Abstract
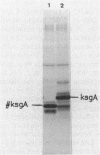
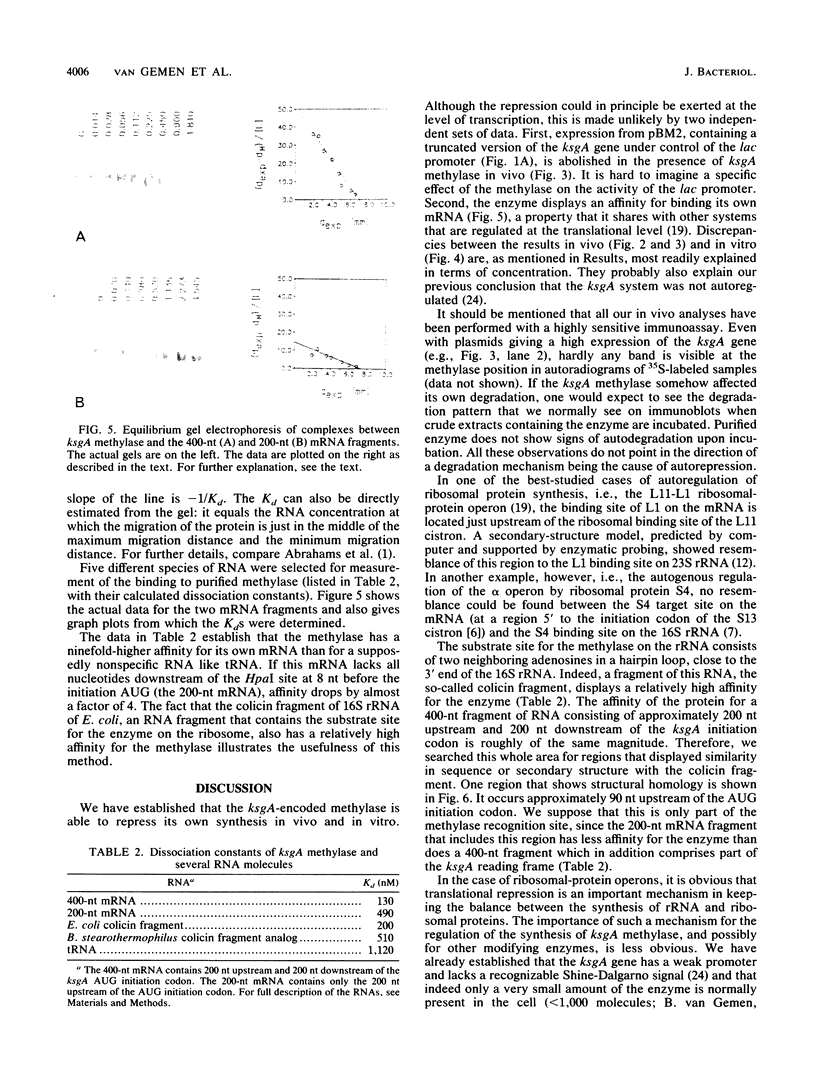
Various plasmids that contain the Escherichia coli ksgA gene, which encodes a 16S rRNA adenosine dimethyltransferase (methylase), were constructed. In one of these plasmids, the DNA encoding the N-terminal part of the methylase was fused to the lacZ gene, and in another construct, the ksgA gene contained a deletion which resulted in a truncated version of the methylase. When a cell contained one plasmid directing the synthesis of the intact, active methylase and another plasmid encoding the methylase-beta-galactosidase protein, production of the latter product became strongly reduced. Likewise, synthesis of the truncated version of the methylase was diminished when the cell at the same time contained a plasmid producing the complete enzyme. These results were partly substantiated by in vitro experiments with a coupled transcription-translation assay system. By using a recently developed gel electrophoresis system for measuring protein-nucleic acid interactions, a specific binding of the ksgA methylase with its own mRNA could be established. Our results demonstrate that the expression of the ksgA gene can be, at least partly, autogenously controlled at the level of translation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams J. P., Kraal B., Bosch L. Zone-interference gel electrophoresis: a new method for studying weak protein-nucleic acid complexes under native equilibrium conditions. Nucleic Acids Res. 1988 Nov 11;16(21):10099–10108. doi: 10.1093/nar/16.21.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchin-Roland S., Blanquet S., Schmitter J. M., Fayat G. The gene for Escherichia coli diadenosine tetraphosphatase is located immediately clockwise to folA and forms an operon with ksgA. Mol Gen Genet. 1986 Dec;205(3):515–522. doi: 10.1007/BF00338091. [DOI] [PubMed] [Google Scholar]
- Bosch L., Kraal B., Van der Meide P. H., Duisterwinkel F. J., Van Noort J. M. The elongation factor EF-Tu and its two encoding genes. Prog Nucleic Acid Res Mol Biol. 1983;30:91–126. doi: 10.1016/s0079-6603(08)60684-4. [DOI] [PubMed] [Google Scholar]
- Butler J. S., Springer M., Dondon J., Graffe M., Grunberg-Manago M. Escherichia coli protein synthesis initiation factor IF3 controls its own gene expression at the translational level in vivo. J Mol Biol. 1986 Dec 20;192(4):767–780. doi: 10.1016/0022-2836(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Deckman I. C., Draper D. E. S4-alpha mRNA translation regulation complex. II. Secondary structures of the RNA regulatory site in the presence and absence of S4. J Mol Biol. 1987 Jul 20;196(2):323–332. doi: 10.1016/0022-2836(87)90693-0. [DOI] [PubMed] [Google Scholar]
- Deckman I. C., Draper D. E. Specific interaction between ribosomal protein S4 and the alpha operon messenger RNA. Biochemistry. 1985 Dec 31;24(27):7860–7865. doi: 10.1021/bi00348a002. [DOI] [PubMed] [Google Scholar]
- Denoya C. D., Bechhofer D. H., Dubnau D. Translational autoregulation of ermC 23S rRNA methyltransferase expression in Bacillus subtilis. J Bacteriol. 1986 Dec;168(3):1133–1141. doi: 10.1128/jb.168.3.1133-1141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. Translational attenuation: the regulation of bacterial resistance to the macrolide-lincosamide-streptogramin B antibiotics. CRC Crit Rev Biochem. 1984;16(2):103–132. doi: 10.3109/10409238409102300. [DOI] [PubMed] [Google Scholar]
- Heus H. A., van Kimmenade J. M., van Knippenberg P. H., Haasnoot C. A., de Bruin S. H., Hilbers C. W. High-resolution proton magnetic resonance studies of the 3'-terminal colicin fragment of 16 S ribosomal RNA from Escherichia coli. Assignment of iminoproton resonances by nuclear Overhauser effect experiments and the influence of adenine dimethylation on the hairpin conformation. J Mol Biol. 1983 Nov 15;170(4):939–956. doi: 10.1016/s0022-2836(83)80197-1. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Wilson H. W., Novick W. J., Jr In vitro evaluation of pyridine-2-azo-p-dimethylaniline cephalosporin, a new diagnostic chromogenic reagent, and comparison with nitrocefin, cephacetrile, and other beta-lactam compounds. J Clin Microbiol. 1982 Apr;15(4):677–683. doi: 10.1128/jcm.15.4.677-683.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney K. R., Nomura M. Secondary structure of the autoregulatory mRNA binding site of ribosomal protein L1. Mol Gen Genet. 1987 Nov;210(1):60–68. doi: 10.1007/BF00337759. [DOI] [PubMed] [Google Scholar]
- Looman A. C., de Gruyter M., Vogelaar A., van Knippenberg P. H. Effects of heterologous ribosomal binding sites on the transcription and translation of the lacZ gene of Escherichia coli. Gene. 1985;37(1-3):145–154. doi: 10.1016/0378-1119(85)90267-7. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan C. S., Dubnau D. Evidence for the translational attenuation model: ribosome-binding studies and structural analysis with an in vitro run-off transcript of ermC. Nucleic Acids Res. 1985 Oct 25;13(20):7307–7326. doi: 10.1093/nar/13.20.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldermans B., Roza L., Van Knippenberg P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3' end of 16 S ribosomal RNA of Escherichia coli. III. Purification and properties of the methylating enzyme and methylase-30 S interactions. J Biol Chem. 1979 Sep 25;254(18):9094–9100. [PubMed] [Google Scholar]
- Springer M., Graffe M., Butler J. S., Grunberg-Manago M. Genetic definition of the translational operator of the threonine-tRNA ligase gene in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4384–4388. doi: 10.1073/pnas.83.12.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorov A. N., van Gemen B., van Knippenberg P. H. Increased kasugamycin sensitivity in Escherichia coli caused by the presence of an inducible erythromycin resistance (erm) gene of Streptococcus pyogenes. Mol Gen Genet. 1988 Dec;215(1):152–155. doi: 10.1007/BF00331317. [DOI] [PubMed] [Google Scholar]
- van Buul C. P., van Knippenberg P. H. Nucleotide sequence of the ksgA gene of Escherichia coli: comparison of methyltransferases effecting dimethylation of adenosine in ribosomal RNA. Gene. 1985;38(1-3):65–72. doi: 10.1016/0378-1119(85)90204-5. [DOI] [PubMed] [Google Scholar]
- van Gemen B., Koets H. J., Plooy C. A., Bodlaender J., Van Knippenberg P. H. Characterization of the ksgA gene of Escherichia coli determining kasugamycin sensitivity. Biochimie. 1987 Aug;69(8):841–848. doi: 10.1016/0300-9084(87)90210-0. [DOI] [PubMed] [Google Scholar]