Abstract
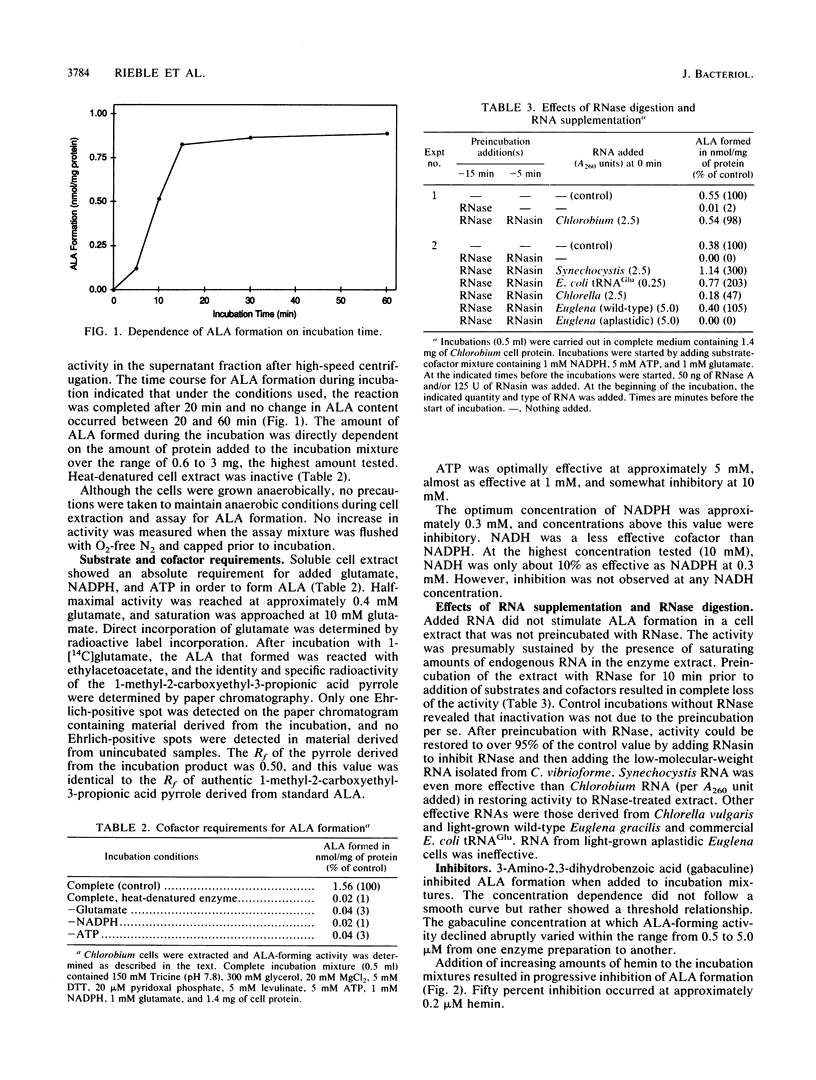
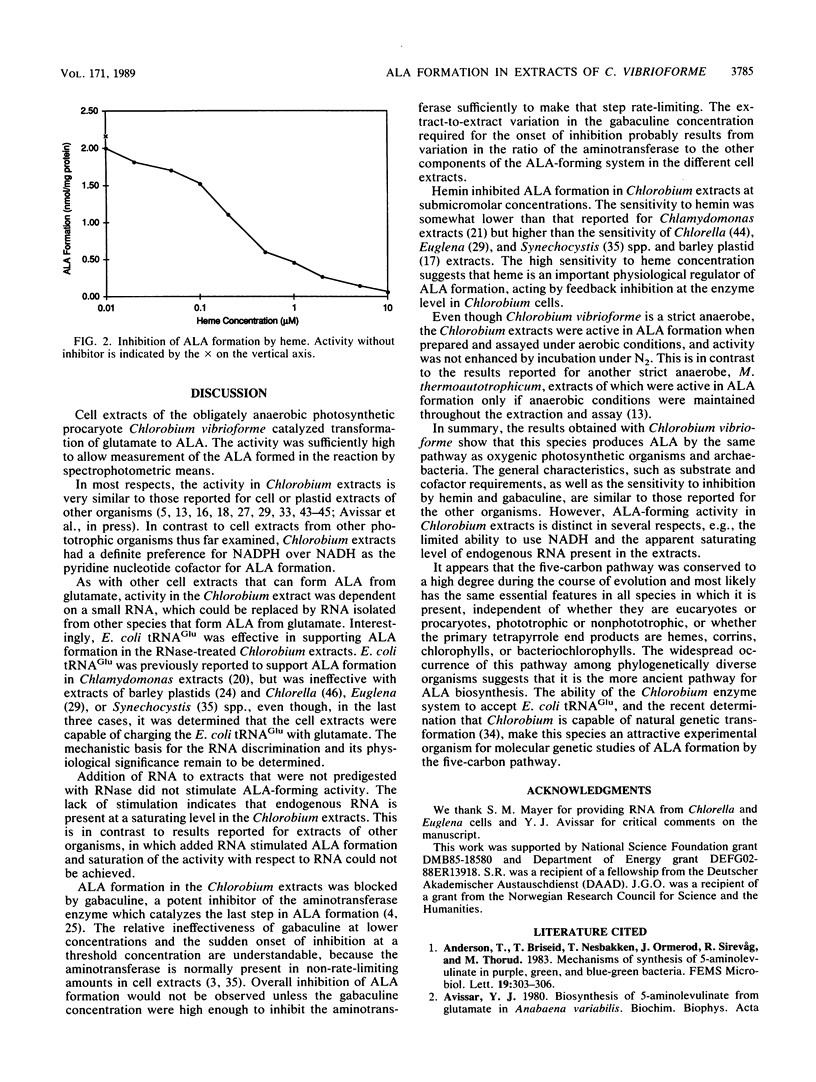
Formation of the tetrapyrrole pigment precursor delta-aminolevulinic acid (ALA) from glutamate was detected and partially characterized in extracts of the strictly anaerobic green photosynthetic bacterial species Chlorobium vibrioforme by using assay methods derived from those developed for algae and cyanobacteria. ALA formation in Chlorobium extracts was saturated at 10 mM glutamate and required NADPH and ATP at optimal concentrations of 0.3 and 3 mM, respectively. Preincubation of the enzyme extract with RNase A destroyed the ALA-forming activity completely. Activity in the RNase-treated extract was restored by supplementation with Chlorobium RNA after addition of RNasin to block further RNase action. RNA from the cyanobacterium Synechocystis sp. strain PCC 6803 and Escherichia coli tRNAGlu also restored activity. Activity was inhibited 50% by 0.2 microM hemin. ALA formation was completely abolished by the addition of 5 microM 3-amino-2,3-dihydrobenzoic acid (gabaculine). These results indicate that Chlorobium extracts share with those of plants, eucaryotic algae, cyanobacteria, prochlorophytes, and methanogens the capacity for RNA-dependent ALA formation from glutamate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Beale S. I. Biosynthesis of Tetrapyrrole Pigment Precursors : Formation and Utilization of Glutamyl-tRNA for delta-Aminolevulinic Acid Synthesis by Isolated Enzyme Fractions from Chlorella Vulgaris. Plant Physiol. 1988 Nov;88(3):879–886. doi: 10.1104/pp.88.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Biosynthesis of Tetrapyrrole Pigment Precursors : Pyridoxal Requirement of the Aminotransferase Step in the Formation of delta-Aminolevulinate from Glutamate in Extracts of Chlorella vulgaris. Plant Physiol. 1989 Mar;89(3):852–859. doi: 10.1104/pp.89.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J Bacteriol. 1989 Jun;171(6):2919–2924. doi: 10.1128/jb.171.6.2919-2924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: II. Formation of C-delta-Aminolevulinic Acid from Labeled Precursors in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):297–303. doi: 10.1104/pp.53.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Rasmussen C. K. Alpha-ketoglutarate metabolism by cytochrome-containing anaerobes. Can J Microbiol. 1983 Jul;29(7):790–796. doi: 10.1139/m83-128. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Rittenberg B., Lascelles J. Cytochrome synthesis and its regulation in Spirillum itersonii. J Bacteriol. 1967 Nov;94(5):1648–1655. doi: 10.1128/jb.94.5.1648-1655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., LAVER W. G., NEUBERGER A. Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J. 1958 Sep;70(1):71–81. doi: 10.1042/bj0700071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel E., Ne'eman E. Alternative Routes for the Synthesis of 5-Aminolevulinic Acid in Maize Leaves : II. Formation from Glutamate. Plant Physiol. 1983 Aug;72(4):1062–1067. doi: 10.1104/pp.72.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y. Chlorophyll biosynthesis in Chlamydomonas starts with the formation of glutamyl-tRNA. J Biol Chem. 1986 Oct 15;261(29):13451–13455. [PubMed] [Google Scholar]
- Jurgenson J. E., Beale S. I., Troxler R. F. Biosynthesis of delta-aminolevulinic acid in the unicellular rhodophyte, cyanidium caldarium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):149–157. doi: 10.1016/s0006-291x(76)80285-9. [DOI] [PubMed] [Google Scholar]
- KIKUCHI G., KUMAR A., TALMAGE P., SHEMIN D. The enzymatic synthesis of delta-aminolevulinic acid. J Biol Chem. 1958 Nov;233(5):1214–1219. [PubMed] [Google Scholar]
- Li J. M., Brathwaite O., Cosloy S. D., Russell C. S. 5-Aminolevulinic acid synthesis in Escherichia coli. J Bacteriol. 1989 May;171(5):2547–2552. doi: 10.1128/jb.171.5.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Mayer S. M., Beale S. I., Weinstein J. D. Enzymatic conversion of glutamate to delta-aminolevulinic acid in soluble extracts of Euglena gracilis. J Biol Chem. 1987 Sep 15;262(26):12541–12549. [PubMed] [Google Scholar]
- Menon I. A., Shemin D. Concurrent decrease of enzymic activities concerned with the synthesis of coenzyme B 12 and of propionic acid in propionibacteria. Arch Biochem Biophys. 1967 Aug;121(2):304–310. doi: 10.1016/0003-9861(67)90080-x. [DOI] [PubMed] [Google Scholar]
- Oh-hama T., Seto H., Miyachi S. 13C NMR evidence for bacteriochlorophyll c formation by the C5 pathway in green sulfur bacterium, Prosthecochloris. Eur J Biochem. 1986 Aug 15;159(1):189–194. doi: 10.1111/j.1432-1033.1986.tb09851.x. [DOI] [PubMed] [Google Scholar]
- Oh-hama T., Seto H., Miyachi S. 13C-NMR evidence of bacteriochlorophyll a formation by the C5 pathway in Chromatium. Arch Biochem Biophys. 1986 Apr;246(1):192–198. doi: 10.1016/0003-9861(86)90463-7. [DOI] [PubMed] [Google Scholar]
- Oh-hama T., Stolowich N. J., Scott A. I. 5-Aminolevulinic acid formation from glutamate via the C5 pathway in Clostridium thermoaceticum. FEBS Lett. 1988 Feb 8;228(1):89–93. doi: 10.1016/0014-5793(88)80591-x. [DOI] [PubMed] [Google Scholar]
- Rieble S., Beale S. I. Transformation of glutamate to delta-aminolevulinic acid by soluble extracts of Synechocystis sp. PCC 6803 and other oxygenic prokaryotes. J Biol Chem. 1988 Jun 25;263(18):8864–8871. [PubMed] [Google Scholar]
- Schneegurt M. A., Beale S. I. Biosynthesis of protoheme and heme a from glutamate in maize. Plant Physiol. 1986 Aug;81(4):965–971. doi: 10.1104/pp.81.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneegurt M. A., Beale S. I. Characterization of the RNA Required for Biosynthesis of delta-Aminolevulinic Acid from Glutamate : Purification by Anticodon-Based Affinity Chromatography and Determination That the UUC Glutamate Anticodon Is a General Requirement for Function in ALA Biosynthesis. Plant Physiol. 1988 Feb;86(2):497–504. doi: 10.1104/pp.86.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C. G., Söll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986 Jul 17;322(6076):281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- Tait G. H. Aminolaevulinate synthetase of Micrococcus denitrificans. Purification and properties of the enzyme, and the effect of growth conditions on the enzyme activity in cells. Biochem J. 1973 Feb;131(2):389–403. doi: 10.1042/bj1310389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Wang W. Y., Huang D. D., Stachon D., Gough S. P., Kannangara C. G. Purification, Characterization, and Fractionation of the delta-Aminolevulinic Acid Synthesizing Enzymes from Light-Grown Chlamydomonas reinhardtii Cells. Plant Physiol. 1984 Mar;74(3):569–575. doi: 10.1104/pp.74.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Mayer S. M., Beale S. I. Formation of delta-Aminolevulinic Acid from Glutamic Acid in Algal Extracts : Separation into an RNA and Three Required Enzyme Components by Serial Affinity Chromatography. Plant Physiol. 1987 Jun;84(2):244–250. doi: 10.1104/pp.84.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Mayer S. M., Beale S. I. Stimulation of delta-Aminolevulinic Acid Formation in Algal Extracts by Heterologous RNA. Plant Physiol. 1986 Dec;82(4):1096–1101. doi: 10.1104/pp.82.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]


