Abstract
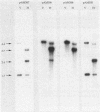
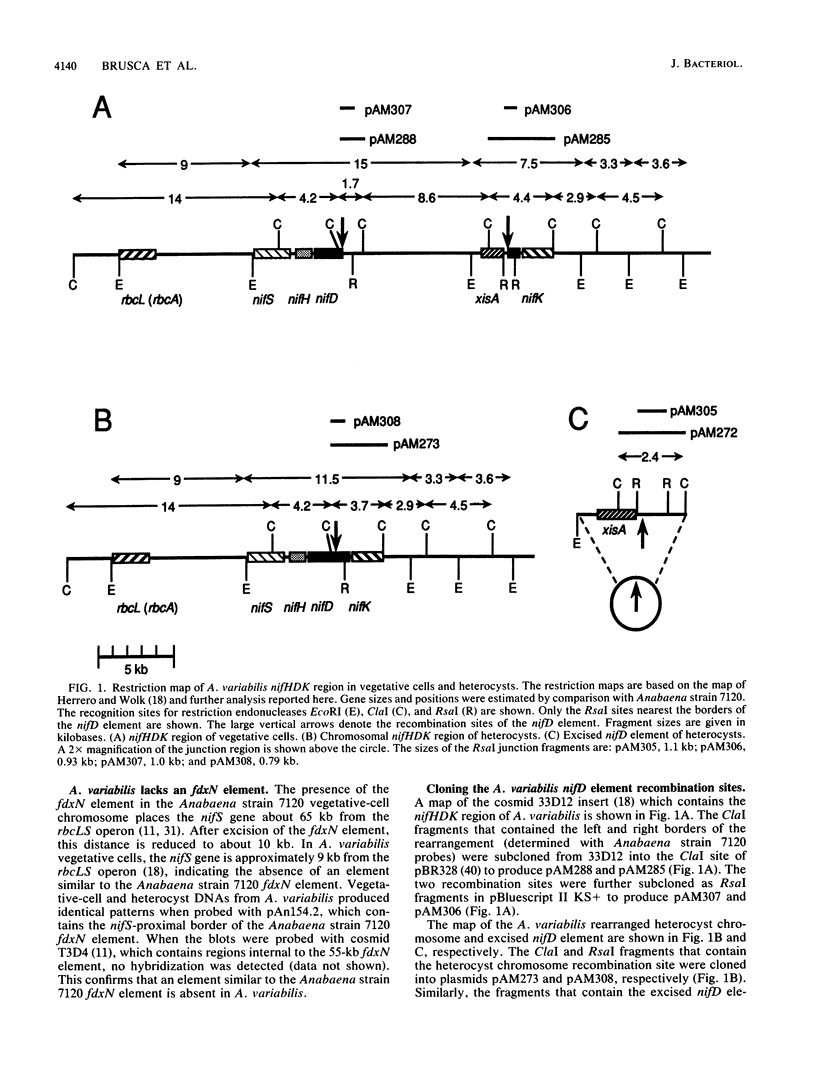
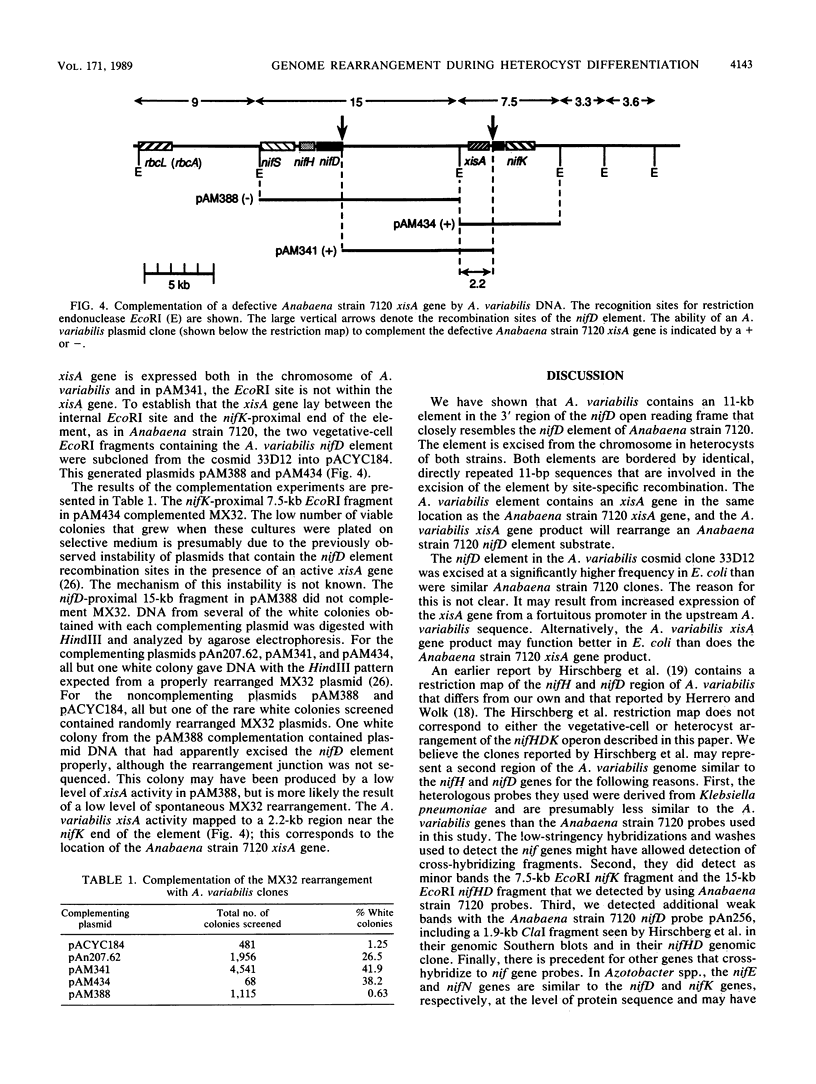
The 3' region of the Anabaena variabilis nifD gene contains an 11-kilobase-pair element which is excised from the chromosome during heterocyst differentiation. We have sequenced the recombination sites which border the element in vegetative cells and the rearranged heterocyst sequences. In vegetative cells, the element was flanked by 11-base-pair direct repeats which were identical to the repeats present at the ends of the nifD element in Anabaena sp. strain PCC 7120 (Anabaena strain 7120). Although Anabaena strain 7120 and A. variabilis are quite distinct in many ways, the overall sequence similarity between the two strains for the regions sequenced was 96%. Like the Anabaena strain 7120 element, the A. variabilis element was excised in heterocysts to produce a functional nifD gene and a free circularized element which was neither amplified nor degraded. The Anabaena strain 7120 xisA gene is located at the nifK-proximal end of the nifD element and is required for excision of the element in heterocysts. The A. variabilis element also contained an xisA gene which could complement a defective Anabaena strain 7120 xisA gene. A. variabilis did not contain the equivalent of the Anabaena strain 7120 fdxN 55-kilobase-pair element.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigle K. E., Newton W. E., Dean D. R. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene. 1985;37(1-3):37–44. doi: 10.1016/0378-1119(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Brigle K. E., Weiss M. C., Newton W. E., Dean D. R. Products of the iron-molybdenum cofactor-specific biosynthetic genes, nifE and nifN, are structurally homologous to the products of the nitrogenase molybdenum-iron protein genes, nifD and nifK. J Bacteriol. 1987 Apr;169(4):1547–1553. doi: 10.1128/jb.169.4.1547-1553.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H., Hill C. W. Attachment site of the genetic element e14. J Bacteriol. 1988 May;170(5):2040–2044. doi: 10.1128/jb.170.5.2040-2044.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Kennedy C., Kondorosi A., Krishnapillai V., Merrick M. Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol Gen Genet. 1977 Nov 29;157(2):189–198. doi: 10.1007/BF00267397. [DOI] [PubMed] [Google Scholar]
- Flores E., Wolk C. P. Identification of facultatively heterotrophic, N2-fixing cyanobacteria able to receive plasmid vectors from Escherichia coli by conjugation. J Bacteriol. 1985 Jun;162(3):1339–1341. doi: 10.1128/jb.162.3.1339-1341.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Carrasco C. D., Mulligan M. E., Schneider G. J., Haselkorn R. Deletion of a 55-kilobase-pair DNA element from the chromosome during heterocyst differentiation of Anabaena sp. strain PCC 7120. J Bacteriol. 1988 Nov;170(11):5034–5041. doi: 10.1128/jb.170.11.5034-5041.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Mulligan M. E., Haselkorn R. Different recombination site specificity of two developmentally regulated genome rearrangements. Nature. 1987 Jun 11;327(6122):526–529. doi: 10.1038/327526a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Wiest D. R. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science. 1988 Dec 9;242(4884):1421–1423. doi: 10.1126/science.3144039. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Greener A., Hill C. W. Identification of a novel genetic element in Escherichia coli K-12. J Bacteriol. 1980 Oct;144(1):312–321. doi: 10.1128/jb.144.1.312-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A., Wolk C. P. Genetic mapping of the chromosome of the cyanobacterium, Anabaena variabilis. Proximity of the structural genes for nitrogenase and ribulose-bisphosphate carboxylase. J Biol Chem. 1986 Jun 15;261(17):7748–7754. [PubMed] [Google Scholar]
- Hu N. T., Thiel T., Giddings T. H., Jr, Wolk C. P. New Anabaena and Nostoc cyanophages from sewage settling ponds. Virology. 1981 Oct 15;114(1):236–246. doi: 10.1016/0042-6822(81)90269-5. [DOI] [PubMed] [Google Scholar]
- Johnson T. R., Haynes J. I., 2nd, Wealand J. L., Yarbrough L. R., Hirschberg R. Structure and regulation of genes encoding phycocyanin and allophycocyanin from Anabaena variabilis ATCC 29413. J Bacteriol. 1988 Apr;170(4):1858–1865. doi: 10.1128/jb.170.4.1858-1865.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C., Snyder L. The lit gene product which blocks bacteriophage T4 late gene expression is a membrane protein encoded by a cryptic DNA element, e14. J Bacteriol. 1988 May;170(5):2056–2062. doi: 10.1128/jb.170.5.2056-2062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. Linkage map of the nitrogen fixation (nif) genes in Klebsiella pneumoniae. Mol Gen Genet. 1977 Nov 29;157(2):199–204. doi: 10.1007/BF00267398. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Lammers P. J., Golden J. W., Haselkorn R. Identification and sequence of a gene required for a developmentally regulated DNA excision in Anabaena. Cell. 1986 Mar 28;44(6):905–911. doi: 10.1016/0092-8674(86)90013-9. [DOI] [PubMed] [Google Scholar]
- Lammers P. J., Haselkorn R. Sequence of the nifD gene coding for the alpha subunit of dinitrogenase from the cyanobacterium Anabaena. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4723–4727. doi: 10.1073/pnas.80.15.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of the gene coding for the beta-subunit of dinitrogenase from the blue-green alga Anabaena. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6782–6786. doi: 10.1073/pnas.79.22.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. C., Joseph C. M., Haselkorn R. Organization of the nif genes in cyanobacteria in symbiotic association with Azolla and Anthoceros. Arch Microbiol. 1988 May;150(1):61–71. doi: 10.1007/BF00409719. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Buikema W. J., Haselkorn R. Bacterial-type ferredoxin genes in the nitrogen fixation regions of the cyanobacterium Anabaena sp. strain PCC 7120 and Rhizobium meliloti. J Bacteriol. 1988 Sep;170(9):4406–4410. doi: 10.1128/jb.170.9.4406-4410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. High recovery of nitrogenase activity and of Fe-labeled nitrogenase in heterocysts isolated from Anabaena variabilis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6271–6275. doi: 10.1073/pnas.75.12.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Saville B., Straus N., Coleman J. R. Contiguous organization of nitrogenase genes in a heterocystous cyanobacterium. Plant Physiol. 1987 Sep;85(1):26–29. doi: 10.1104/pp.85.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Weinman J. J., Fellows F. F., Gresshoff P. M., Shine J., Scott K. F. Structural analysis of the genes encoding the molybdenum-iron protein of nitrogenase in the Parasponia rhizobium strain ANU289. Nucleic Acids Res. 1984 Nov 26;12(22):8329–8344. doi: 10.1093/nar/12.22.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P., Shaffer P. W. Heterotrophic micro- and macrocultures of a nitrogen-fixing cyanobacterium. Arch Microbiol. 1976 Nov 2;110(23):145–147. doi: 10.1007/BF00690221. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van de Putte P., Plasterk R., Kuijpers A. A Mu gin complementing function and an invertible DNA region in Escherichia coli K-12 are situated on the genetic element e14. J Bacteriol. 1984 May;158(2):517–522. doi: 10.1128/jb.158.2.517-522.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]