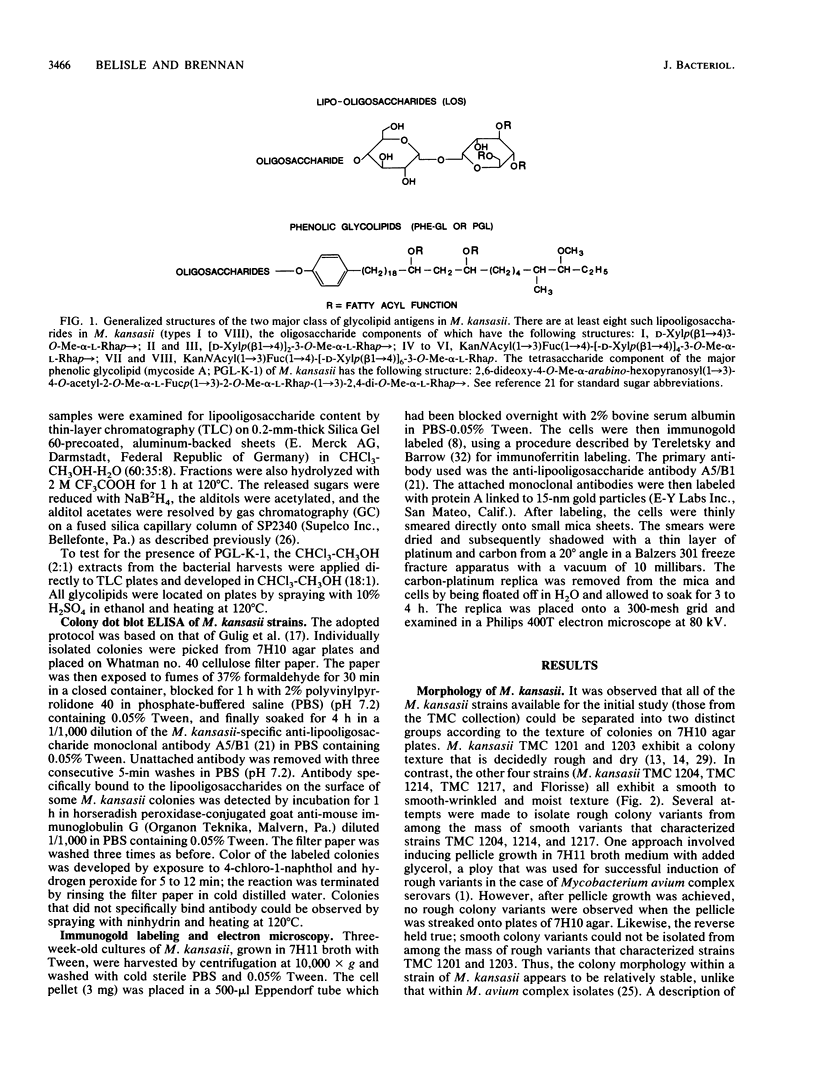
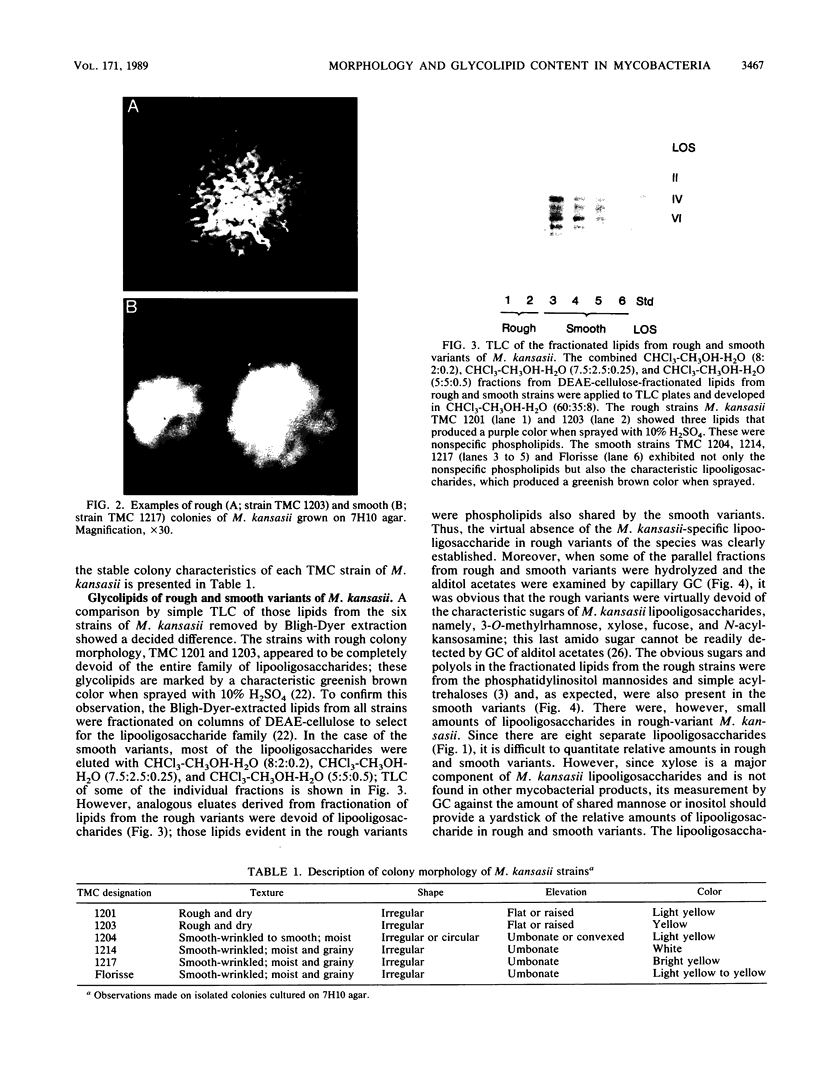
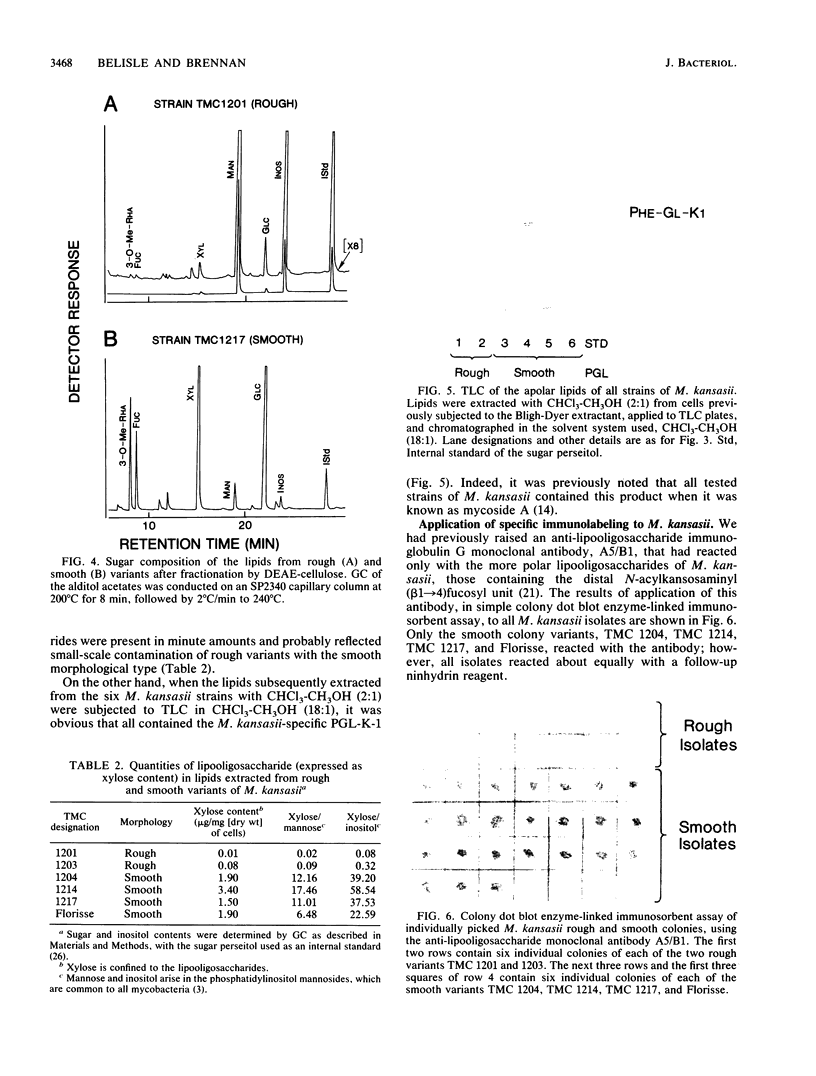
Abstract
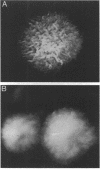
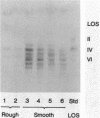
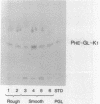
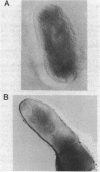
Rough and smooth colony variants of Mycobacterium kansasii were compared with respect to surface glycolipid composition. Thin-layer chromatography of the native glycolipid antigens, gas chromatography of the constituent sugars, and in situ probing with an appropriate monoclonal antibody by colony dot blot enzyme-linked immunosorbent assay and immunogold labeling demonstrated that all M. kansasii strains of smooth colony morphology contain on their surfaces the recently described trehalose-containing lipooligosaccharides, whereas all rough variants were devoid of such surface antigens. Yet all strains, rough and smooth, contained another glycolipid, the M. kansasii-specific phenolic glycolipid. Previous studies by others had shown that the rough forms of M. kansasii persist longer than smooth variants in experimentally infected mice. Therefore, this study may provide some insight into the question of the chemical basis of pathogenesis in certain mycobacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BUHLER V. B., POLLAK A. Human infection with atypical acid-fast organisms; report of two cases with pathologic findings. Am J Clin Pathol. 1953 Apr;23(4):363–374. doi: 10.1093/ajcp/23.4.363. [DOI] [PubMed] [Google Scholar]
- Barrow W. W., Brennan P. J. Isolation in high frequency of rough variants of Mycobacterium intracellulare lacking C-mycoside glycopeptidolipid antigens. J Bacteriol. 1982 Apr;150(1):381–384. doi: 10.1128/jb.150.1.381-384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Souhrada M., Ullom B., McClatchy J. K., Goren M. B. Identification of atypical mycobacteria by thin-layer chromatography of their surface antigens. J Clin Microbiol. 1978 Oct;8(4):374–379. doi: 10.1128/jcm.8.4.374-379.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Cunningham D. S. Systemic Mycobacterium kansasii infection and regulation of the alloantigenic response. Infect Immun. 1981 May;32(2):614–624. doi: 10.1128/iai.32.2.614-624.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FREGNAN G. B., SMITH D. W. Description of various colony forms of mycobacteria. J Bacteriol. 1962 Apr;83:819–827. doi: 10.1128/jb.83.4.819-827.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREGNAN G. B., SMITH D. W., RANDALL H. M. A mutant of a scotochromogenic Mycobacterium detected by colony morphology and lipid studies. J Bacteriol. 1962 Apr;83:828–836. doi: 10.1128/jb.83.4.828-836.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREGNAN G. B., SMITH D. W., RANDALL H. M. Biological and chemical studies on mycobacteria. Relationship of colony morphology to mycoside content for Mycobacterium kansasil and Mycobacterium fortuitum. J Bacteriol. 1961 Oct;82:517–527. doi: 10.1128/jb.82.4.517-527.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Taylor G. M. An immunocolloid method for the electron microscope. Immunochemistry. 1971 Nov;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Fournie J. J., Riviere M., Puzo G. Absolute configuration of the unique 2,6-dideoxy-4-O-methyl-arabino-hexopyranose of the major phenolic glycolipid antigen from Mycobacterium kansasii. Eur J Biochem. 1987 Oct 1;168(1):181–183. doi: 10.1111/j.1432-1033.1987.tb13402.x. [DOI] [PubMed] [Google Scholar]
- Fournié J. J., Rivière M., Papa F., Puzo G. Structural elucidation of the major phenolic glycolipid from Mycobacterium kansasii. II. Presence of a novel dideoxyhexose. J Biol Chem. 1987 Mar 5;262(7):3180–3184. [PubMed] [Google Scholar]
- Fournié J. J., Rivière M., Puzo G. Structural elucidation of the major phenolic glycolipid from Mycobacterium kansasii. I. Evidence for tetrasaccharide structure of the oligosaccharide moiety. J Biol Chem. 1987 Mar 5;262(7):3174–3179. [PubMed] [Google Scholar]
- Gastambide-Odier M., Sarda P. Contribution à l'étude de la structure et de la biosynthèse de glycolipides spécifiques isolés de mycobactéries: les mycosides a et b. Pneumonologie. 1970;142(2):241–255. doi: 10.1007/BF02095222. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., Frisch C. F., Hansen E. J. A set of two monoclonal antibodies specific for the cell surface-exposed 39K major outer membrane protein of Haemophilus influenzae type b defines all strains of this pathogen. Infect Immun. 1983 Nov;42(2):516–524. doi: 10.1128/iai.42.2.516-524.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Murphy R. C., Brennan P. J. N-acylkansosamine. A novel N-acylamino sugar from the trehalose-containing lipooligosaccharide antigens of Mycobacterium kansasii. J Biol Chem. 1984 Aug 10;259(15):9729–9734. [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Hunter S. W., Jardine I., Yanagihara D. L., Brennan P. J. Trehalose-containing lipooligosaccharides from mycobacteria: structures of the oligosaccharide segments and recognition of a unique N-acylkanosamine-containing epitope. Biochemistry. 1985 May 21;24(11):2798–2805. doi: 10.1021/bi00332a030. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Murphy R. C., Clay K., Goren M. B., Brennan P. J. Trehalose-containing lipooligosaccharides. A new class of species-specific antigens from Mycobacterium. J Biol Chem. 1983 Sep 10;258(17):10481–10487. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- McCarthy C. Spontaneous and Induced Mutation in Mycobacterium avium. Infect Immun. 1970 Sep;2(3):223–228. doi: 10.1128/iai.2.3.223-228.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Neill M. A., Klebanoff S. J. The effect of phenolic glycolipid-1 from Mycobacterium leprae on the antimicrobial activity of human macrophages. J Exp Med. 1988 Jan 1;167(1):30–42. doi: 10.1084/jem.167.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière M., Fournié J. J., Puzo G. A novel mannose containing phenolic glycolipid from Mycobacterium kansasii. J Biol Chem. 1987 Nov 5;262(31):14879–14884. [PubMed] [Google Scholar]
- Runyon E. H. Identification of mycobacterial pathogens utilizing colony characteristics. Am J Clin Pathol. 1970 Oct;54(4):578–586. doi: 10.1093/ajcp/54.4.578. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereletsky M. J., Barrow W. W. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983 Sep;41(3):1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal A. L., Kubica G. P. Differential colonial characteristics of mycobacteria on Middlebrook and Cohn 7H10 agar-base medium. Am Rev Respir Dis. 1966 Aug;94(2):247–252. doi: 10.1164/arrd.1966.94.2.247. [DOI] [PubMed] [Google Scholar]