Abstract
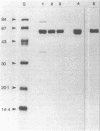
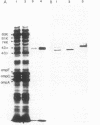
Antigen 43 has been identified as a unique protein complex in the outer membrane of Escherichia coli. The complex contains two different polypeptides, alpha (Mr, 60,000) and beta (Mr, 53,000), in equal stoichiometry (P. Owen, P. Caffrey, and L.-G. Josefsson, J. Bacteriol. 169:3770-3777, 1987). The alpha subunit was released in a water-soluble form upon heating of outer membranes to 60 degrees C and was purified to apparent homogeneity by gel filtration and ion-exchange chromatography. The purified protein was acidic (pI 4.6) and had a polarity of 49.2%. The N-terminal sequence showed homology with the N termini of certain enterobacterial fimbrial subunits. In addition, antigen 43 underwent a reversible phase variation similar to that of type 1 fimbriae. By use of subunit-specific antisera, it was shown that the purified alpha subunit was capable of reassociating with the beta polypeptide. However, electron microscopic examination indicated that antigen 43 does not form a recognizable surface structure. The available evidence supports the view that antigen 43 is a complex consisting of a peripheral membrane protein (alpha) anchored to a subunit (beta) that is integral to the outer membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S. N., Goguen J. D., Beachey E. H. Hyperadhesive mutant of type 1-fimbriated Escherichia coli associated with formation of FimH organelles (fimbriosomes). Infect Immun. 1988 May;56(5):1023–1029. doi: 10.1128/iai.56.5.1023-1029.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty H., Yamada H., Caffrey P., Owen P. Identification, immunochemical characterization, and purification of a major lipoprotein antigen associated with the inner (cytoplasmic) membrane of Escherichia coli. J Bacteriol. 1986 Jun;166(3):1072–1082. doi: 10.1128/jb.166.3.1072-1082.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Dykes C. W., Halliday I. J., Read M. J., Hobden A. N., Harford S. Nucleotide sequences of four variants of the K88 gene of porcine enterotoxigenic Escherichia coli. Infect Immun. 1985 Oct;50(1):279–283. doi: 10.1128/iai.50.1.279-283.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Fader R. C., Duffy L. K., Davis C. P., Kurosky A. Purification and chemical characterization of type 1 pili isolated from Klebsiella pneumoniae. J Biol Chem. 1982 Mar 25;257(6):3301–3305. [PubMed] [Google Scholar]
- Hanson M. S., Hempel J., Brinton C. C., Jr Purification of the Escherichia coli type 1 pilin and minor pilus proteins and partial characterization of the adhesin protein. J Bacteriol. 1988 Aug;170(8):3350–3358. doi: 10.1128/jb.170.8.3350-3358.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans D. A., Gibson F. Cloning of the structural genes of the Escherichia coli adenosinetriphosphatase complex. Methods Enzymol. 1983;97:176–187. doi: 10.1016/0076-6879(83)97131-8. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987 Jul;208(3):439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Klemm P. The complete amino-acid sequence of the K88 antigen, a fimbrial protein from Escherichia coli. Eur J Biochem. 1981 Jul;117(3):617–627. doi: 10.1111/j.1432-1033.1981.tb06382.x. [DOI] [PubMed] [Google Scholar]
- Klemm P. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur J Biochem. 1984 Sep 3;143(2):395–399. doi: 10.1111/j.1432-1033.1984.tb08386.x. [DOI] [PubMed] [Google Scholar]
- Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986 Jun;5(6):1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt K. A., Klemm P. Investigation of minor components of Escherichia coli type 1 fimbriae: protein chemical and immunological aspects. Microb Pathog. 1988 Mar;4(3):231–238. doi: 10.1016/0882-4010(88)90073-3. [DOI] [PubMed] [Google Scholar]
- Krogfelt K. A., Meldal M., Klemm P. K88 fimbrial antigens: identification of antigenic determinants by the use of synthetic peptides. Microb Pathog. 1987 Jun;2(6):465–472. doi: 10.1016/0882-4010(87)90053-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., Claassen I., Bakker D., Kuipers H., de Graaf F. K. Regulation and structure of an Escherichia coli gene coding for an outer membrane protein involved in export of K88ab fimbrial subunits. Nucleic Acids Res. 1986 Mar 25;14(6):2443–2457. doi: 10.1093/nar/14.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Oudega B., De Graaf F. K. Genetic organization and biogenesis of adhesive fimbriae of Escherichia coli. Antonie Van Leeuwenhoek. 1988;54(4):285–299. doi: 10.1007/BF00393521. [DOI] [PubMed] [Google Scholar]
- Owen P., Caffrey P., Josefsson L. G. Identification and partial characterization of a novel bipartite protein antigen associated with the outer membrane of Escherichia coli. J Bacteriol. 1987 Aug;169(8):3770–3777. doi: 10.1128/jb.169.8.3770-3777.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Smit J., Grano D. A., Glaeser R. M., Agabian N. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J Bacteriol. 1981 Jun;146(3):1135–1150. doi: 10.1128/jb.146.3.1135-1150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]