Abstract
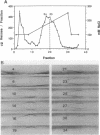
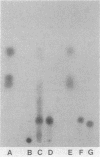
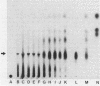
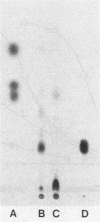
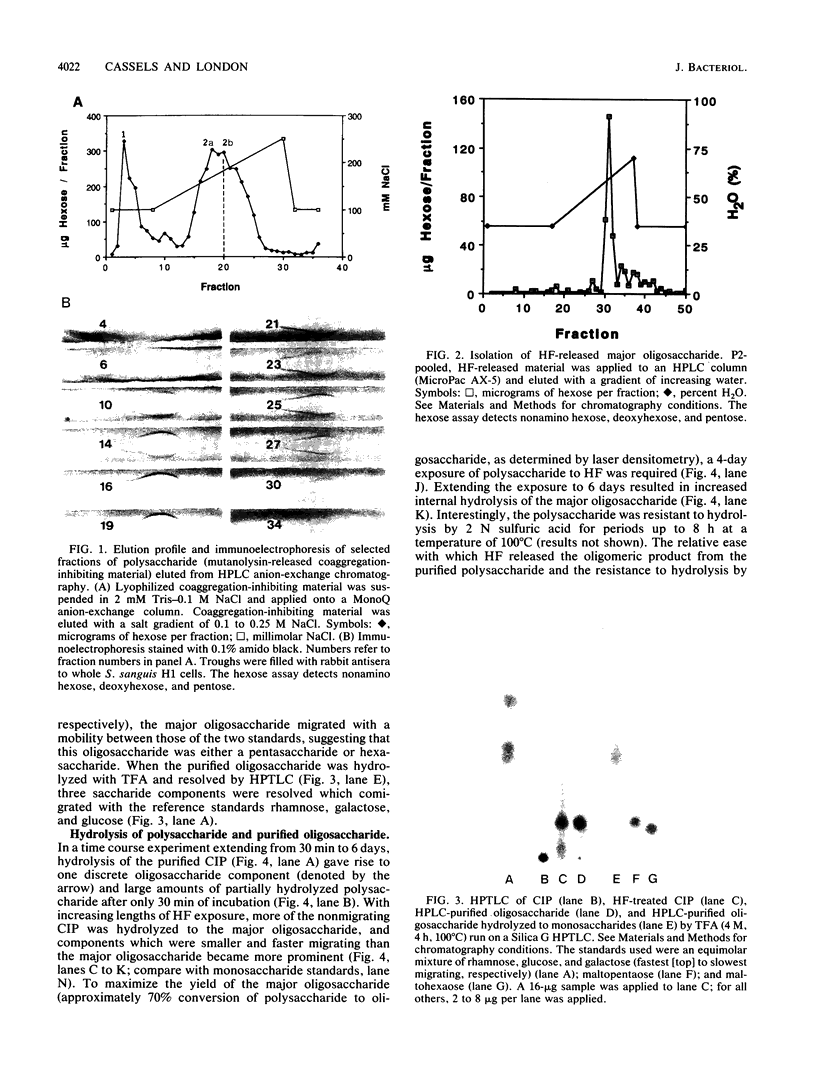
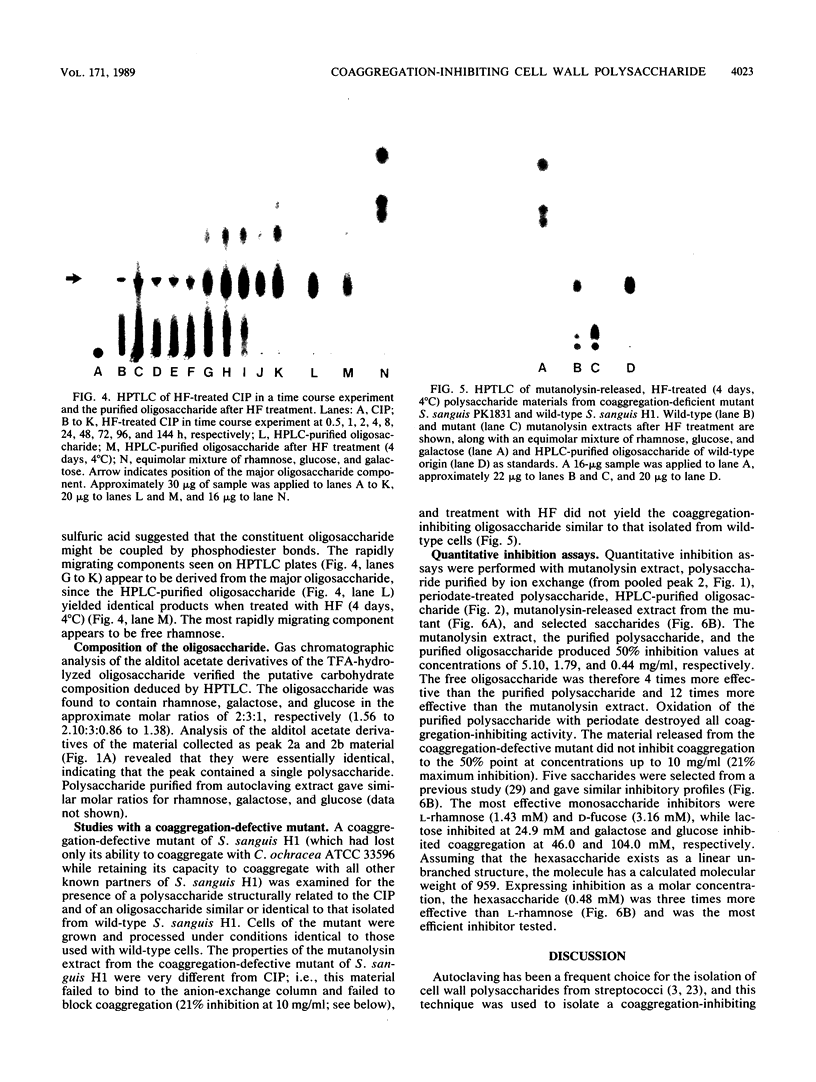
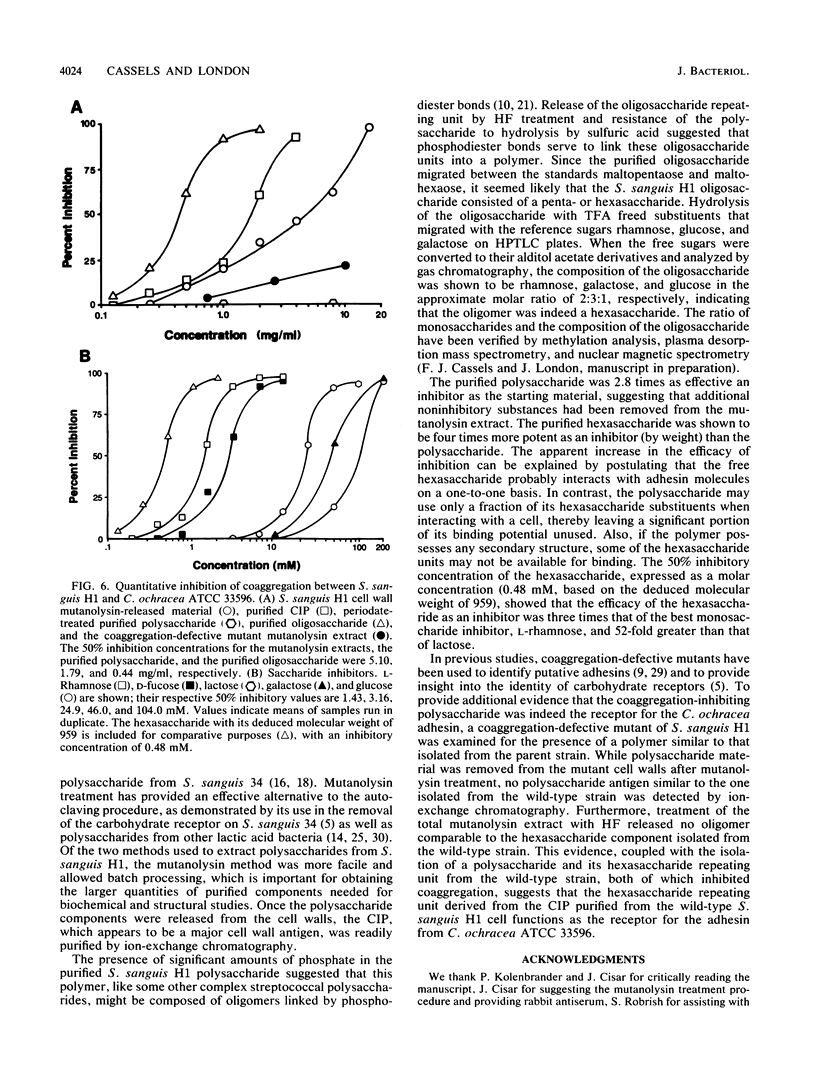
Coaggregation between Streptococcus sanguis H1 and Capnocytophaga ochracea ATCC 33596 cells is mediated by a carbohydrate receptor on the former and an adhesin on the latter. Two methods were used to release the carbohydrate receptor from the gram-positive streptococcus, autoclaving and mutanolysin treatment. The polysaccharide released from the streptococcal cell wall by either treatment was purified by ion-exchange chromatography; this polysaccharide inhibited coaggregation when preincubated with the gram-negative capnocytophaga partner. After hydrolysis of the polysaccharide by hydrofluoric acid (HF), the major oligosaccharide of the polysaccharide was purified by high-performance liquid chromatography. By analysis of the HF hydrolysis of the polysaccharide and the purified oligosaccharide, this major oligosaccharide appeared to be the repeating unit of the polysaccharide, with minor components resulting from internal hydrolysis of the major oligosaccharide. Gas chromatography results showed that the oligomer was a hexasaccharide, consisting of rhamnose, galactose, and glucose, in the ratio of 2:3:1, respectively. By weight, the purified hexasaccharide was a fourfold-more-potent inhibitor of coaggregation than the native polysaccharide. Resistance to hydrolysis by sulfuric acid alone and susceptibility to hydrolysis by HF suggested that oligosaccharide chains of the polysaccharide are linked by phosphodiester bonds. Studies with a coaggregation-defective mutant of S. sanguis H1 revealed that the cell walls of the mutant contained neither the polysaccharide nor the hexasaccharide repeating unit. The purification of both a polysaccharide and its constituent hexasaccharide repeating unit, which both inhibited coaggregation, and the absence of this polysaccharide or hexasaccharide on a coaggregation-defective mutant strongly suggest that the hexasaccharide derived from the polysaccharide functions as the receptor for the adhesin from C. ochracea ATCC 33596.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell L. K., Knox K. W., Wicken A. J. Extractability of cell wall polysaccharide from lactobacilli and streptococci by autoclaving and by dilue acid. Infect Immun. 1978 Dec;22(3):842–851. doi: 10.1128/iai.22.3.842-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Morgan S. L., Hudson J. R., Zhu Z. T., Lau P. Y. Capillary gas chromatographic analysis of alditol acetates of neutral and amino sugars in bacterial cell walls. J Chromatogr. 1983 Feb 18;256(3):429–438. doi: 10.1016/s0021-9673(01)88260-1. [DOI] [PubMed] [Google Scholar]
- Kagermeier A., London J. Identification and preliminary characterization of a lectinlike protein from Capnocytophaga gingivalis (emended). Infect Immun. 1986 Feb;51(2):490–494. doi: 10.1128/iai.51.2.490-494.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- Linzer R., Reddy M. S., Levine M. J. Structural studies of the rhamnose-glucose polysaccharide antigen from Streptococcus sobrinus B13 and 6715-T2. Infect Immun. 1985 Nov;50(2):583–585. doi: 10.1128/iai.50.2.583-585.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Bush C. A., Wu S. S., Li S. C., Li Y. T., McNeil M., Tjoa S. S., Fennessey P. V. Structure of a new hexasaccharide from the coaggregation polysaccharide of Streptococcus sanguis 34. Carbohydr Res. 1987 Aug 15;166(1):133–143. doi: 10.1016/0008-6215(87)80050-2. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Crosby L. K., Vatter A. E., Cisar J. O., McNeil M. R., Bush C. A., Tjoa S. S., Fennessey P. V. A polysaccharide from Streptococcus sanguis 34 that inhibits coaggregation of S. sanguis 34 with Actinomyces viscosus T14V. J Bacteriol. 1988 May;170(5):2229–2235. doi: 10.1128/jb.170.5.2229-2235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):276–280. doi: 10.1016/0003-2697(81)90480-2. [DOI] [PubMed] [Google Scholar]
- Michon F., Katzenellenbogen E., Kasper D. L., Jennings H. J. Structure of the complex group-specific polysaccharide of group B Streptococcus. Biochemistry. 1987 Jan 27;26(2):476–486. doi: 10.1021/bi00376a020. [DOI] [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K. Cell-wall lipopolysaccharide from Escherichia coli B. Eur J Biochem. 1975 Aug 1;56(1):41–55. doi: 10.1111/j.1432-1033.1975.tb02205.x. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- SCHIFFMAN G., KABAT E. A., THOMPSON W. IMMUNOCHEMICAL STUDIES ON BLOOD GROUPS. XXX. CLEAVAGE OF A, B, AND H BLOOD-GROUP SUBSTANCES BY ALKALI. Biochemistry. 1964 Jan;3:113–120. doi: 10.1021/bi00889a018. [DOI] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Warren C. D., Schmit A. S., Jeanloz R. W. Chromatographic separation of oligosaccharides from mannosidosis urine. Carbohydr Res. 1983 Jun 1;116(2):171–182. doi: 10.1016/0008-6215(83)88107-5. [DOI] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Kagermeier A. S., Andersen R. N. Characterization of lectinlike surface components on Capnocytophaga ochracea ATCC 33596 that mediate coaggregation with gram-positive oral bacteria. Infect Immun. 1987 May;55(5):1198–1202. doi: 10.1128/iai.55.5.1198-1202.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]