Abstract
The hippocampus is a malleable brain region that responds to external agents such as hormones and stressors. Investigations that began in our laboratories with the Golgi technique and an appreciation of hippocampal neuroanatomy at the light and electron microscopic levels have led us down a path that has uncovered unexpected structural plasticity in the adult brain along with unanticipated cellular and molecular mechanisms of this plasticity and of hormone mediation of these effects. This chapter reviews the history of discoveries in our two laboratories involving the actions of estradiol and stress hormones on neuronal structure and function and then discusses the insight to hormone-brain interactions that this has engendered. These discoveries have led us to a new view of brain structural plasticity and the role and mechanism of steroid hormone action involving both genomic and non-genomic pathways. This new view is consistent with the predictions of Cajal in his book “The Structure of Ammon’s horn”, 1892.
Keywords: hippocampus, estrogen, glucocorticoid, Golgi, electron microscopy
Introduction
In his book “The structure of Ammon’s horn”, 1892, [pg 726 (Cajal, S. R. 1995)], Ramony Cajal wrote:
“If the ability of neurons to grow and create new associations in the adult explains human adaptability and the facility to change ideational systems, the end of neuronal changes in the aged or in people rigid from lack of education or any other reason, also explains unshakable convictions, an inability to conform to moral standards and even misoneism. One also might imagine that amnesia, a paucity of thought associations, retardation and dementia could result when synapses between neurons are weakened as a result of more or less pathological cortical conditions, that is, when processes atrophy and no longer form contacts, when cortical mnemonic or association areas suffer partial disorganization. Our hypothesis even accounts for the greater conservation of older memories – memories of youth in the aged, amnestic and demented – because association pathways created long ago and repeated for many years obviously acquire even greater strength. In addition, they were formed during a period of life when neuronal plasticity was maximal.”
Camillo Golgi, who shared the Nobel Prize with Cajal in 1907, provided a very important technique for neuroanatomical investigations and Cajal was the visionary neuroanatomist who predicted many aspects of brain structure and plasticity that we and others are finding today. As reflected in the above passage, Cajal had incredible insight into brain plasticity that foreshadowed our current studies of the hippocampus and other brain regions involved in cognition and emotion.
Cajal predicted that structural details of the hippocampal formation ”may shed light on the interpretation of arrangements in other regions of the nervous system” (Cajal, S. R. 1968). Consistent with this prediction, the hippocampal formation has proven to be one of the most intensely studied structures in the brain. The hippocampus is a malleable brain region that responds to external agents such as hormones and stressors. Investigations that began in our laboratories with the Golgi technique and an appreciation of hippocampal neuroanatomy at the light and electron microscopic levels have led us down a path that has uncovered unexpected structural plasticity in the adult brain along with unanticipated cellular and molecular mechanisms of this plasticity and of hormone mediation of these effects. This chapter reviews the history of discoveries in our two laboratories and then discusses the new view of hormone-brain interactions that this has helped to engender.
Studies of neuronal structural plasticity using the Golgi method
In 1968, we discovered that the hippocampus has receptors for adrenal steroid hormones (McEwen et al., 1968). This finding has led to many studies on the behavioral, physiological and molecular actions of stress and stress hormones on this brain region (McEwen, 2007). One missing element in studies of stress effects on the hippocampus was a neuroanatomical analysis, and this issue was dealt with very effectively when Elizabeth Gould introduced the Golgi technique to the McEwen lab in 1990. Serendipitously, many unexpected findings have come from the use of this method that could not have been discovered by other methods very easily, if at all.
First, we found sex differences in the morphology of CA3 pyramidal neurons that altered with thyroid hormone treatment in neonatal life (Gould et al., 1990a;Gould et al., 1990b). Then, Catherine Woolley and Gould (Gould et al., 1990c;Woolley et al., 1990) discovered that estradiol treatment increased the spine synapse density of CA1 pyramidal neurons, a finding that has opened a new area of exploration of estrogen effects on cognitive function (McEwen et al., 1999;Woolley, 1999) (Fig. 1). Next came the result that glucocorticoids and stress cause CA3 neuronal dendritic shrinkage in male rat hippocampus (Woolley et al., 1990), a finding that has opened another area of study on stress effects not only in hippocampus but also in prefrontal cortex and amygdala and led to structural imaging studies on the human hippocampus (McEwen, 2007) (Fig. 2).
Figure 1.
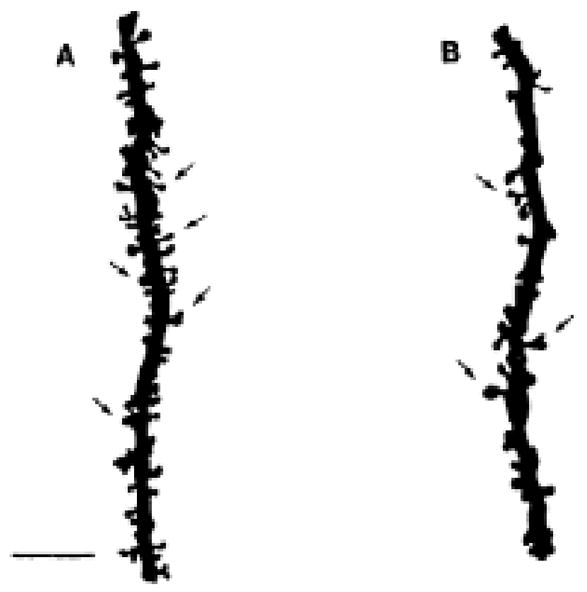
Camera lucida drawings of Golgi impregnated apical dendrites from CA1 pyramidal cells of animals in the proestrus (A) or estrus (B) phase of the estrous cycle. The arrows indicate some of the dendritic spines. Note the greater density of dendritic spines in A compared to B. Scale bar, 10 μm for both A and B. Reprinted from (Woolley et al., 1990) by permission.
Figure 2.
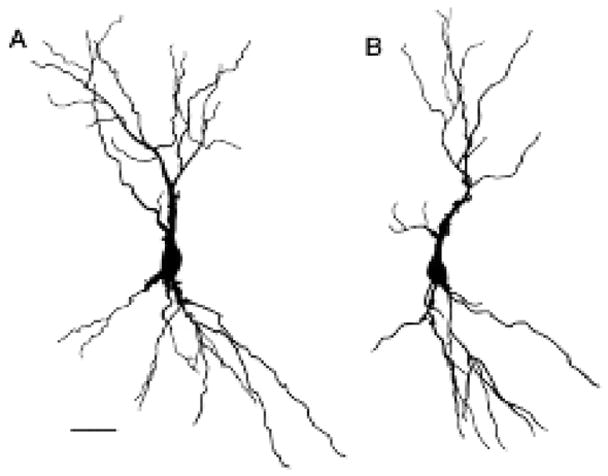
Camera lucida drawings of CA3c pyramidal cells from the brains of sham-injected (A) and corticosterone-injected (B) animals. For these cells, numbers of dendritic branch points are: (A) 15 apical, and 10 basal; (B) 9 apical, and 9 basal. Dendritic length values for these cells are: (A) 1636.9 μm apical, and 1164.7 μm basal; (B) 1136.3 μm apical, and 1285.0 μm basal. Note the decrease in both the number of dendritic branch points and dendritic length in the apical tree of (B) compared to (A) while the basal tree shows no change in these parameters. Scale bar = 50 μm for A and B. Reprinted from (Woolley et al., 1990).
Since the Golgi method requires complete impregnation of neurons and separation between stained neurons to permit the visualization of dendrites, confirmation of the basic findings by other methods is necessary. For spine synapse formation, electron microscopy and stereological evaluation of spine density have confirmed the Golgi results for the hippocampus and also for prefrontal cortex (Leranth et al., 2003; Tang et al., 2004; Woolley et al., 1992). For dendritic remodeling, the use of the dye-filling method has enabled studies of stress effects in prefrontal cortex and confirmation of the ability of stress to cause retraction of dendrites of certain neuron types (Liston et al., 2006;Radley et al., 2004) since, in prefrontal cortex, another laboratory has shown dendritic retraction using the Golgi method (Cook et al., 2004).
Indirectly, the Golgi studies led to the rediscovery by Elizabeth Gould and Heather Cameron that neurogenesis in the dentate gyrus is regulated by adrenal steroids (Cameron et al., 1994). This finding was an extension of a study in which we found that removal of the adrenal glands caused programmed cell death of dentate neurons and atrophy of dendrites of surviving dentate neurons, which could be prevented by giving low dose of adrenal steroids (Gould et al., 1991) (Fig. 3). The follow-up studies using 3H-thymidine led to the reinvestigation of cell proliferation in the dentate gyrus, which could be inhibited by adrenal steroids interacting with excitatory amino acids (EAA) (Cameron et al., 1995).
Figure 3.
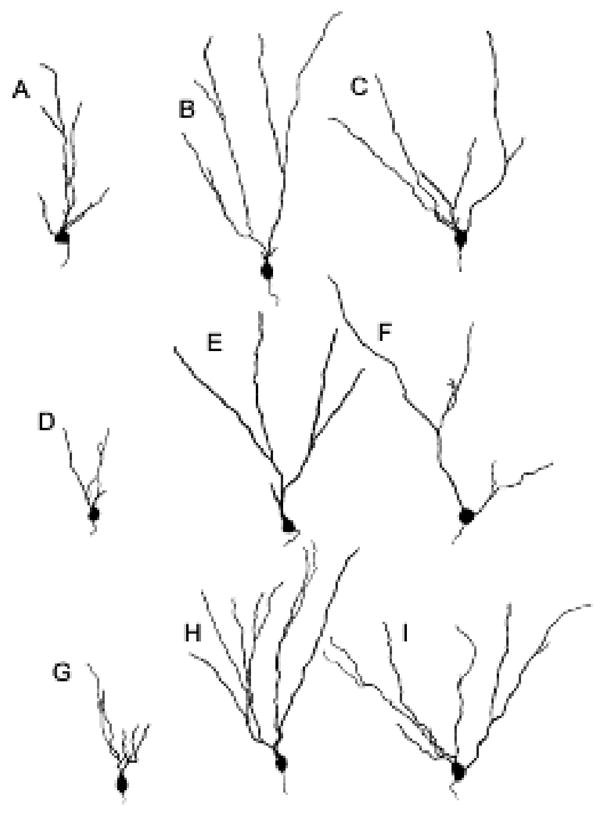
Camera lucida drawings of representative Golgi-impregnated dentate gyrus granule cells from brains of sham operated (A, B, C), adrenalectomized (I, E, F) and adrenalectomized plus corticosterone (G, H, I) animals. There is a decrease in dendritic branch points in all three cell types [granule cells located in the genu (A, D, G), granule cells with single primary dendrites (B, E, H) and granule cells with multiple primary dendrites (C, F, I) with adrenalectomy compared with sham operation and adrenalectomy plus corticosterone. From (Gould et al., 1990d) by permission.
Stress-induced retraction of dendrites in CA3 neurons and estrogen-induced synapse formation are dependent on EAA activity via NMDA receptors, since NMDA blockers will prevent each of these types of changes (Magarinos et al., 1995;Woolley et al., 1994) and estradiol up-regulates NMDA receptor expression (Daniel et al., 2001;Gazzaley et al., 1996;Weiland, 1992;Woolley et al., 1997). In addition to a role for EAA, there is evidence that GABA and acetylcholine are involved in estrogen-induced synapse formation (Daniel et al., 2001;Murphy et al., 1998;Rudick et al., 2003;Rudick et al., 2001) and that GABAergic activity may play an important inhibitory role in stress-induced remodeling of hippocampal neurons (Magarinos et al., 1999;Orchinik et al., 1995). Initial findings in hippocampus using the Golgi method led to the discovery of structural plasticity in other brain regions. One of the most striking findings has been that, in amygdala, repeated immobilization stress causes dendrite expansion and increased anxiety (Vyas et al., 2002). Perhaps even more remarkable and unexpected is that stress causes dendritic expansion in neurons of the orbitofrontal cortex while causing retraction of dendrites in anterior cingulate, prelimbic and infralimbic regions (Cook et al., 2004;Liston et al., 2006;Radley et al., 2004). A behavioral correlate of the mPFC changes is a striking impairment of performance on an attention set shifting task that is impaired by mPFC lesions (Birrell et al., 2000;Liston et al., 2006;McEwen, 2005;Radley et al., 2004). Hence, the reduction in dendritic length and spine synapse density resulting from stress appears to have a strong effect on behavior.
Although the involvement of steroid hormones as well as neurotransmitters in estrogen- and stress-induced structural remodeling is clear, the mechanisms are complex and intriguing. One of the first issues is what type of receptors are involved in mediating the steroid hormone contribution and where are they located? Light microscopic localization of steroid receptors has largely been limited to the detection of cell nuclei, which focus attention on the direct gene regulation effects that steroid hormones have on neuronal function (Pfaff, 1980). Such nuclear actions of steroid hormones have been known since the1960’s (Jensen et al., 1962). However, there is an emerging story of non-nuclear steroid receptors in the brain and other tissues that has come about, in part, via immunocytochemistry using the electron microscope (Milner et al., 2001). As noted above, Woolley and Gould, using the Golgi method, discovered that the hippocampus, a brain region known to have few cell nuclear estrogen receptors (Weiland et al., 1997), shows a strong effect of estradiol on spine synapse formation (Gould et al., 1990c). This investigation (Woolley et al., 1990) led us to look for other types of estrogen receptors, and that, in turn, has led to a unique view of how estradiol and other steroid hormones exert their effects on the brain.
Towards a novel mechanism of hormone- regulated structural plasticity
Localization of gonadal steroid receptors in the hippocampal formation
The existence of estrogen effects in hippocampal formation where there is a paucity of nuclear receptors has led to a search for other mechanisms and sites of estrogen action. Somewhat serendipitously, we conducted a study with electron microscopic immunocytochemistry to localize non-nuclear estrogen receptor (ER) α in the rat hippocampal formation (Milner et al., 2001). We have subsequently gone on to examine the localization of ERβ as well as androgen and progestin receptors in the hippocampal formation. Below is a brief synopsis of these findings.
ERα
Our earlier light microscopic studies showed that ERα-immunoreactivity (ERα-ir) in the hippocampal formation is found in the nuclei of scattered inhibitory GABAergic interneurons (Weiland et al., 1997) (Fig. 4A). Our ultrastructural analysis (Milner et al., 2001) revealed that in addition to interneuron nuclei, ERα-ir was affiliated with the cytoplasmic face of the plasmalemma in select interneurons and with endosomes of a few principal (pyramidal and granule) cells. Moreover, extranuclear ERα labeling was found in profiles that were dispersed throughout the hippocampal formation, but were slightly more numerous in CA1 stratum radiatum (Figs. 5, 8); this latter region is the same area that undergoes estrogen-induced synaptic plasticity (Gould et al., 1990c; Woolley et al., 1990). About half of the ERα-labeled profiles were unmyelinated axons and axon terminals that contained numerous small, synaptic vesicles. ERα-labeled terminals formed symmetric and asymmetric synapses on dendritic shafts and spines suggesting that ERα-containing profiles arise from inhibitory as well as excitatory neurons. As described later, some ERα-containing terminals were cholinergic (Towart et al., 2003). Approximately one-quarter of the ERα-labeled profiles were dendritic spines, many originating from principal cells; ERα-ir was often associated with spine apparati, suggesting that estrogen might act locally through the ERα to influence protein synthesis during synaptic remodeling, topics that will be touched on below. The remaining quarter of ERα-labeled profiles were glia that resembled astrocytes and were often located near the spines of principal cells. Recently, low levels of ERα–ir were identified in microglia using c-fms-EGFP mice as a means of identifying microglial processes (A. Sierra-Saavedra. A.C. Gottfried-Blackmore, T.A. Milner, B.S. McEwen, K. Bulloch, unpublished).
Figure 4.
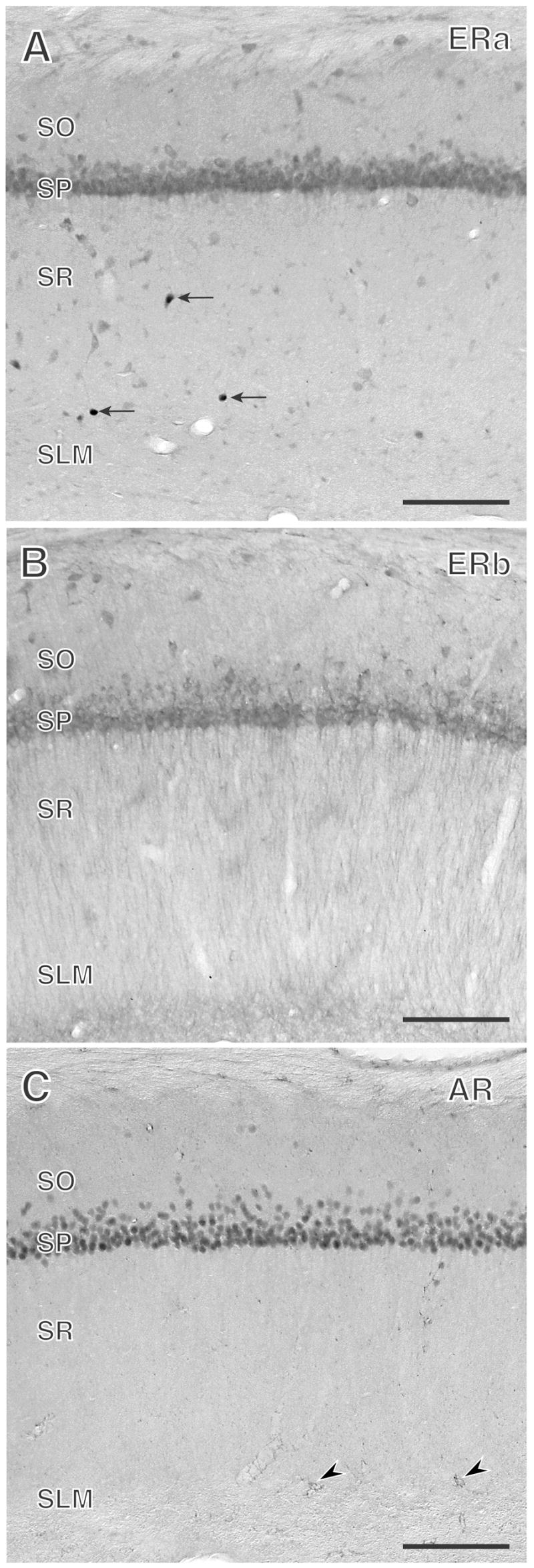
ERα-, ERβ-, and AR- immunoreactivities have overlapping, yet distinct, distributions in the CA1 region of the rat hippocampus. A. ERα-immunoreactive nuclei (arrows) are found primarily in the border of strata radiatum (SR) and lacunosum-moleculare (SLM). B. Extranuclear ERβ-immunoreactivity is evident in pyramidal cell perikarya and their apical dendrites in SR. A few, scattered ERβ-labeled interneuron perikarya also are found. C. AR-immunoreactivity is primarily observed in pyramidal cell nuclei and in a few scattered glial processes (arrowheads). SO, stratum oriens; SP, stratum pyramidale. Bars, 500 μm
Figure 5.
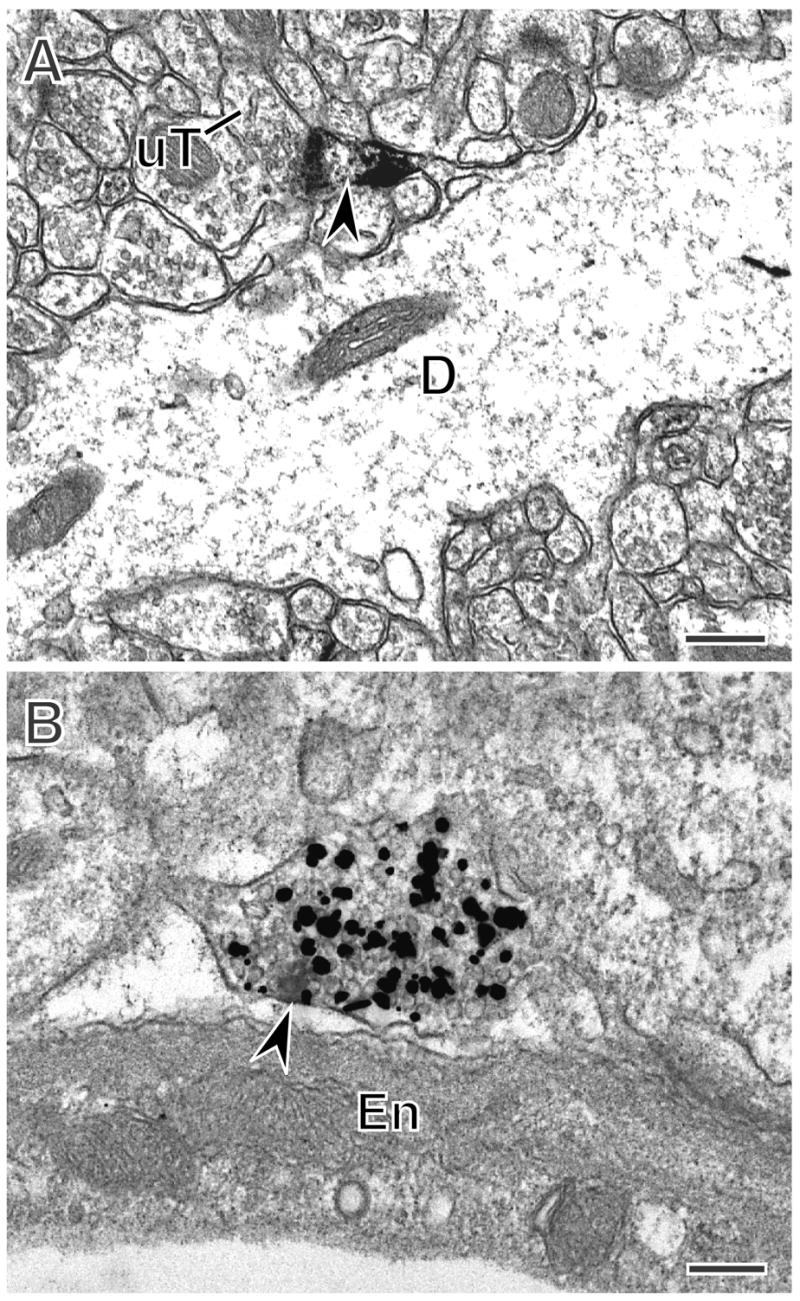
In the rat hippocampus, extranuclear ERα-is found postsynaptically in dendritic spines and presynaptically in terminals, some of which are cholinergic. A. ERα-immunoreactivity (arrowhead) is found in a dendritic spine emanating from a dendritic shaft. The spine is contacted (curved arrow) by an unlabeled terminal. B. A terminal with ERα-immunoreactivity (arrowhead) also contains silver intensified immunogold (black particles) labeling the cholinergic marker, vesicular acetylcholine transporter (VAChT). A, B, CA1 Stratum radiatum. Bars, 500 nm.
Figure 8.
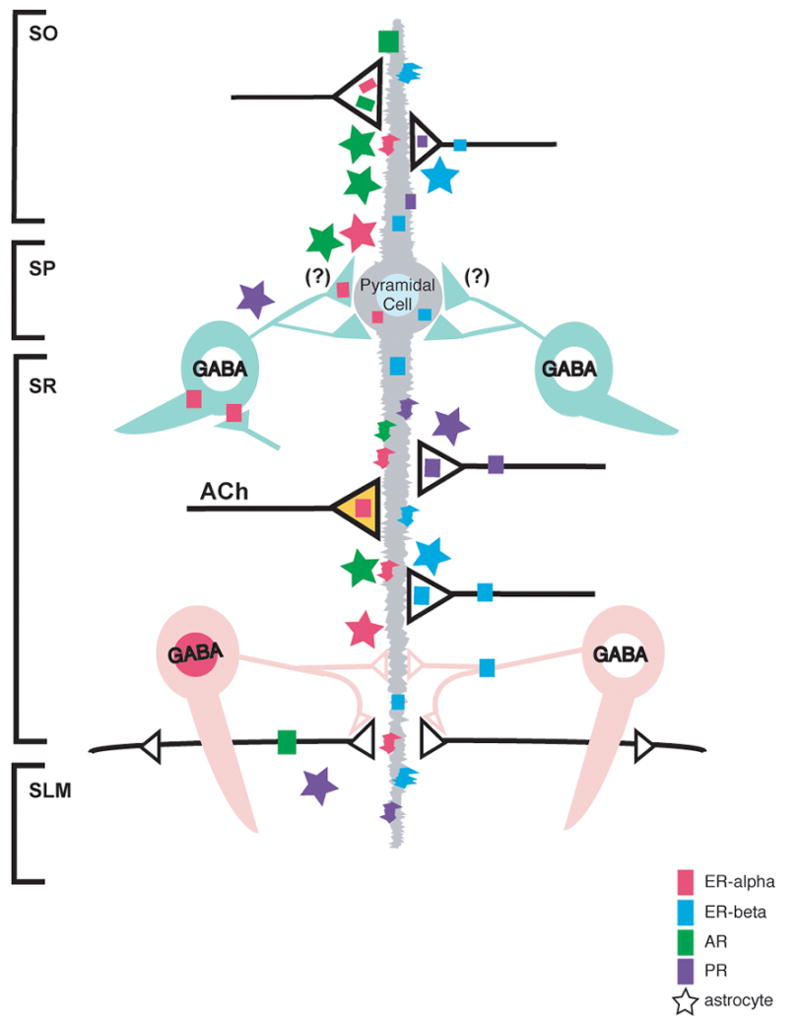
Schematic diagram depicting the cellular and subcellular localization of ERs, PRs and ARs in the hippocampal CA1 region. Pyramidal cells contain nuclear labeling for AR (dark green) and a subset of GABAergic interneurons contains nuclear ERαs (dark pink). Pyramidal cells and some GABAergic interneurons contain cytosolic and plasma membrane-associated ERβs (blue). Dendritic spines, many belonging to pyramidal cells, contain ERαs, ERβs, ARs and PRs (purple). ERαs, ERβs, ARs and PRs are found in axons and axon terminals. Some ERα-containing terminals are cholinergic (acetylcholine; orange); some ERβ-containing terminals resemble monoaminergic terminals. Astrocytes (stars), mostly in the molecular layer, also contain ERαs, ERβs, ARs and PRs. (Summarized from Milner et al., 2001; 2005; Tabori et al., 2005; Towart et al., 2003; Waters et al., 2005).
ERβ
The cellular and subcellular location of ERβ-ir in the hippocampal formation is generally similar to that of ERα-ir except that ERβ-ir was more extensively found at extranuclear sites (Milner et al., 2005). By light microscopy, ERβ mRNA and protein is found in the perikarya of pyramidal cells (Fig 4B.), granule cells as well as some interneurons in adult rodents (Milner et al., 2005;Mitra et al., 2003;Shughrue et al., 1997). Electron microscopic analysis revealed ERβ-ir at several extranuclear sites in pyramidal and granule cells (Milner et al., 2005) and, as described below, some newly born cells (Herrick et al., 2006) (Fig. 8). Within the perikarya and proximal dendrites of these cells, ERβ-ir was associated with cytoplasmic organelles, especially endomembranes and mitochondria, suggesting that ERβ may be involved in generating Ca++ signals that control processes such as neuronal excitability and synaptic plasticity (see discussion in Milner et al., 2005). ERβ-ir was also found on plasma membranes and in dendritic spines, many arising from pyramidal and granule cell dendrites. In both dendritic shafts and spines, ERβ-ir was near the perisynaptic zone adjacent to synapses formed by unlabeled terminals. These results are consistent with the possibility of rapid activation by extracellular ligands (see discussion Wang et al., 2006). ERβ-ir also was associated with clusters of small, synaptic vesicles in preterminal axons that were particularly prominent in the mossy fiber pathway. ERβ-labeled terminals had morphological features resembling monoaminergic terminals: they rarely formed synapses, however, those that did formed asymmetric and symmetric synapses with dendrites. ERβ-ir also was detected in glial profiles.
Progestin Receptors (PR)
Progesterone that follows estrogen exposure rapidly decreases spine density in the CA1 region of the hippocampus, an action that is blocked by the PR antagonist, RU486 (Woolley et al., 1993). Although cells containing PR mRNA are seen in hippocampal formation (Hagihara et al., 1992), PR protein is undetectable in the rat hippocampus at the light microscopic level. However, ultrastructural analysis of the hippocampal formation revealed PR-ir at several extranuclear sites (Waters et al., 2005). PR-ir was present in dendritic spines, many arising from principal cell dendrites, and was closely associated with the postsynaptic density. PR-ir was found in axons and axon terminals that contained small synaptic vesicles. Terminals and en passant axonal boutons with PR-ir formed predominantly asymmetric synapses with dendritic spines. These localizations suggest that PR activation influences excitatory neurotransmission. PR-ir was found in glia, many resembling astrocytes.
Androgen Receptors (AR)
Like estrogens in female rats, androgens can affect dendritic spine density in the CA1 subfield of the male rat hippocampus (Kovacs et al., 2003;Leranth et al., 2003). The cellular and subcellular localization of ARs in the hippocampal formation was different from the ERs (Fig. 8) (Tabori et al, 2005). In agreement with other light microscopic studies (Commins et al., 1985;Kerr et al., 1995;Pouliot et al., 1996;Sar et al., 1990;Simerly et al., 1990;Stumpf et al., 1978), AR immunoreactivity was present in pyramidal cell nuclei (Fig. 4C). AR-ir also was present in disperse, punctate processes that resembled glia (Fig. 4C) and in the mossy fiber pathway (localizations which were later confirmed by electron microscopy). Ultrastructural analysis confirmed the light microscopic localization and revealed AR-ir at several extranuclear sites in all hippocampal subregions (Tabori et al., 2005) (Figs. 7, 8). AR-ir was found in dendritic spines, many arising from pyramidal and granule cell dendrites. AR-ir was affiliated with clusters of small, synaptic vesicles within preterminal axons and axon terminals. Unlike ER-containing terminals, AR-labeled terminals almost exclusively formed asymmetric synapses with dendrites and spines, suggesting that they primarily arise from excitatory afferents. AR-labeled astrocytic profiles often apposed terminals synapsing on unlabeled dendritic spines or formed gap junctions with other AR-labeled or unlabeled astrocytes. Both localizations suggest additional mechanisms were androgens could influence synaptic plasticity (see discussion in Tabori et al., 2005)
Figure 7.
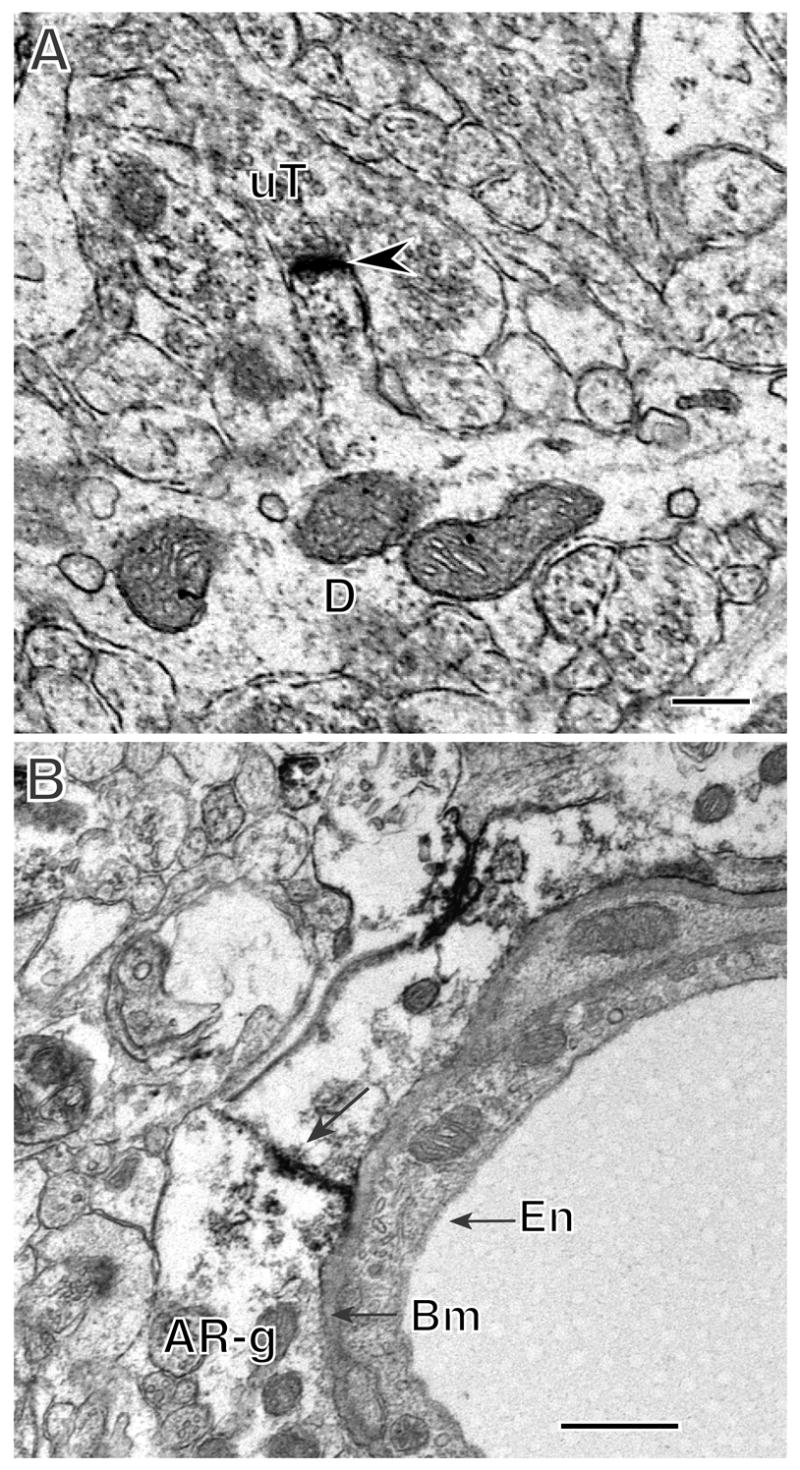
In the hippocampus, AR is found at nuclear sites in CA1 pryamidal cells and in extranuclear sites in dendritic spinces and glial cells. A. AR-immunoreactivity (arrowheads) is found in the nucleus of a pyramidal cell perikaryon. B. AR-immunoreactivity (arrowhead) is in a dendritic spine emanating from a dendritic shaft (D). The labeled spine is contacted by an unlabeled terminal (uT). C. AR immunoreactivity is found in two glial profiles (AR-g) that form a gap junction (arrow). The glial profiles abut the basement membrane (bm) of a blood vessel endothelial cell. A, B, CA1 Stratum radiatum. Bars, 500 nm.
Functional implications of non-nuclear steroid receptors
The localization studies of steroid receptors in hippocampus have also led to the discovery of some functional relationships with neurotransmitter release, signaling pathways and protein synthesis regulation, as well as neurogenesis. In addition, there are indications that declines in non-nuclear ERs are important in the aging process. Moreover, sex differences are apparent.
ERα and acetylcholine
Estrogen action on basal forebrain cholinergic neurons is critically involved in mediating the effects of estrogen in the hippocampal formation (Rudick et al., 2003). Cholinergic presynaptic profiles (identified by vesicular acetylcholine transporter (VAChT) immunoreactivity) are most concentrated in stratum oriens of the hippocampal CA1 region and in inner molecular layer and hilus of the dentate gyrus (Towart et al., 2003). Quantitative ultrastructural analysis revealed that VAChT-ir presynaptic profiles contained ERα-ir (Figs. 5B, 8) suggesting that estrogens could rapidly and directly affect the local release and/or uptake of acetylcholine. VAChT-labeled presynaptic profiles also apposed ERα-immunoreactive dendritic spines, presynaptic profiles and glial profiles; many of the latter two types of profiles abutted unlabeled dendritic spines that receive asymmetric (excitatory-type) synapses from unlabeled terminals. The affiliation of cholinergic terminals with excitatory terminals near ERα-labeled dendritic spines or glial profiles suggests that alterations in acetylcholine release could indirectly affect estrogen-modulated structural plasticity.
Estradiol and growth factors
Neurotrophins are important modulators of structural synaptic plasticity. Through trophic action (Jordan, 1999), astrocytes serve as permissive substrates to support axonal regrowth (Ridet et al., 1997) and are involved in estrogen-induced synaptic structural plasticity (Garcia-Segura et al., 1999). In addition to presynaptic neuronal processes, immunoreactivity for tyrosine kinase A (TrkA), the primary receptor for nerve growth factor (NGF), was present in select astrocytes of the rat hippocampal formation (Barker-Gibb et al., 2001). Moreover, the number of TrkA-labeled astrocytes in female rats fluctuated across the estrous cycle in dendritic fields of the hippocampal formation, with the greatest number found at estrus after the peak plasma estradiol concentration of proestrus (McCarthy et al., 2002). Few TrkA-labeled astrocytes were found in ovariectomized animals; after estradiol replacement, this number increased by 12-fold in the hippocampal formation, indicating estrogen-mediated induction. Electron microscopic analysis of the dentate gyrus molecular layer demonstrated that TrkA-labeled astrocytes are positioned primarily next to dendrites and unmyelinated axons. Because NGF is known to stimulate astrocytes to act as substrates for axon growth (Kawaja et al., 1991), these findings support the theory that TrkA-containing astrocytes serve a role in structural plasticity, synaptic regeneration and axon guidance across the estrous cycle in the hippocampal formation.
Signaling pathways - AKT
In addition to genomic mechanisms, estrogens may regulate gene expression by activating specific signal transduction pathways, such as that involving phosphatidylinositol 3-kinase and the subsequent phosphorylation of Akt. The Akt pathway regulates a variety of cellular events, including the initiation of protein synthesis. Our studies (Znamensky et al., 2003) revealed that the density of pAkt-ir in CA1 stratum radiatum was significantly higher in proestrus rats (or in estrogen-supplemented ovariectomized females) compared to diestrus, estrus or male rats. Ultrastructurally, pAkt-ir was found throughout the shafts and in select spines of stratum radiatum dendrites. Moreover, proestrus rats compared to diestrus, estrus and male rats contained a significantly more pAkt-ir associated with: (1) dendritic spines; (2) spine apparati located within 0.1 μm of dendritic spine bases; (3) endoplasmic reticula and polyribosomes in the cytoplasm of dendritic shafts; and (4) the plasmalemma of dendritic shafts. These findings suggest that estrogens may regulate spine formation in CA1 pyramidal neurons via Akt-mediated signaling events.
Progress in the study of non-genomic estrogen actions has been made possible by the use of in vitro models which enable pharmacological and genetic manipulations (Chen et al., 2006;Razandi et al., 1999). For example, the investigation of the role of Akt phosphorylation in rapid estrogen effects on the translation of PSD-95 was carried out using NG-108-15 cells (Akama et al., 2003) and then extended to the rat hippocampus by electron microscopic immunocytochemistry (Znamensky et al., 2003).
Estradiol and protein synthesis
The synaptogenesis phase of estrous is driven by estrogen and may require newly synthesized proteins. Ribosomes synthesize soluble proteins when free in the cytoplasm, and integral membrane or secretory proteins when membrane-associated (Blobel et al., 1979). We (McCarthy et al., 2003) found that ribosomal protein immunoreactivity and the percentage associated with membranes increases locally in CA1 stratum radiatum dendrites during estrogen-induced synaptogenesis. These findings support a role for estrogen in regulating the local synthesis of integral membrane proteins, e.g. receptors, in dendrites for newly developing synapses across the estrous cycle.
ERβ and neurogenesis
During development, cells in or near the granule cell layer transiently express high levels of estrogen binding and nuclear ERs (Solum et al., 2001). Moreover, mRNA for ERs, especially ERβ, is expressed in proliferating and differentiating cells in the subgranular zone of the dentate gyrus (Isgor et al., 2005). Our recent studies revealed that neuronal perikarya and dendrites labeled with doublecortin, a marker of newly generated cells, contained extranuclear ERβ-ir in both adult and neonatal dentate gyrus (Herrick et al., 2006) (Figs. 6,7). Dual labeled perikarya had the morphological characteristics of granule cells, although a few resembled interneurons. ERβ-ir also was in glial profiles that apposed DCX-labeled perikarya and dendrites. These findings are consistent with data showing that estrogens can exert non-genomic effects directly and indirectly on newly generated cells in neonatal and adult rat dentate gyrus.
Figure 6.
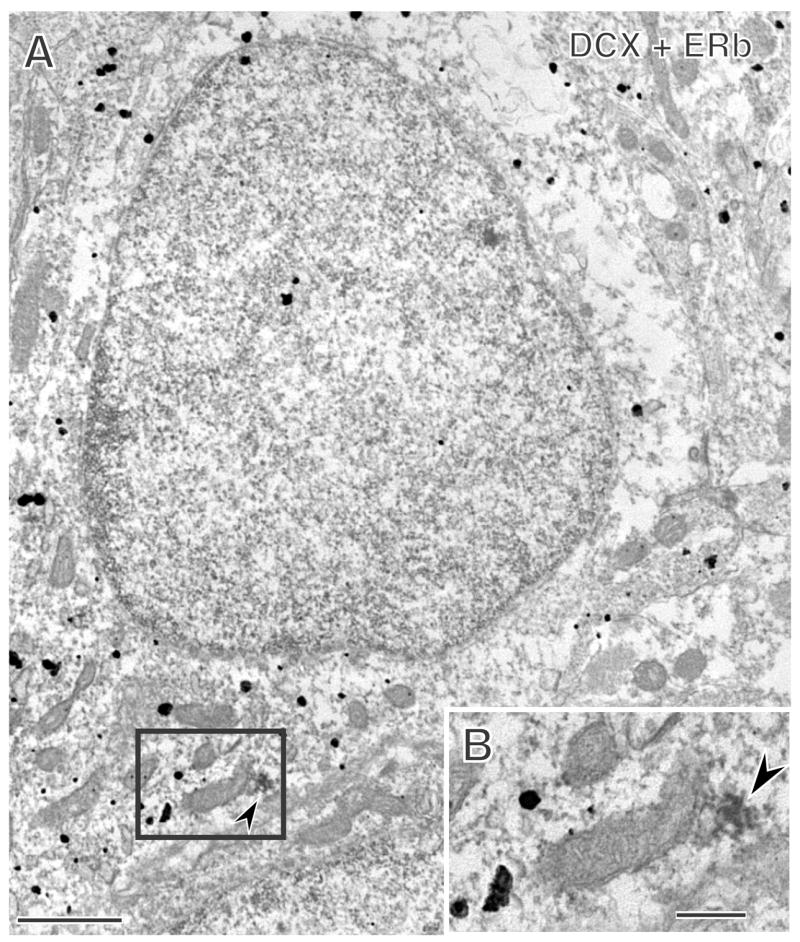
Extranuclear ERβ-immunoreactivity is detected in newly born cells in the rat dentate gyrus. A. A cell with silver intensified immunogold (black particles) for the new cell marker, doublecortin (DCX), also contains immunoreactivity for ERβ (arrowhead). B. Enlargement of the boxed region shows that the ERβ-immunoreactivity (arrowhead) is associated with a mitochondrion (m). Bars, 500 nm.
ERα and aging
The effects of estrogens on the distribution of ERα in CA1 of young (3–4 mo.) and aged (22–23 mo.) ovariectomized rats was examined using post-embedding immunoelectron microscopy (Adams et al., 2002). Within dendritic spines, most ERα-ir was seen in plasmalemmal and cytoplasmic regions of spine heads, with a smaller proportion within 60 nm of the post-synaptic density. In the presynaptic terminal, ERα-ir was clustered around synaptic vesicles. Non-synaptic pools of ERα-ir within the pre- and post-synaptic compartments were significantly reduced (to 35% and 27%, respectively) in the young animals treated with estradiol compared to those that received vehicle. However, no estrogen treatment-related differences were seen in the aged animals. Also, 50% fewer ERα –labeled spines were seen in the hippocampi of aged rats compared to young rats. The reduced responsiveness of ERα to estrogen in aging rats may contribute to decreased hippocampal function and synaptic plasticity seen in aging.
ERα and sex differences
Estradiol-mediated increases in dendritic spine density and synaptogenesis in the CA1 region are specific to females, as estradiol-treated males fail to show increases in hippocampal spine density (Leranth et al., 2003). As noted earlier, estradiol-induced spinogenesis in the female is dependent upon up-regulation of the NMDA-type glutamate receptor. We (Romeo et al., 2005) found that while estradiol increases NMDA binding in gonadectomized females, estradiol fails to modulate NMDA binding in gonadectomized males. Moreover, proestrus (high estrogen) females had a significantly increased number of ERα-labeled dendritic spines compared to diestrus females and intact males. These studies suggest that the ability of estrogen to increase NMDA binding in the hippocampus and the presence of ERα in dendritic spines may contribute to the observed sex difference in estradiol-induced hippocampal spinogenesis.
Presence of non-genomic receptors in other brain regions
The line of research outlined above that led us to the discovery of non-genomic receptors for steroid hormones in the hippocampal formation has paved the way for the finding of non-nuclear receptors in other brain regions, in which nuclear receptors are absent or sparsely present. This, in turn, has synergized with progress using cell culture models and studies of non-neural tissues which have revealed many examples of rapid, non-genomic actions of estrogens as well as other steroids (Kelly et al., 2001; Norman, 2006; Tasker et al., 2006). The brain regions that have been studied thus far are ones in which functional effects of estrogens were recognized even if the nuclear receptor density was negligible, and now, using electron microscopic immunocytochemistry, non-genomic estrogen receptors are visible. The results of these studies are summarized below.
Estrogen receptors in brainstem
Bulbospinal C1 adrenergic neurons in the rostral ventrolateral medulla (RVLM) are critically involved in blood pressure control (Reis et al., 1988). Local injection of 17β-estradiol into the RVLM region decreases sympathetic tone within minutes (Saleh et al., 2000). We (Wang et al., 2006) investigated the anatomical and physiological basis for estrogen effects in the RVLM. Immunoelectron microscopy revealed that ERα and ERβ were extranuclear and in particular suggest that estrogens can modulate the function of RVLM C1 bulbospinal neurons either directly through extranuclear ERβ, or indirectly through extranuclear ERα in selected afferents. In isolated bulbospinal RVLM neurons, 17β-estradiol specifically and dose-dependently reduced voltage-gated Ca++ currents, especially the long-lasting (L-type) component via an ERβ-selective mechanism. This Ca++ current inhibition may underlie the decrease in sympathetic tone evoked by local 17β-estradiol application. Together, these findings provide a structural and functional basis for the effects of estrogens on blood pressure control and suggest a mechanism for the modulation cardiovascular function by estrogen in women.
Estrogen receptors in dorsal raphe
Estrogens influence serotonergic activity in rodent and monkey brain and there is evidence that estrogen affect mood and potentiate the actions of serotonin-related antidepressant drugs (Schneider et al., 1997). In rat dorsal raphe, cell nuclear ERα-ir was found in non-serotonergic neurons, whereas in mouse some ERα-ir is in the nuclei of serotonergic (5HT) neurons (Alves et al., 1998;Alves et al., 2000). In the rat dorsal raphe, extranuclear ERα-ir was associated with the plasmalemma of 5HT-containing neurons identified by tryptophan hydroxylase (TPH) immunostaining (Milner et al., 2003) suggesting that ERα may be shuttled to extranuclear sites. One such site is the synaptic junction of non-5HT neurons and another is near dense core vesicles that abut TPH immunoreactive dendrites. Thus, non-5HT neurons have non-nuclear ERα and one function might be to regulate neuropeptide release from efferents that modulate 5HT neurons.
Estrogen receptor in striatum
The rat striatum is devoid of any nuclear ER labeling and yet shows powerful effects of estrogens on dopamine release and neuronal firing. Moreover, estrogen effects on dopamine release and on D1 and D2 receptor binding have been demonstrated, along with rapid estrogen effects on Ca++ currents in striatal neurons (Mermelstein et al., 1996). ERα-ir was detected in dendritic spines contacted by unlabeled terminals that form asymmetric synapses, suggesting that the terminals were glutamatergic (Milner et al., 2003). In addition, some ERα–ir was detected in terminals with small synaptic vesicles that contacted cholinergic perikarya identified by VAChT-ir, suggesting that estrogen could directly influence the activity of cholinergic neurons in striatum.
Glucocorticoid receptors in amydgala
Glucocorticoids, released in high concentrations from the adrenal cortex during stressful experiences, bind to glucocorticoid receptors in nuclear and peri-nuclear sites in neuronal somata (McEwen, 2005). Their classically known mode of action is to induce gene promoter receptors to alter gene transcription. Using electron microscopy to examine the lateral amygdala, we (Johnson et al., 2005) found glucocorticoid receptors in non-genomic sites, including glia processes, presynaptic terminals, neuronal dendrites, and post-synaptic densities of dendritic spines. The lateral nucleus of the amygdala is specifically implicated in the formation of memories for stressful experiences (LeDoux, 2003). The presence of glucocorticoid receptors in non-nuclear-membrane translocation sites, particularly dendritic spines, where they show an affinity for postsynaptic membrane densities, suggests that glucocorticoids may have a specialized role in modulating synaptic transmission plasticity related to fear and emotional memory.
Rapid non-genomic effects of glucocorticoids have been recognized for some years in the newt, where G-protein coupled receptors have been identified that regulate reproductive and aggressive behaviors (Orchinik et al., 1994). More recently, rapid non-genomic effects of glucocorticoids have been described on endocannabinoid release (Di et al., 2003). Furthermore, rapid mineralocorticoid actions on sodium flux are known (Wehling, 1997), and, quite recently, rapid actions of corticosterone on glutamate neurotransmission have been shown by genetic deletion to depend on the mineralocorticoid receptor that affects cell nuclear events (Karst et al., 2005).
Conclusion
We have described a path of discovery that began with the use of the Golgi method to demonstrate heretofore unrecognized aspects of the structural plasticity of the adult hippocampal formation and which has led us to a new view of brain structural plasticity and the role and mechanism of steroid hormone action. This new perspective is consistent with the predictions of Cajal, as reflected in the quotation at the beginning of this article. Our findings were largely unexpected given the bias against structural plasticity of the adult brain that has existed until recently and also given what was known at the time concerning the distribution and mechanism of action of nuclear steroid receptors. The dissonance of finding a steroid action where few or no nuclear receptors could be found prompted us to seek, and find, non-nuclear steroid receptors and estrogen effects on second messenger pathways of the type that are now more widely recognized in endocrinology but which were controversial at the time. It is safe to say that we would never be where we are now without the Golgi method and the insights of Cajal that got us started down this pathway.
Non-genomic effects of steroids are now an increasingly accepted aspect of steroid action in brain and other tissues and organs. Evidence points to rapid, non-genomic actions of aldosterone and Vitamin D (Funder, 2005;Norman et al., 1999;Wehling, 1997) and this complements evidence that estradiol, progesterone, glucocorticoids and androgens also have non-genomic actions.
The discovery of non-genomic localizations of epitopes for estrogen, androgen and progestin receptors in brain regions not previously associated with the actions of so-called “sex hormones” has supported functional evidence for the widespread actions of these steroids throughout the nervous system. Thus, in addition to reproductive behavior and neuroendocrine function, these steroids are now known to affect motor coordination, mood, pain and cognitive function and to have neuroprotective effects (Handelsman et al., 2005;McEwen et al., 1999;Sherwin, 2003;Stein, 2001;Wise et al., 2001). The extensive actions in brain of estrogens, progestins and androgens raise the possibility, on the one hand, for the development of pharmaceutical agents that mimic some action of these hormones. On the other hand, they also indicate that developmentally-programmed sex differences and “sex hormone” actions in adult life may play a role in the actions of other pharmaceutical agents for, and the progression of, a variety of neurological and behavioral disorders (Baker et al., 1974;Cahill, 2006;Grossi et al., 2000;Hamilton, 1989;McEwen et al., 2005;Wilson, 1999).
Considerable progress has been made in the development of pharmaceutical agents that mimic selective steroid hormone actions. These include SERM’S (selective estrogen response modulators) (McDonnell, 1999), SARM’s (selective androgen response modulators)(Kearby et al., 2007;Rosen et al., 2002) as well as agents that mimic non-genomic actions of Vitamin D (Norman, 2006). Just as is the case for exploration of non-genomic actions of steroids (Akama et al., 2003;Kelly et al., 2001), the development of these selective modulators has required in vitro models and immortalized cell systems (Razandi et al., 1999;Simoncini et al., 2000) with later translation to in vivo models where differences in organ and tissue responsiveness to these agents have been revealed (Brinton, 2004;Shang et al., 2002).
As a result of the newly recognized complexity of steroid hormone action in brain, there are many open questions for future research. For example, to what extent are there other receptors for non-genomic effects of steroids and how are they related to the genomic steroid receptors? For PRs, are there not only functions for the non-nuclear locations of receptors with the nuclear receptor epitope (see above) as well as functions and possibly unique cellular locations of receptors unrelated to the genomic receptors, besides the well-know actions of Aring reduced progestins via the GABAa receptor (Wagner, 2006)? For Vitamin D and for mineralocorticoid actions, do the rapid effects depend on the genomic receptors because they are the products of the same gene but with different cellular localizations (Karst et al., 2005;Norman, 2006) or are there separate non-genomic receptors that depend on expression of the genomic receptors? For glucocorticoids, are there rapidly acting non-genomic receptors and actions in mammals that are unrelated to the genomic receptors (Tasker et al., 2006) as well as non-genomic localizations of the genomic GR (see above)? A related question is do the rapid effects of all steroids synergize with the genomic actions, as for example, has been shown for estrogens (Vasudevan et al., 2001)?
Although the prospects for development of new pharmaceutical are exciting because of the diversity of genomic and non-genomic steroid actions, there are potential problems stemming from the widespread actions of steroids in the brain and body. Nowhere is this better illustrated than for estrogens. SERM’s have the ability to protect bone mineral loss because of their partial agonist effects and to protect against breast cancer because of their partial estrogen antagonist effects (Riggs et al., 2003). In brain, SERM’s mimic the effects of estrogens on the cholinergic system which is involved in cognitive function and attention (Gibbs et al., 2004;Luine et al., 1977;McMillan et al., 2002;Voytko, 2002;Wu et al., 1999). Yet at least some SERM’s have the ability to block estrogen-induced synapse formation in the hippocampus (McEwen et al., 1999), which would, unlike estradiol treatment, not enhance processes related to memory storage or retrieval. One alternative to this problem is the development of SERM’s that cannot enter the brain (Labrie et al., 2003) and thus produce the beneficial effects on bone and cancer progression without compromising brain function. Yet, we know so little about the molecular mechanisms for each of the steroid actions that much more research is needed on this aspect, and the possibilities to develop new, selective compounds are thus very attractive (Brinton, 2004).
The broader view of hormone action in brain and other tissue has engendered a new view of hormone-brain interactions. Neuroscience has moved forward by recognizing the importance of external hormonal influences on brain and also the importance of the remodeling of the brain throughout the lifespan not only as a naturally occurring process but also as a potential mechanism for the action of pharmaceutical agents and non-pharmaceutical therapies such as physical activity, cognitive behavior and psychotherapy and physical therapy. At a mechanistic level, the revelations about how steroid hormones affect many signaling pathways, neurotransmitters and growth factors have enriched the study of hormone action and brought it more in line with other aspects of neurochemistry and neuropharmacology.
Finally, the new understanding of the role of hormones in brain structural plasticity has translational implications for a better understanding of stress and stress-related disorders such as depressive illness and sex differences in these conditions as well as for age-related cognitive decline and dementia. With improved methods for brain imaging, there is increased ability to translate findings from animal models to the human condition and vice versa.
Acknowledgments
We thank Dr. Carrie T. Drake for her helpful comments on the manuscript and Ms. Katherine Mitterling for technical assistance and her comments on the manuscript.
Support: NIH grants: NS0007080 and MH41256 (B.S.M.); DA08259 and HL18974 (T.A.M.)
Abbreviations
- AR
androgen receptor
- EAA
excitatory amino acids
- ER
estrogen receptor
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartic acid
- PR
progestin receptor
- RVLM
rostral ventrolateral medulla
- TH
tyrosine hydroxylase
- TPH
tryptophan hydroxylase
- Trk A
tyrosine kinase A
- VAChT
vesicular acetylcholine transporter
Footnotes
Classification terms: Theme E – Endocrine and autonomic regulation; neuroendocine regulation, other
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Fink SE, Shah RA, Janssen WGM, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves S, Weiland NG, Hayashi S, McEwen B. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ERα) gene-disrupted mice. J Comp Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Baker SW, Ehrhardt AA. Prenatal androgen, intelligence, and cognitive sex differences. In: Friedman RC, Richart RM, Vande Wiele RL, editors. Sex Differences in Behavior. New York: John Wiley & Sons, Inc; 1974. pp. 53–76. [Google Scholar]
- Barker-Gibb AL, Dougherty KD, Einheber S, Drake CT, Milner TA. Hippocampal tyrosine kinase A receptors are restricted primarily to presynaptic vesicle clusters. J Comp Neurol. 2001;430:182–199. doi: 10.1002/1096-9861(20010205)430:2<182::aid-cne1024>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G, Walter P, Chang CN, Goldman BM, Erickson AH, Lingappa VR. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Brinton RD. Requirements of a brain selective estrogen: Advances and remaining challenges for developing a NeuroSERM. J Alzheimer’s Disease. 2004;6:S27–S35. doi: 10.3233/jad-2004-6s607. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cajal SR. The structure of Ammon’s horn. Springfield, IL: Charles C. Thomas; 1968. [Google Scholar]
- Cajal SR. Histology of the Nervous System. New York: Oxford University Press; 1995. [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Nilsen J, Diaz Brinton R. Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: Therapeutic implications. Endocrinology. 2006;147:5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J Comp Neurol. 1985;231:473–489. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KCs, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder JW. The nongenomic actions of aldosterone. Endocrine Rev. 2005;26:313–321. doi: 10.1210/er.2005-0004. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA. Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J Neurobiol. 1999;40:574–584. [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gould E, Frankfurt M, Westlind-Danielsson A, McEwen BS. Developing forebrain astrocytes are sensitive to thyroid hormone. Glia. 1990a;3:283–292. doi: 10.1002/glia.440030408. [DOI] [PubMed] [Google Scholar]
- Gould E, Westlind-Danielsson A, Frankfurt M, McEwen BS. Sex differences and thyroid hormone sensitivity of hippocampal pyramidal neurons. J Neurosci. 1990b;10:996–1003. doi: 10.1523/JNEUROSCI.10-03-00996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley C, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990c;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley C, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990d;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley C, McEwen BS. The hippocampal formation: morphological changes induced by thyroid, gonadal and adrenal hormones. Psychoneuroendocrinology. 1991;16:67–84. doi: 10.1016/0306-4530(91)90071-z. [DOI] [PubMed] [Google Scholar]
- Grossi G, Soares JJF, Lundberg U. Gender differences in coping with musculoskeletal pain. Int J Behav Med. 2000;7:305–321. [Google Scholar]
- Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Mol Brain Res. 1992;14:239–249. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. Reproductive pharmacology: perspectives on gender as a complex variable in clinical research. Social Pharmacology. 1989;3:181–200. [Google Scholar]
- Handelsman DJ, Liu PY. Andropause: invention, prevention, rejuvenation. Trends Endocrin & Metab. 2005;16:39–45. doi: 10.1016/j.tem.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res. 2006;1121:46–58. doi: 10.1016/j.brainres.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Isgor C, Watson SJ. Estrogen receptor α and β mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience. 2005;134:847–856. doi: 10.1016/j.neuroscience.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Jensen E, Jacobson H. Basic guides to the mechanism of estrogen action. Rec Prog Horm Res. 1962;18:387–408. [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Jordan CL. Glia as mediators of steroid hormone action on the nervous system: An overview. J Neurobiol. 1999;40:434–445. doi: 10.1002/(sici)1097-4695(19990915)40:4<434::aid-neu2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaja MD, Gage FH. Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron. 1991;7:1019–1030. doi: 10.1016/0896-6273(91)90346-2. [DOI] [PubMed] [Google Scholar]
- Kearby JD, Gao W, Narayanan R, Fisher SJ, Wu D, Miller DD, Dalton JT. Selective androgen receptor modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharmaceut Res. 2007;24:328–335. doi: 10.1007/s11095-006-9152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endo & Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kovacs EG, MacLusky NJ, Leranth C. Effects of testosterone on hippocampal CA1 spine synaptic density in the male are inhibited by fimbria/fornix transection. Neuroscience. 2003;122:807–810. doi: 10.1016/j.neuroscience.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Labrie F, El-Alfy M, Berger L, Labrie C, Martel C, Belanger A, Candas B, Pelletier G. The combination of a novel selective estrogen receptor modulator with an estrogen protects the mammary gland and uterus in a rodent model: The future of postmenopausal women’s health? Endocrinology. 2003;144:4700–4706. doi: 10.1210/en.2003-0269. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, McEwen BS. Effects of an estrogen antagonist on enzyme activities and 3H estradiol nuclear binding in uterus, pituitary and brain. Endocrinology. 1977;100:903–910. doi: 10.1210/endo-100-4-903. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharm. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- McCarthy JB, Barker-Gibb AL, Alves SE, Milner TA. TrkA immunoreactive astrocytes in dendritic fields of the hippocampal formation across estrous. Glia. 2002;38:36–44. doi: 10.1002/glia.10060. [DOI] [PubMed] [Google Scholar]
- McCarthy JB, Milner TA. Dendritic ribosomes suggest local protein synthesis during estrous synaptogenesis. NeuroReport. 2003;14:1357–1360. doi: 10.1097/01.wnr.0000078380.40088.99. [DOI] [PubMed] [Google Scholar]
- McDonnell DP. The molecular pharmacology of SERMs. TEM. 1999;10:301–311. doi: 10.1016/s1043-2760(99)00177-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007 doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SH. Estrogen actions in the central nervous system. Endocrine Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lasley EN. Cerebrum. The Dana Forum on Brain Science Dana Press; 2005. The end of sex as we know it. [Google Scholar]
- McEwen BS, Tanapat P, Weiland NG. Inhibition of dendritic spine induction on hippocampal CA1 pyramidal neurons by a non-steroidal estrogen antagonist in female rats. Endocrinology. 1999;140:1044–1047. doi: 10.1210/endo.140.3.6570. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McMillan PJ, LeMaster AM, Dorsa DM. Tamoxifen enhances choline acetyltransferase mRNA expression in rat basal forebrain cholinergic neurons. Mol Brain Res. 2002;103:140–145. doi: 10.1016/s0169-328x(02)00195-x. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Alves SE, Hayashi S, McEwen BS. The Identities of Membrane Steroid Receptors. Boston, MA: Kluwer Academic Publishers; 2003. An expanded view of estrogen receptor localization in neurons; pp. 21–25. [Google Scholar]
- Milner TA, Ayoola K, Drake K, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagen L, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor β in the mouse brain: Comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- Norman AW, Song XD, Zanello L, Bula C, Okamura WH. Rapid and genomic biological responses are mediated by different shapes of the agonist steroid hormone, 1·,25(OH)2 vitamin D3. Steroids. 1999;64:120–128. doi: 10.1016/s0039-128x(98)00091-9. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Moore FL, Rose JD. Mechanistic and functional studies of rapid corticosteroid actions. Ann NY Acad Sci. 1994;746:101–114. doi: 10.1111/j.1749-6632.1994.tb39219.x. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Weiland NG, McEwen BS. Chronic exposure to stress levels of corticosterone alters GABAA receptor subunit mRNA levels in rat hippocampus. Mol Brain Res. 1995;34:29–37. doi: 10.1016/0169-328x(95)00118-c. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Estrogens and Brain Function. N.Y: Springer-Verlag; 1980. [Google Scholar]
- Pouliot wA, Handa RJ, Beck SG. Androgen modulates N-methyl-D-aspartate-mediated depolarization in CA1 hippocampal pyramidal Cells. Synapse. 1996;23:10–19. doi: 10.1002/(SICI)1098-2396(199605)23:1<10::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Morrison S, Ruggiero DA. The C1 area of the brainstem in tonic and reflex control of blood pressure. Hypertension. 1988;11:I8–I13. doi: 10.1161/01.hyp.11.2_pt_2.i8. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. TINS. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Hartmann LC. Selective estrogen-receptor modulators - Mechanisms of action and application to clinical practice. New Engl J Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- Rosen J, Negro-Vilar A. Novel, non-steroidal, selective androgen receptor modulators (SARMs) with anabolic activity in bone and muscle and improved safety profile. J Musculoskel Neuron Interact. 2002;2:222–224. [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23:4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MC, Connell BJ, Saleh TM. Medullary and intrathecal injections of 17β-estradiol in male rats. Brain Res. 2000;867:200–209. doi: 10.1016/s0006-8993(00)02313-1. [DOI] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 1990;127:3180–3186. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS. Estrogen replacement and the response to fluoxetine in a multicenter geriatric depression trial. Am J Geriatr Psychiatry. 1997;5:97–106. [PubMed] [Google Scholar]
- Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endocrine Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-α knockout mouse. Endocrinology. 1997;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramastsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;29:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ERα) in pyramidal neurons of the developing rat hippocampus. Devel Brain Res. 2001;128:165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? TINS. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- Stumpf WW, Sar M. Anatomical distribution of estrogen, androgen, progestin, corticosteroid and thyroid hormone target sites in the brain of mammals: phylogeny and ontogeny. Amer Zool. 1978;18:435–445. [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WGM, Han J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female Rhesus monkeys. Cerebral Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: Rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-α in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow L-M, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci USA. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML. Estrogen and the cholinergic system modulate visuospatial attention in monkeys (Macaca fascicularis) Behav Neurosci. 2002;116:187–197. doi: 10.1037//0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK. The many faces of progesterone: A role in adult and developing male brain. Front Neuroendocrin. 2006;27:340–359. doi: 10.1016/j.yfrne.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res. 2006;1094:163–178. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]
- Waters EM, Herrick SP, McEwen BS, Milner TA. Program No 403.12, Abstract Viewer/Itinerary planner. Washington DC: Society for Neuroscience; 2005. Subcellular localization of progestin receptors in the rat hippocampus. Online. [Google Scholar]
- Wehling M. Specific, nongenomic actions of steroid hormones. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131:662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wilson JD. The role of androgens in male gender role behavior. Endocrine Rev. 1999;20:726–737. doi: 10.1210/edrv.20.5.0377. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M. Minireview: Neuroprotective effects of estrogen - New insights into mechanisms of action. Endocrinology. 2001;142:969–973. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- Woolley C, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley C, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Current Opinion Neurobiol. 1999;9:349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Glinn MA, Ostrowski NL, Su Y, Ni B, Cole HW, Bryant HU, Paul SM. Raloxifene and estradiol benzoate both full restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. 1999;847:98–104. doi: 10.1016/s0006-8993(99)02062-4. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal Ca1 dendrites. J Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


