Abstract
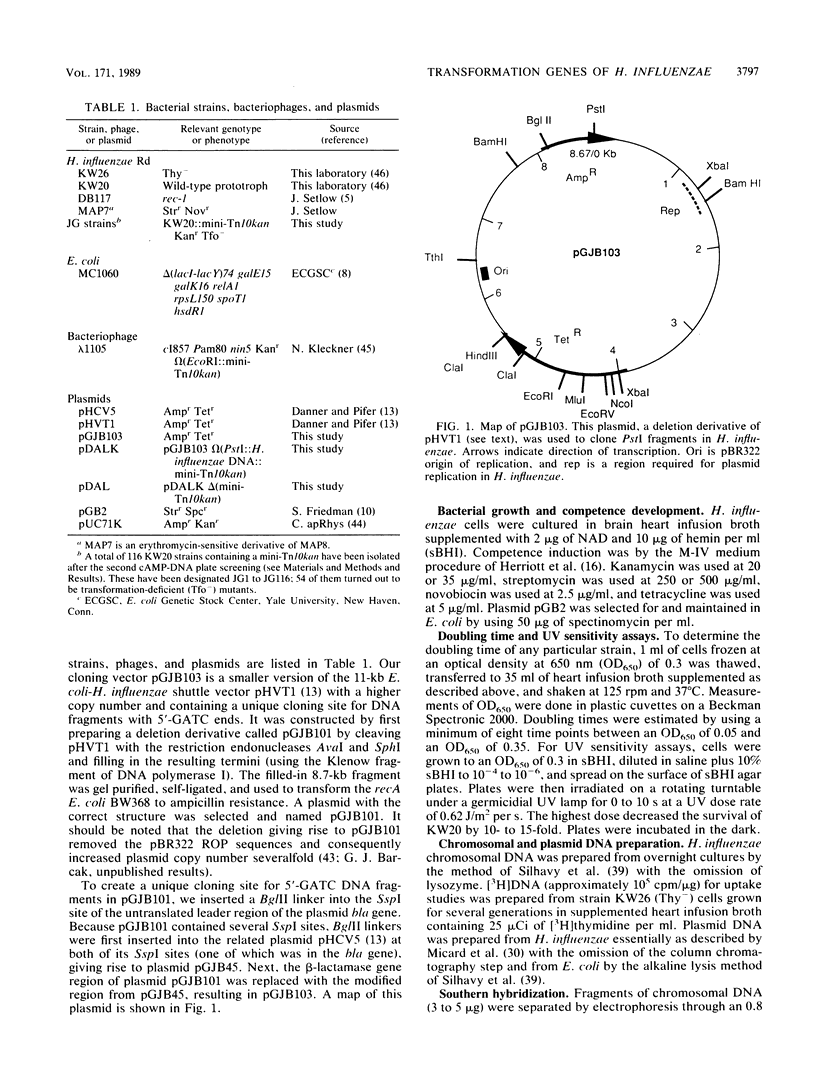
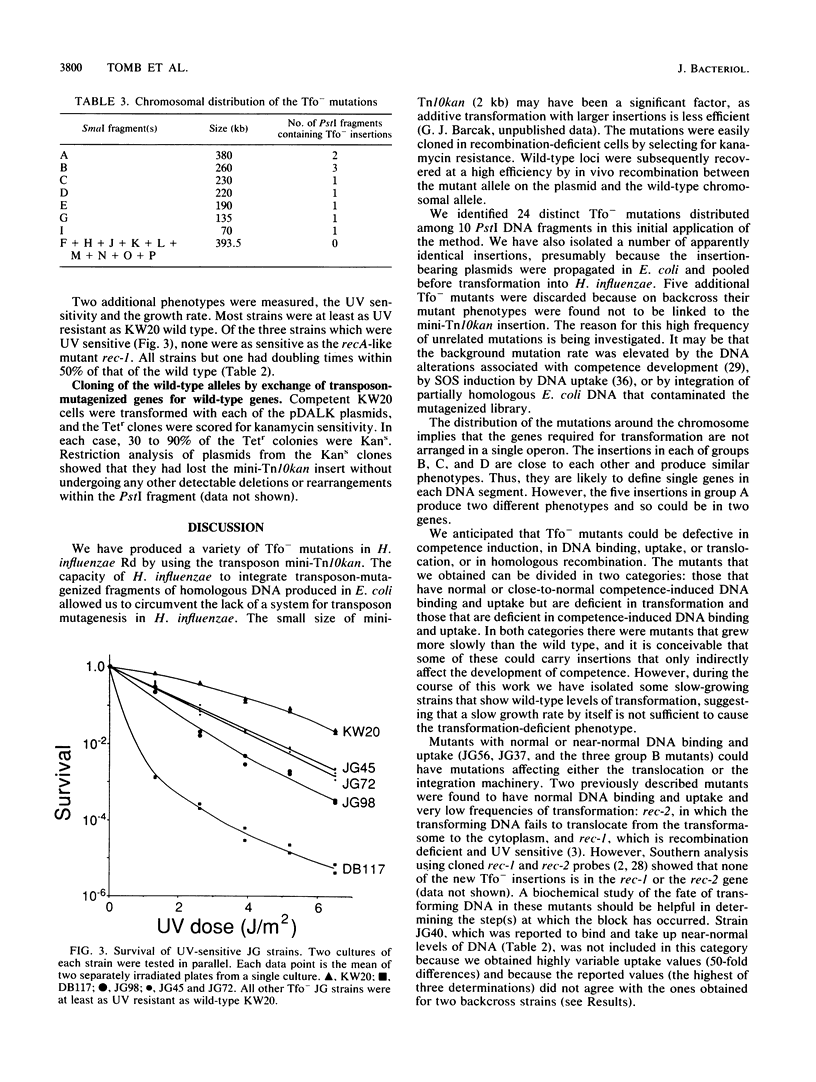
A plasmid library of PstI fragments of Haemophilus influenzae Rd genomic DNA was mutagenized in Escherichia coli with mini-Tn10kan. The mutagenized PstI fragments were introduced by transformation into the H. influenzae chromosome, and kanamycin-resistant transformants were screened for the transformation-deficient phenotype by a cyclic AMP-DNA plate method. Fifty-four mutant strains containing 24 unique insertions that mapped to 10 different PstI fragments were isolated. Strains carrying unique insertions were tested individually for DNA uptake, transformation efficiency, UV sensitivity, and growth rate. The transformation frequencies of these mutants were decreased by factors of 10(-2) to 10(-6). Five of the mutants had normal competence-induced DNA uptake, and the rest were variably deficient in competence development. Three strains were moderately UV sensitive. All strains but one had doubling times within 50% of that of the wild type. Mutated genes were cloned into an H. influenzae-E. coli shuttle vector, and wild-type loci were recovered by in vivo recombinational exchange. Hybridization of these clones to SmaI genomic fragments separated in pulsed-field gels showed that these insertions were not clustered in a particular region of the chromosome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F., Kahn M. E., Smith H. O. Directional transport and integration of donor DNA in Haemophilus influenzae transformation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7274–7278. doi: 10.1073/pnas.80.23.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Tomb J. F., Laufer C. S., Smith H. O. Two Haemophilus influenzae Rd genes that complement the recA-like mutation rec-1. J Bacteriol. 1989 May;171(5):2451–2457. doi: 10.1128/jb.171.5.2451-2457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R., Smith H. O. Initial steps in Haemophilus influenzae transformation. Donor DNA binding in the com10 mutant. J Biol Chem. 1986 Jul 5;261(19):8617–8623. [PubMed] [Google Scholar]
- Barouki R., Smith H. O. Reexamination of phenotypic defects in rec-1 and rec-2 mutants of Haemophilus influenzae Rd. J Bacteriol. 1985 Aug;163(2):629–634. doi: 10.1128/jb.163.2.629-634.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie K. L., Setlow J. K. Transformation-defective strains of Haemophilus influenzae. Nat New Biol. 1971 Jun 9;231(23):177–179. doi: 10.1038/newbio231177a0. [DOI] [PubMed] [Google Scholar]
- Beattie K. L., Wakil A. E., Driggers P. H. Action of restriction endonucleases on transforming DNA of Haemophilus influenzae. J Bacteriol. 1982 Oct;152(1):332–337. doi: 10.1128/jb.152.1.332-337.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K., Allison D. P. Bacteriophage of Haemophilus influenzae. I. Differences between infection by whole phage, extracted phage DNA and prophage DNA extracted from lysogenic cells. J Mol Biol. 1972 Feb 14;63(3):335–348. doi: 10.1016/0022-2836(72)90431-7. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Caster J. H., Postel E. H., Goodgal S. H. Competence mutants: isolation of transformation deficient strains of Haemophilus influenzae. Nature. 1970 Aug 1;227(5257):515–517. doi: 10.1038/227515a0. [DOI] [PubMed] [Google Scholar]
- Churchward G., Belin D., Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984 Nov;31(1-3):165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. DNA-binding vesicles released from the surface of a competence-deficient mutant of Haemophilus influenzae. J Bacteriol. 1982 Oct;152(1):441–450. doi: 10.1128/jb.152.1.441-450.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. Haemophilus influenzae polypeptides involved in deoxyribonucleic acid uptake detected by cellular surface protein iodination. J Bacteriol. 1981 Oct;148(1):220–231. doi: 10.1128/jb.148.1.220-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Pifer M. L. Plasmid cloning vectors resistant to ampicillin and tetracycline which can replicate in both E. coli and Haemophilus cells. Gene. 1982 Apr;18(1):101–105. doi: 10.1016/0378-1119(82)90062-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Barany F., Smith H. O. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6927–6931. doi: 10.1073/pnas.80.22.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Maul G., Goodgal S. H. Possible mechanism for donor DNA binding and transport in Haemophilus. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6370–6374. doi: 10.1073/pnas.79.20.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Smith H. O. Transformation in Haemophilus: a problem in membrane biology. J Membr Biol. 1984;81(2):89–103. doi: 10.1007/BF01868974. [DOI] [PubMed] [Google Scholar]
- Kooistra J., Venema G. Fate of donor DNA in some poorly transformable strains of Haemophilus influenzae. Mutat Res. 1970 Mar;9(3):245–253. doi: 10.1016/0027-5107(70)90126-0. [DOI] [PubMed] [Google Scholar]
- Kooistra J., Venema G. Properties of Haemophilus influenzae mutants that are slightly recombination deficient and carry a mutation in the rec-1 gene region. J Bacteriol. 1980 Jun;142(3):829–835. doi: 10.1128/jb.142.3.829-835.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., van Boxel T., Venema G. Characterization of a conditionally transformation-deficient mutant of Haemophilus influenzae that carries a mutation in the rec-1 gene region. J Bacteriol. 1983 Feb;153(2):852–860. doi: 10.1128/jb.153.2.852-860.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O. Sizing of the Haemophilus influenzae Rd genome by pulsed-field agarose gel electrophoresis. J Bacteriol. 1988 Sep;170(9):4402–4405. doi: 10.1128/jb.170.9.4402-4405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. Cloning of the rec-2 locus of Haemophilus influenzae. Gene. 1989 Jan 30;75(1):135–143. doi: 10.1016/0378-1119(89)90390-9. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Kupfer D. M. Electron microscopy of single-stranded structures in the DNA of competent Haemophilus influenzae cells. J Bacteriol. 1987 Feb;169(2):565–571. doi: 10.1128/jb.169.2.565-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. Plasmid recombination in Haemophilus influenzae. J Mol Biol. 1982 Jun 5;157(4):577–596. doi: 10.1016/0022-2836(82)90500-9. [DOI] [PubMed] [Google Scholar]
- Micard D., Sobrier M. L., Couderc J. L., Dastugue B. Purification of RNA-free plasmid DNA using alkaline extraction followed by Ultrogel A2 column chromatography. Anal Biochem. 1985 Jul;148(1):121–126. doi: 10.1016/0003-2697(85)90636-0. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Huang P. C. Identification of competence-repressing factors during log-phase growth of Haemophilus influenzae. J Bacteriol. 1972 Feb;109(2):560–564. doi: 10.1128/jb.109.2.560-564.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifer M. L., Smith H. O. Processing of donor DNA during Haemophilus influenzae transformation: analysis using a model plasmid system. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3731–3735. doi: 10.1073/pnas.82.11.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Herriott R. M. Inosine and lactate: factors critical during growth for development of competence in Haemophilus influenzae. Biochem Biophys Res Commun. 1966 Mar 8;22(5):591–596. doi: 10.1016/0006-291x(66)90316-0. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M., Lichstein H. C. Effect of selected antibiotics and other inhibitors on competence development in Haemophilus influenzae. J Gen Microbiol. 1969 Jan;55(1):37–43. doi: 10.1099/00221287-55-1-37. [DOI] [PubMed] [Google Scholar]
- Scocca J. J., Habersat M. Synchronous division and rates of macromolecular synthesis in Haemophilus influenzae competent for genetic transformation. J Bacteriol. 1978 Sep;135(3):961–967. doi: 10.1128/jb.135.3.961-967.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Cabrera-Juárez E., Griffin K. Mechanism of acquisition of chromosomal markers by plasmids in Haemophilus influenzae. J Bacteriol. 1984 Nov;160(2):662–667. doi: 10.1128/jb.160.2.662-667.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H., Walter R. B. Addition, deletion, and substitution of long nonhomologous deoxyribonucleic acid segments by genetic transformation of Haemophilus influenzae. J Bacteriol. 1981 Nov;148(2):565–571. doi: 10.1128/jb.148.2.565-571.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H., Walter R. B. Effect of glycerol on plasmid transfer in genetically competent Haemophilus influenzae. Mol Gen Genet. 1986 May;203(2):296–299. doi: 10.1007/BF00333969. [DOI] [PubMed] [Google Scholar]
- Sutrina S. L., Scocca J. J. Haemophilus influenzae periplasmic protein which binds deoxyribonucleic acid: properties and possible participation in genetic transformation. J Bacteriol. 1979 Sep;139(3):1021–1027. doi: 10.1128/jb.139.3.1021-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Smith H. O. Isolation and characterization of mutants of Haemophilus influenzae deficient in an adenosine 5'-triphosphate-dependent deoxyribonuclease activity. J Bacteriol. 1975 May;122(2):443–453. doi: 10.1128/jb.122.2.443-453.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Alexander S. P., Powers M. Adenosine 3':5'-cyclic monophosphate as a regulator of bacterial transformation. Proc Natl Acad Sci U S A. 1973 Feb;70(2):471–474. doi: 10.1073/pnas.70.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Habersat M., Scocca J. J. Multiple regulatory events in the development of competence for genetic transformation in Haemophilus influenzae. J Bacteriol. 1975 Dec;124(3):1607–1609. doi: 10.1128/jb.124.3.1607-1609.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Habersat M., Scocca J. J. Synthesis of envelope polypeptides by Haemophilus influenzae during development of competence for genetic transformation. J Bacteriol. 1976 Jul;127(1):545–554. doi: 10.1128/jb.127.1.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Scocca J. J. Constitution of the cell envelope of Haemophilus influenzae in relation to competence for genetic transformation. J Bacteriol. 1975 Aug;123(2):666–677. doi: 10.1128/jb.123.2.666-677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


