Abstract
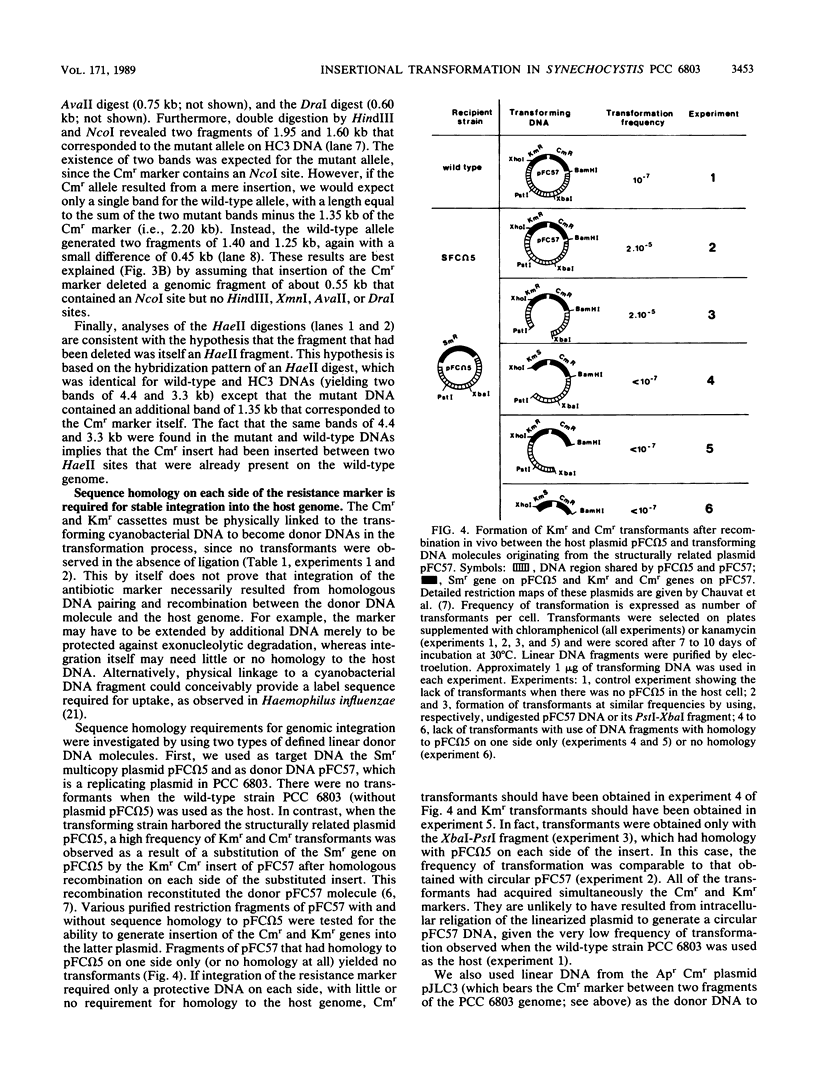
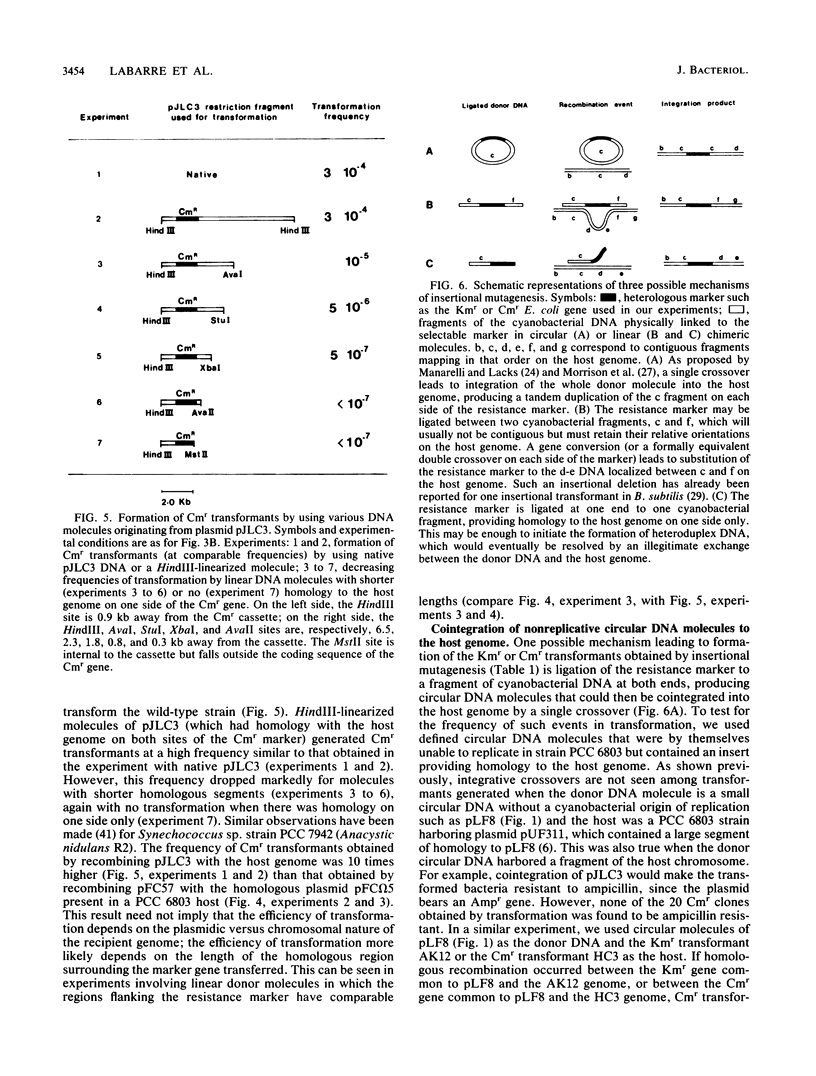
The facultative heterotrophic cyanobacterium Synechocystis sp. strain PCC 6803 was transformed by HaeII Cmr fragments ligated at random to HaeII DNA fragments of the host genome. A similar transformation was done with an AvaII Kmr marker ligated to AvaII host DNA fragments. Integration of the resistance markers into the host genome led to a high frequency of stable Kmr and Cmr transformants. Physical analysis of individual transformants indicated that this result was due to homologous recombination by conversionlike events leading to insertion of the Cmr (or Kmr) gene between two HaeII (or AvaII) sites of the host genome, with precise deletion of the host DNA between these sites. In contrast, integrative crossover of circular DNA molecules with homology to the host DNA is very rare in this cyanobacterium. Strain PCC 6803 was shown to have about 12 genomic copies per cell in standard growth conditions, which complicates the detection of recessive mutations induced by chemical or UV mutagenesis. Random disruption of the host DNA by insertional transformation provides a convenient alternative to transposon mutagenesis in cyanobacteria and may help to overcome the difficulties encountered in generating recessive mutants by classical mutagenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astier C., Elmorjani K., Meyer I., Joset F., Herdman M. Photosynthetic mutants of the cyanobacteria Synechocystis sp. strains PCC 6714 and PCC 6803: sodium p-hydroxymercuribenzoate as a selective agent. J Bacteriol. 1984 May;158(2):659–664. doi: 10.1128/jb.158.2.659-664.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvat F., Rouet P., Bottin H., Boussac A. Mutagenesis by random cloning of an Escherichia coli kanamycin resistance gene into the genome of the cyanobacterium Synechocystis PCC 6803: selection of mutants defective in photosynthesis. Mol Gen Genet. 1989 Mar;216(1):51–59. doi: 10.1007/BF00332230. [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Lacks S. A. Heteroduplex deoxyribonucleic acid base mismatch repair in bacteria. Microbiol Rev. 1986 Jun;50(2):133–165. doi: 10.1128/mr.50.2.133-165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Schilling-Cordaro C., Mergia A., Houck C. M. A new technique for genetic engineering of Agrobacterium Ti plasmid. Plasmid. 1983 Jul;10(1):21–30. doi: 10.1016/0147-619x(83)90054-9. [DOI] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Babcock G. T., McIntosh L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1988 Jan;85(2):427–430. doi: 10.1073/pnas.85.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. H., Wilson G. A., Young F. E. Mechanism of integrating foreign DNA during transformation of Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3664–3668. doi: 10.1073/pnas.75.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzelzkalns V. A., Bogorad L. Molecular analysis of a mutant defective in photosynthetic oxygen evolution and isolation of a complementing clone by a novel screening procedure. EMBO J. 1988 Feb;7(2):333–338. doi: 10.1002/j.1460-2075.1988.tb02817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzelzkalns V. A., Bogorad L. Stable transformation of the cyanobacterium Synechocystis sp. PCC 6803 induced by UV irradiation. J Bacteriol. 1986 Mar;165(3):964–971. doi: 10.1128/jb.165.3.964-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. T. Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol. 1978 Apr;134(1):71–75. doi: 10.1128/jb.134.1.71-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson C., Debus R. J., Osiewacz H. D., Gurevitz M., McIntosh L. Construction of an Obligate Photoheterotrophic Mutant of the Cyanobacterium Synechocystis 6803 : Inactivation of the psbA Gene Family. Plant Physiol. 1987 Dec;85(4):1021–1025. doi: 10.1104/pp.85.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Smith H. O. Transformation in Haemophilus: a problem in membrane biology. J Membr Biol. 1984;81(2):89–103. doi: 10.1007/BF01868974. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Toukdarian A. Genetics of azotobacters: applications to nitrogen fixation and related aspects of metabolism. Annu Rev Microbiol. 1987;41:227–258. doi: 10.1146/annurev.mi.41.100187.001303. [DOI] [PubMed] [Google Scholar]
- Labarre J., Thuriaux P., Chauvat F. Genetic analysis of amino acid transport in the facultatively heterotrophic cyanobacterium Synechocystis sp. strain 6803. J Bacteriol. 1987 Oct;169(10):4668–4673. doi: 10.1128/jb.169.10.4668-4673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Mann N., Carr N. G. Control of macromolecular composition and cell division in the blue-green algae Anacystis nidulans. J Gen Microbiol. 1974 Aug;83(2):399–405. doi: 10.1099/00221287-83-2-399. [DOI] [PubMed] [Google Scholar]
- Mannarelli B. M., Lacks S. A. Ectopic integration of chromosomal genes in Streptococcus pneumoniae. J Bacteriol. 1984 Dec;160(3):867–873. doi: 10.1128/jb.160.3.867-873.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Trombe M. C., Hayden M. K., Waszak G. A., Chen J. D. Isolation of transformation-deficient Streptococcus pneumoniae mutants defective in control of competence, using insertion-duplication mutagenesis with the erythromycin resistance determinant of pAM beta 1. J Bacteriol. 1984 Sep;159(3):870–876. doi: 10.1128/jb.159.3.870-876.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean V., Claverys J. P., Vasseghi H., Sicard A. M. Rapid cloning of specific DNA fragments of Streptococcus pneumoniae by vector integration into the chromosome followed by endonucleolytic excision. Gene. 1981 Nov;15(2-3):289–293. doi: 10.1016/0378-1119(81)90139-6. [DOI] [PubMed] [Google Scholar]
- Niaudet B., Goze A., Ehrlich S. D. Insertional mutagenesis in Bacillus subtilis: mechanism and use in gene cloning. Gene. 1982 Oct;19(3):277–284. doi: 10.1016/0378-1119(82)90017-8. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Williams J. G., Arntzen C. J. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988 Feb;7(2):325–332. doi: 10.1002/j.1460-2075.1988.tb02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbrick J. B., Zilinskas B. A. Cloning, nucleotide sequence and mutational analysis of the gene encoding the Photosystem II manganese-stabilizing polypeptide of Synechocystis 6803. Mol Gen Genet. 1988 Jun;212(3):418–425. doi: 10.1007/BF00330845. [DOI] [PubMed] [Google Scholar]
- Porter R. D. Transformation in cyanobacteria. Crit Rev Microbiol. 1986;13(2):111–132. doi: 10.3109/10408418609108736. [DOI] [PubMed] [Google Scholar]
- Postgate J. R., Kent H. M., Robson R. L., Chesshyre J. A. The genomes of Desulfovibrio gigas and D. vulgaris. J Gen Microbiol. 1984 Jul;130(7):1597–1601. doi: 10.1099/00221287-130-7-1597. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Shimel B., Ellis S. Characterization of Azotobacter vinelandii deoxyribonucleic acid and folded chromosomes. J Bacteriol. 1979 Jun;138(3):871–877. doi: 10.1128/jb.138.3.871-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Stewart G. J., Carlson C. A. The biology of natural transformation. Annu Rev Microbiol. 1986;40:211–235. doi: 10.1146/annurev.mi.40.100186.001235. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Borrias W. E., Kuhlemeier C. J., Castets A. M., van Arkel G. A., van den Hondel C. A. A new approach for molecular cloning in cyanobacteria: cloning of an Anacystis nidulans met gene using a Tn901-induced mutant. Gene. 1982 Nov;20(1):111–119. doi: 10.1016/0378-1119(82)90092-0. [DOI] [PubMed] [Google Scholar]
- Ubben D., Schmitt R. Tn1721 derivatives for transposon mutagenesis, restriction mapping and nucleotide sequence analysis. Gene. 1986;41(2-3):145–152. doi: 10.1016/0378-1119(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Vermaas W. F., Williams J. G., Rutherford A. W., Mathis P., Arntzen C. J. Genetically engineered mutant of the cyanobacterium Synechocystis 6803 lacks the photosystem II chlorophyll-binding protein CP-47. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9474–9477. doi: 10.1073/pnas.83.24.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]