Abstract
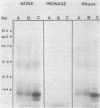
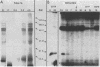
Protein phosphorylation was demonstrated in Bradyrhizobium japonicum bacteroids in vivo and in cultures in vivo and in vitro. Comparison of in vivo-labeled phosphoproteins of bacteroids and of cultured cells showed differences in both the pattern and intensity of labeling. In cultured cells, comparison of the labeling patterns and intensities of in vivo- and in vitro-labeled phosphoproteins showed a number of similarities; however, several phosphoproteins were found only after one of the two labeling conditions. The labeling intensity was time dependent in both in vivo and in vitro assays and was dependent on the presence of magnesium in in vitro assays. Differences in the rates of phosphorylation and dephosphorylation were noted for a number of proteins. The level of incorporation of 32P into protein was only 2% or less of the total phosphate accumulated during the in vivo labeling period. Several isolation and sample preparation procedures resulted in differences in labeling patterns. Phosphatase inhibitors and several potential metabolic effectors had negligible effects on the phosphorylation pattern. There were no significant changes in the phosphorylation patterns of cells cultured on mannitol, acetate, and succinate, although the intensity of the labeling did vary with the carbon source.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Dilworth M. J. Ammonia assimilation by rhizobium cultures and bacteroids. J Gen Microbiol. 1975 Jan;86(1):39–48. doi: 10.1099/00221287-86-1-39. [DOI] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Fox D. K., Meadow N. D., Roseman S. Phosphate transfer between acetate kinase and enzyme I of the bacterial phosphotransferase system. J Biol Chem. 1986 Oct 15;261(29):13498–13503. [PubMed] [Google Scholar]
- Garnak M., Reeves H. C. Phosphorylation of Isocitrate dehydrogenase of Escherichia coli. Science. 1979 Mar 16;203(4385):1111–1112. doi: 10.1126/science.34215. [DOI] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Matsumura P., Simon M. I. Protein phosphorylation is involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7609–7613. doi: 10.1073/pnas.84.21.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Karr D. B., Emerich D. W. Uniformity of the microsymbiont population from soybean nodules with respect to buoyant density. Plant Physiol. 1988 Mar;86(3):693–699. doi: 10.1104/pp.86.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manai M., Cozzone A. J. Two-dimensional separation of phosphoamino acids from nucleoside monophosphates. Anal Biochem. 1982 Jul 15;124(1):12–18. doi: 10.1016/0003-2697(82)90213-5. [DOI] [PubMed] [Google Scholar]
- McAllister C. F., Lepo J. E. Succinate transport by free-living forms of Rhizobium japonicum. J Bacteriol. 1983 Mar;153(3):1155–1162. doi: 10.1128/jb.153.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G. The regulatory properties of isocitrate dehydrogenase kinase and isocitrate dehydrogenase phosphatase from Escherichia coli ML308 and the roles of these activities in the control of isocitrate dehydrogenase. Eur J Biochem. 1984 Jun 1;141(2):409–414. doi: 10.1111/j.1432-1033.1984.tb08206.x. [DOI] [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Rahmsdorf H. J., Pai S. H., Hirsch-Kauffmann M., Herrlich P., Schweiger M. Control of gene expression in bacteriophage T7: transcriptional controls. Mol Gen Genet. 1974;134(4):281–297. doi: 10.1007/BF00337463. [DOI] [PubMed] [Google Scholar]
- Robertson E. F., Hoyt J. C., Reeves H. C. Evidence of histidine phosphorylation in isocitrate lyase from Escherichia coli. J Biol Chem. 1988 Feb 15;263(5):2477–2482. [PubMed] [Google Scholar]
- Schieven G., Martin G. S. Nonenzymatic phosphorylation of tyrosine and serine by ATP is catalyzed by manganese but not magnesium. J Biol Chem. 1988 Oct 25;263(30):15590–15593. [PubMed] [Google Scholar]
- Vallejos R. H., Holuigue L., Lucero H. A., Torruella M. Evidence of tyrosine kinase activity in the photosynthetic bacterium Rhodospirillum rubrum. Biochem Biophys Res Commun. 1985 Jan 31;126(2):685–691. doi: 10.1016/0006-291x(85)90239-6. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr Evidence for protein kinase activities in the prokaryote Salmonella typhimurium. J Biol Chem. 1978 Nov 10;253(21):7605–7608. [PubMed] [Google Scholar]
- Zillig W., Fujiki H., Blum W., Janeković D., Schweiger M., Rahmsdorf H., Ponta H., Hirsch-Kauffmann M. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage-T7-induced protein kinase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2506–2510. doi: 10.1073/pnas.72.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]