Abstract
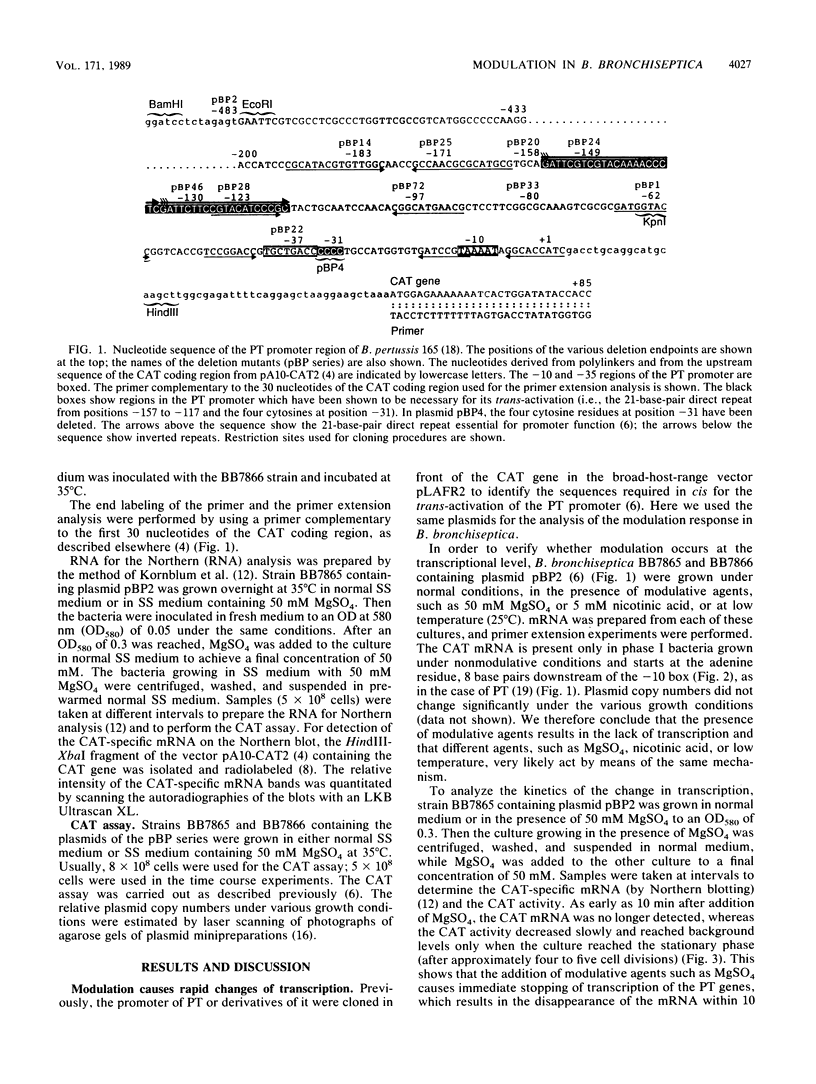
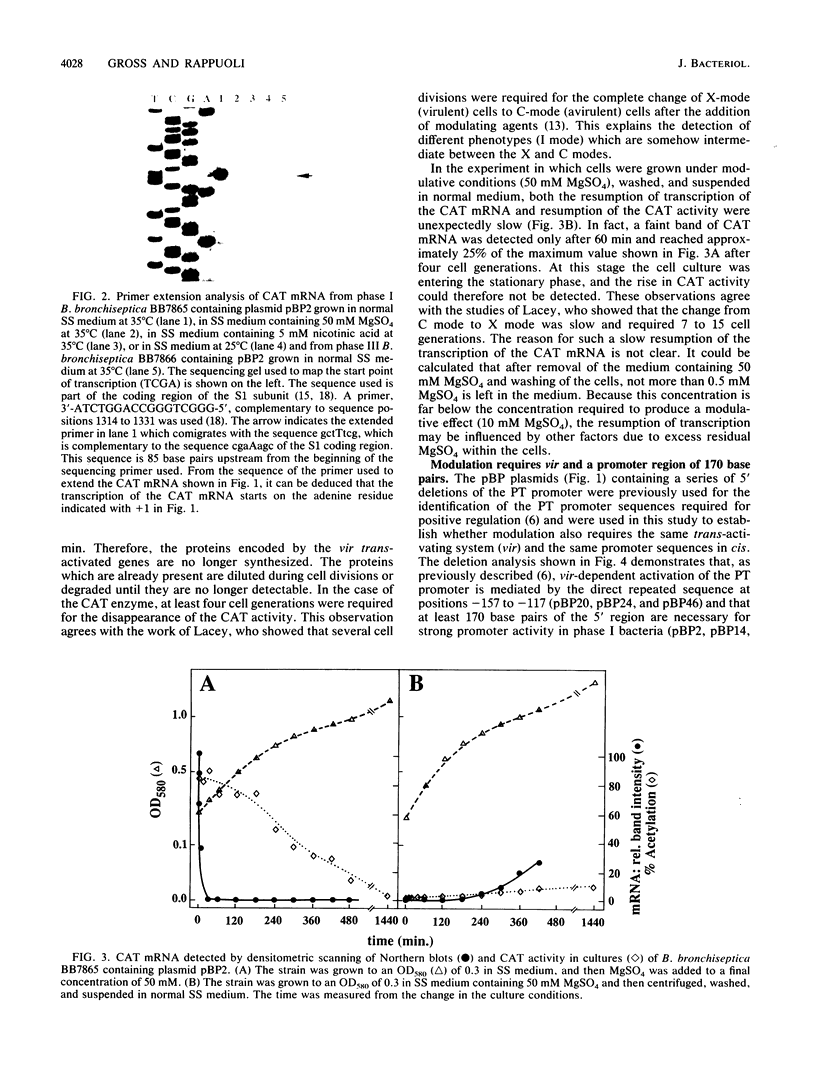
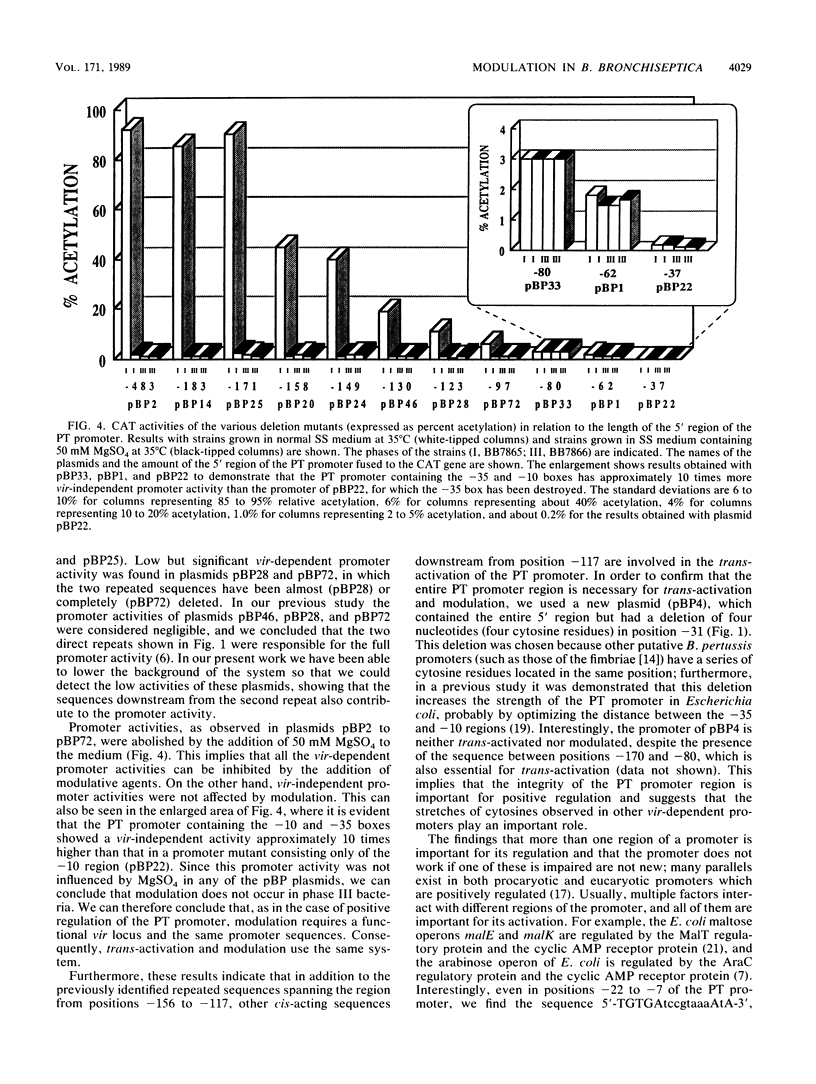
Previous analysis of the pertussis toxin (PT) promoter has shown that expression of PT requires a trans-activating factor encoded by the vir locus and a 170-base-pair DNA sequence upstream from the transcription start site containing a 21-base-pair direct repeat sequence crucial trans-activation (R. Gross and R. Rappuoli, Proc. Natl. Acad. Sci. USA 85:3913-3917, 1988). In this paper we extend the analysis to the modulative response to environmental stimuli. We show that modulation acts at the transcriptional level and occurs only in phase I bacteria. Modulation also requires a functional vir locus and the same promoter region of 170 base pairs. We show that, in addition to the previously identified direct repeat, even the sequences downstream from position -117 are required for trans-activation and modulation and that the deletion of four cytosine residues at position -31 causes the inactivation of the promoter. The kinetics of the change in transcription show that the PT promoter can be shut off very rapidly by adding 50 mM MgSO4 to the medium, whereas resumption of transcription after removal of the modulative agents from the medium is slow.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aricò B., Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987 Jun;169(6):2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio C., Colantuoni V., Cortese R. Structure and cell-specific expression of a cloned human retinol binding protein gene: the 5'-flanking region contains hepatoma specific transcriptional signals. EMBO J. 1985 Aug;4(8):1981–1989. doi: 10.1002/j.1460-2075.1985.tb03881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Gross R., Rappuoli R. Positive regulation of pertussis toxin expression. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3913–3917. doi: 10.1073/pnas.85.11.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Hendrickson W., Schleif R. Transcription of Escherichia coli ara in vitro. The cyclic AMP receptor protein requirement for PBAD induction that depends on the presence and orientation of the araO2 site. J Mol Biol. 1986 Apr 5;188(3):355–367. doi: 10.1016/0022-2836(86)90160-9. [DOI] [PubMed] [Google Scholar]
- Hodgson C. P., Fisk R. Z. Hybridization probe size control: optimized 'oligolabelling'. Nucleic Acids Res. 1987 Aug 11;15(15):6295–6295. doi: 10.1093/nar/15.15.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. An E. coli promoter that regulates transcription by DNA superhelix-induced cruciform extrusion. Science. 1988 Aug 5;241(4866):703–705. doi: 10.1126/science.2456617. [DOI] [PubMed] [Google Scholar]
- Husslein V., Huhle B., Jarchau T., Lurz R., Goebel W., Chakraborty T. Nucleotide sequence and transcriptional analysis of the aerCaerA region of Aeromonas sobria encoding aerolysin and its regulatory region. Mol Microbiol. 1988 Jul;2(4):507–517. doi: 10.1111/j.1365-2958.1988.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Idigbe E. O., Parton R., Wardlaw A. C. Rapidity of antigenic modulation of Bordetella pertussis in modified Hornibrook medium. J Med Microbiol. 1981 Nov;14(4):409–418. doi: 10.1099/00222615-14-4-409. [DOI] [PubMed] [Google Scholar]
- Kornblum J. S., Projan S. J., Moghazeh S. L., Novick R. P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63(1):75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livey I., Duggleby C. J., Robinson A. Cloning and nucleotide sequence analysis of the serotype 2 fimbrial subunit gene of Bordetella pertussis. Mol Microbiol. 1987 Sep;1(2):203–209. doi: 10.1111/j.1365-2958.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Monaci P., Nicosia A., Cortese R. Two different liver-specific factors stimulate in vitro transcription from the human alpha 1-antitrypsin promoter. EMBO J. 1988 Jul;7(7):2075–2087. doi: 10.1002/j.1460-2075.1988.tb03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Rappuoli R. Promoter of the pertussis toxin operon and production of pertussis toxin. J Bacteriol. 1987 Jun;169(6):2843–2846. doi: 10.1128/jb.169.6.2843-2846.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]