an amount of iron and sulfide equivalent to the amount now present in the 2Fe cluster observed. The “lost” Fe appears in the signals as indicated in Table 1 (columns 3 and 4). The calculated value for loss of sulfide at 2 min agrees well with the values found in the experiment of Fig. 5. As oxidation proceeds, complete cluster destruction competes with 4Fe → 2Fe cluster conversion, and after 17 min we find ≈95% loss of the 4Fe cluster with little further net formation of the 2Fe cluster. On the other hand, sulfide analysis shows only a very slow decline of sulfide values (Fig. 5). We suspect, therefore, that during further decay, iron sulfides arise, which are also detected as sulfide by the analytical method used (see Materials and Methods). By Mössbauer spectroscopy, we detect undefined species with broad absorption, which may well include iron sulfides. These observations agree with our result that on oxidation of FNR in air, no significant amounts of S0 are generated. Formation of S0 would have to involve the cysteine cluster ligands, which would appear to be occupied if a 2Fe cluster is indeed formed. This consideration brings up the question as to whether the 2Fe cluster uses the same four of the total of five cysteine residues present in FNR. We have investigated this by analyzing a mutant form of FNR, in which the only noncluster cysteine (Cys-16) of FNR was mutated to alanine (D. Glodowski and P.J.K., unpublished data). The formation of the 2Fe cluster in air proceeded unimpaired with this mutant.
Figure 5.
Loss of sulfide from FNR upon exposure to air. Disappearance of inorganic sulfide as a function of time of exposure of FNR to air. The reaction with air was quenched as described in Materials and Methods.
Reconstitution of the [4Fe-4S] Cluster in Oxygen-Exposed FNR.
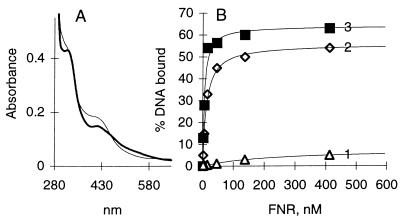
We have investigated whether DNA binding and the spectral characteristics of the protein containing the [4Fe-4S] cluster could be restored to the air-oxidized (10 min) FNR by reconstitution (see above, Materials and Methods). Reconstitution of the [4Fe-4S] cluster was observed by reappearance of the olive–green–yellowish color characteristic of the native form of FNR and restoration of its absorption spectrum (Fig. 6A). Upon reconstitution of the Fe-S cluster, DNA binding by FNR was also significantly restored, since reconstituted FNR maximally bound 53% of DNA with apparent affinity (S0.5) of 12.8 nM as compared with anaerobically purified FNR protein that maximally bound 64% of the DNA with an S0.5 of 5.0 nM (Fig. 6B). The data are in reasonable agreement with the spectroscopic findings according to which approximately 68% of the absorption initially present at 409 nm was restored upon reconstitution. Furthermore, addition of dithionite to air-oxidized protein (17 min) containing the [2Fe-2S]2+ cluster resulted in restoration of the [4Fe-4S]2+ cluster, such that about 90% of the 57Fe in 2Fe clusters was reassembled into the 4Fe form as judged by the Mössbauer spectra; addition of dithionite did not affect the iron contained in the broad magnetic component (data not shown).
Figure 6.
Reconstitution of the [4Fe-4S] cluster in FNR from the [2Fe-2S] cluster. (A) Absorption spectrum of air-oxidized FNR (thick line) and the same after reconstitution of the [4Fe-4S] cluster with Azotobacter vinelandii NifS protein as described (thin line). The reference cuvette contained the same mixture except for FNR. (B) DNA binding of air-oxidized (10 min) FNR lacking the 4Fe cluster (1), air-oxidized FNR following reconstitution of the [4Fe-4S] cluster (2), and FNR as obtained on purification (3).
DISCUSSION
FNR requires a Fe-S cluster for its regulatory functions as a transcription factor and in vitro is inactivated within a few minutes when exposed to air. We have followed this process by UV/visible, EPR, and Mössbauer spectroscopies, by determinations of DNA-binding ability and analyses for Fe, sulfide, and protein. From the stoichiometries of iron and sulfide relative to the number of cysteine residues of anaerobically purified FNR (3), we had previously concluded that FNR contains one [4Fe-4S] rather than two [2Fe-2S] clusters per monomer. The optical and Mössbauer spectra reported here support this conclusion and show that it is a [4Fe-4S]2+ cluster. We also found that, on reaction of FNR with air, the [4Fe-4S]2+ cluster decayed and a new cluster species was formed in high yield (60–65%), which, according to UV/VIS and Mössbauer spectroscopies, exhibited properties of [2Fe-2S]2+ clusters. In agreement with this, determination of sulfide showed that ≈70% of the original labile sulfide is still present after 2 min of aeration. However, even when all biological activity had been lost after about 10 min of aeration, the sulfide values changed little. Moreover, all the sulfide present followed the protein fraction on a Sephadex column (data not shown). These findings would be compatible with the spectroscopic results if iron sulfides are formed during the 4Fe → 2Fe cluster conversion concomitant with loss of activity and that these iron sulfides are adsorbed to the protein. We have observed similar behavior previously in reconstitution type of experiments, when excess of iron and sulfide had been added to apo- or partly occupied protein (11). Concerning the cluster ligands, we have found no indication that the 2Fe cluster might use the single alternative cysteine ligand available in FNR by studying the Cys-16 to Ala FNR mutant.
Fe-S cluster interconversions are not a new phenomenon. Most common with proteins are 4Fe → 3Fe conversions (17, 18). 4Fe → 2Fe conversions in proteins have, to our knowledge, not been observed without outside intervention, such as adding chelators (19), detergents, alkali (8), or other nonphysiological agents. With FNR we now have a 4Fe → 2Fe conversion occurring simply with a pure protein exposed to air in an aqueous solution at neutral pH. Since the 2Fe form appears relatively stable in the presence of air (half-life at least 12 h at 0°C) and this cluster (or its components) could be used to regenerate the 4Fe form in vitro, it is possible that the 2Fe form could have some physiological relevance if the 2Fe form was more readily reactivated in vivo. However, inactive FNR, as purified from aerobically grown bacteria, contains no significant amounts of labile sulfide and at best substoichiometric amounts of Fe (3, 5). This may suggest that in cellular systems further disassembly of the 2Fe form proceeds more rapidly under the influence of in vivo conditions or that the 2Fe cluster is not stable to purification. Further work will be necessary to determine whether in an in vitro reconstitution system, the iron in the 2Fe cluster is preferentially used as a scaffold in building up the active 4Fe form.
We have thus further supported our proposed model for the function of FNR, namely that the active form of the FNR protein contains a [4Fe-4S]2+ cluster, and that this cluster serves as the O2 sensor by virtue of its extreme sensitivity toward O2. Furthermore, we found that the [4Fe-4S]2+ cluster in wild-type FNR was very difficult to reduce, suggesting that its redox potential is rather low and that such a reduced cluster (+1 oxidation state) would not occur in vivo (see above and ref. 4). Fe-S clusters have been known to be unstable toward oxidants; however, their sensitivity toward O2 covers a wide range from their high stability in high-potential Fe-S proteins (20) to the high sensitivity in proteins such as FNR. Previous analysis of an FNR mutant protein (2) suggests that sensitivity toward O2 is primarily determined by subtle details of protein structure, since substitution of Leu-28 for His allowed for purification in the presence of oxygen of FNR containing a [4Fe-4S] cluster. Thus, differences in protein structure may likely provide O2 sensors of a wide range of sensitivities.
Acknowledgments
We dedicate this paper to the memory of Natalia Khoroshilova, who lost her life in a highway accident while on her way to help complete this paper. We thank Dennis Dean (Virginia Polytechnic Institute and State University) for the gift of NifS protein and Kafryn Lieder and Frank Ruzicka (University of Wisconsin) for assistance with EPR. This work was supported by U.S. Public Health Service, National Institutes for Health Grants GM45844 (to P.J.K.) and GM22701 (to E.M.). P.J.K. was also a recipient of a Young Investigator Award from the National Science Foundation and the Shaw Scientist Award from the Milwaukee Foundation.
ABBREVIATION
- FNR
fumarate nitrate reduction
Footnotes
For this batch, we have passed the sample that was exposed to air for 20 min through an anaerobic Sephadex G-25 column. This treatment removed most of iron associated with the broad magnetic component, but retained the central doublet of Figs. 1C and 4A, showing that the doublet assigned to the [2Fe-2S]2+ cluster belongs to a species tightly bound to protein. Approximately 30–35% of the iron of the column-treated sample belonged to the [2Fe-2S]2+ cluster, 40% to adventitious bound Fe2+ with ΔEQ ≈ 3.2 mm/s and δ ≈ 1.3 mm/s; the remainder belonged to a variety of unidentified species.
References
- 1.Spiro S. Antonie van Leeuwenhoek. 1994;66:23–36. doi: 10.1007/BF00871630. [DOI] [PubMed] [Google Scholar]
- 2.Khoroshilova N, Beinert H, Kiley P J. Proc Natl Acad Sci USA. 1995;92:2499–2503. doi: 10.1073/pnas.92.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazazzera B A, Beinert H, Khoroshilova N, Kennedy M C, Kiley P J. J Biol Chem. 1996;271:2762–2768. doi: 10.1074/jbc.271.5.2762. [DOI] [PubMed] [Google Scholar]
- 4.Green J, Bennett B, Jordan P, Ralph E T, Thomson A J, Guest J R. Biochem J. 1996;316:887–892. doi: 10.1042/bj3160887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green J, Trageser M, Six S, Unden G, Guest J R. Proc R Soc London B. 1991;244:137–144. doi: 10.1098/rspb.1991.0062. [DOI] [PubMed] [Google Scholar]
- 6.Lazazzera B A, Bates D M, Kiley P J. Genes Dev. 1993;7:1993–2005. doi: 10.1101/gad.7.10.1993. [DOI] [PubMed] [Google Scholar]
- 7.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 8.Kennedy M C, Kent T A, Emptage M, Merkle H, Beinert H, Münck E. J Biol Chem. 1984;259:14463–14471. [PubMed] [Google Scholar]
- 9.Beinert H. Anal Biochem. 1983;131:373–378. doi: 10.1016/0003-2697(83)90186-0. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai H, Lien S, San Pietro A. Anal Biochem. 1982;119:372–377. doi: 10.1016/0003-2697(82)90600-5. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy M C, Emptage M H, Beinert H. J Biol Chem. 1984;259:3145–3151. [PubMed] [Google Scholar]
- 12.Emptage M, Dreyer J-L, Kennedy M C, Beinert H. J Biol Chem. 1983;258:11106–11111. [PubMed] [Google Scholar]
- 13.Zheng L, Dean D R. J Biol Chem. 1994;269:18723–18726. [PubMed] [Google Scholar]
- 14.Bertini I, Ciurli S, Luchinat C. Struct Bonding (Berlin) 1995;83:1–53. [Google Scholar]
- 15.Ziegelhoffer E C, Kiley P J. J Mol Biol. 1995;245:351–361. doi: 10.1006/jmbi.1994.0029. [DOI] [PubMed] [Google Scholar]
- 16.Wong G B, Kurtz D M, Jr, Holm R H, Mortensen L E, Upchurch R G. J Am Chem Soc. 1979;101:3078–3091. [Google Scholar]
- 17.Beinert H, Thomson A J. Arch Biochem Biophys. 1983;222:333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- 18.Moura J J G, Moura I, Kent T A, Lipscomb J D, Huynh B H, LeGall J, Xavier A V, Münck E. J Biol Chem. 1982;257:6259–6267. [PubMed] [Google Scholar]
- 19.Anderson G L, Howard J B. Biochemistry. 1984;23:2118–2122. doi: 10.1021/bi00305a002. [DOI] [PubMed] [Google Scholar]
- 20.Maskiewicz R, Bruice T C, Bartsch R G. Biochem Biophys Res Commun. 1975;65:407–412. doi: 10.1016/s0006-291x(75)80108-2. [DOI] [PubMed] [Google Scholar]