Abstract
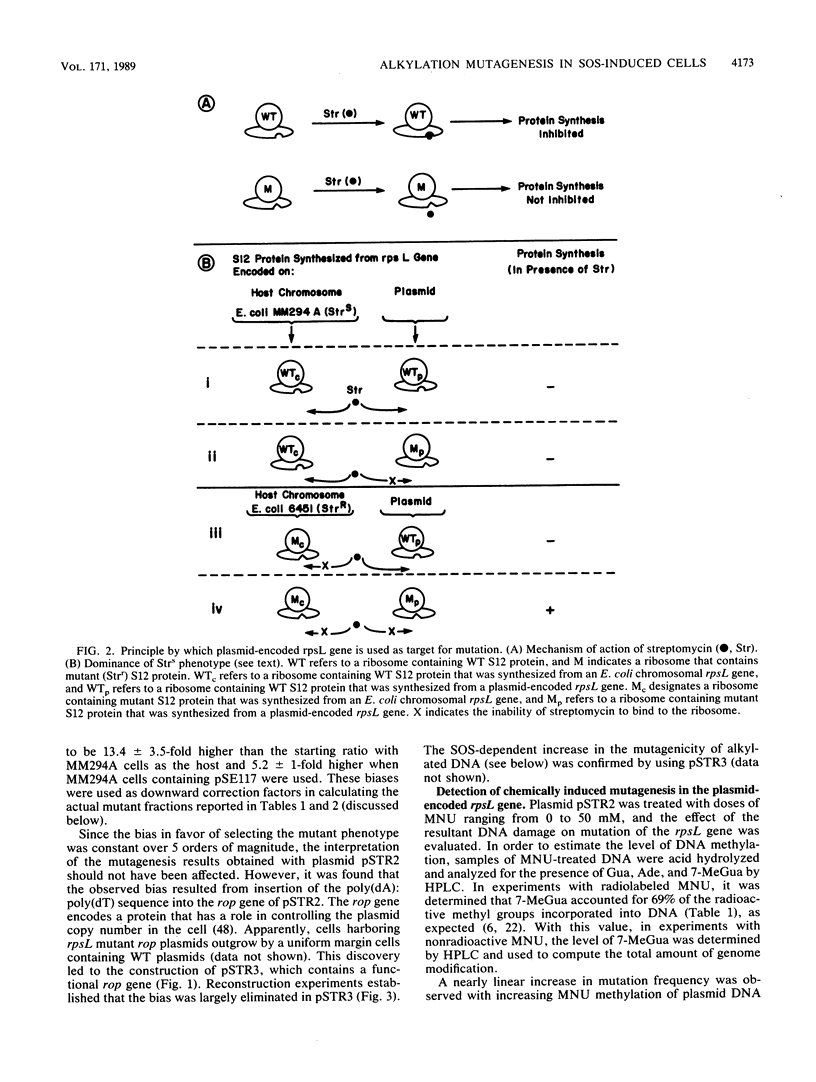
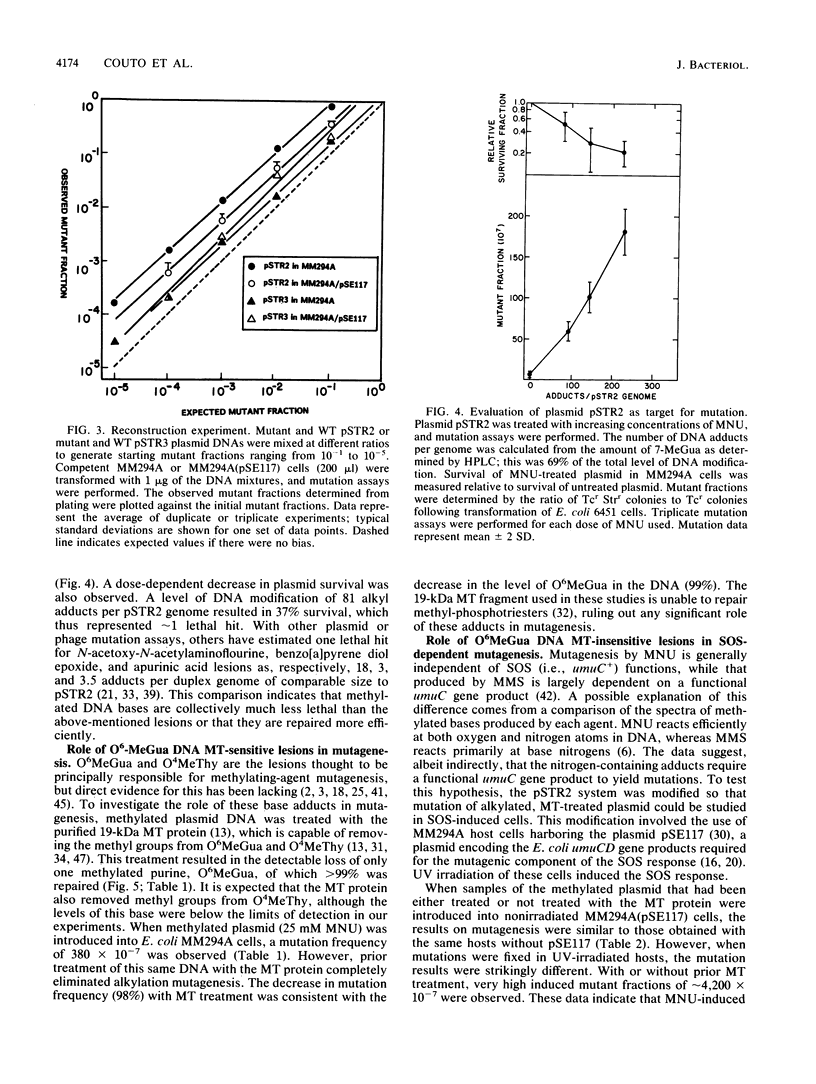
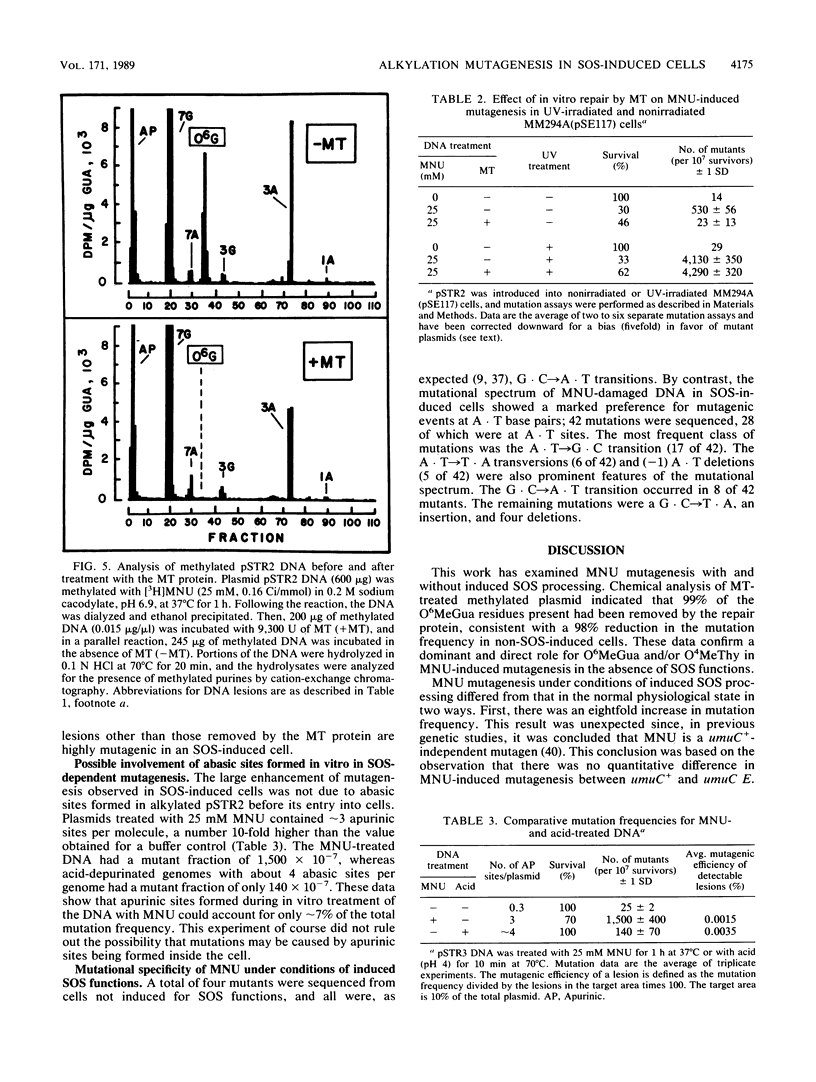
Escherichia coli plasmids containing the rpsL+ gene (Strs phenotype) as the target for mutation were treated in vitro with N-methyl-N-nitrosourea. Following fixation of mutations in E. coli MM294A cells (recA+ Strs), an unselected population of mutant and wild-type plasmids was isolated and transferred into a second host, E. coli 6451 (recA Strr). Strains carrying plasmid-encoded forward mutations were then selected as Strr isolates, while rpsL+ plasmids conferred the dominant Strs phenotype in the second host. Mutation induction and reduced survival of N-methyl-N-nitrosourea-treated plasmids were shown to be dose dependent. Because this system permitted analysis and manipulation of the levels of certain methylated bases produced in vitro by N-methyl-N-nitrosourea, it afforded the opportunity to assess directly the relative roles of these bases and of SOS functions in mutagenesis. The methylated plasmid DNA gave a mutation frequency of 6 X 10(-5) (a 40-fold increase over background) in physiologically normal cells. When the same methylated plasmid was repaired in vitro by using purified O6-methylguanine DNA methyltransferase (to correct O6-methylguanine and O4-methylthymine), no mutations were detected above background levels. In contrast, when the methylated plasmid DNA was introduced into host cells induced by UV light for the SOS functions, rpsL mutagenesis was enhanced eightfold over the level seen without SOS induction. This enhancement of mutagenesis by SOS was unaffected by prior treatment of the DNA with O6-methylguanine DNA methyltransferase. These results demonstrate a predominant mutagenic role for alkylation lesions other than O6-methylguanine or O4-methylthymine when SOS functions are induced. The mutation spectrum of N-methyl-N-nitrosourea under conditions of induced SOS functions revealed a majority of mutagenic events at A . T base pairs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J. Mutational and recombinational events in carcinogen-modified plasmid DNA. Influence of host-cell repair genes. Mutat Res. 1985 Jan-Mar;145(1-2):25–34. doi: 10.1016/0167-8817(85)90036-7. [DOI] [PubMed] [Google Scholar]
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Abbott P. J., Saffhill R. DNA-synthesis with methylated poly(dA-dT) templates: possible role of O4-methylthymine as a pro-mutagenic base. Nucleic Acids Res. 1977 Mar;4(3):761–769. doi: 10.1093/nar/4.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Kenyon C. J., Walker G. C. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5749–5753. doi: 10.1073/pnas.78.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranek D. T., Heflich R. H., Kodell R. L., Morris S. M., Casciano D. A. Correlation between specific DNA-methylation products and mutation induction at the HGPRT locus in Chinese hamster ovary cells. Mutat Res. 1983 Jun-Jul;110(1):171–180. doi: 10.1016/0027-5107(83)90026-x. [DOI] [PubMed] [Google Scholar]
- Beranek D. T., Weis C. C., Swenson D. H. A comprehensive quantitative analysis of methylated and ethylated DNA using high pressure liquid chromatography. Carcinogenesis. 1980 Jul;1(7):595–606. doi: 10.1093/carcin/1.7.595. [DOI] [PubMed] [Google Scholar]
- Bichara M., Fuchs R. P. DNA binding and mutation spectra of the carcinogen N-2-aminofluorene in Escherichia coli. A correlation between the conformation of the premutagenic lesion and the mutation specificity. J Mol Biol. 1985 Jun 5;183(3):341–351. doi: 10.1016/0022-2836(85)90005-1. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Dean D. A plasmid cloning vector for the direct selection of strains carrying recombinant plasmids. Gene. 1981 Oct;15(1):99–102. doi: 10.1016/0378-1119(81)90108-6. [DOI] [PubMed] [Google Scholar]
- Defais M., Caillet-Fauquet P., Fox M. S., Radman M. Induction kinetics of mutagenic DNA repair activity in E. coli following ultraviolet irradiation. Mol Gen Genet. 1976 Oct 18;148(2):125–130. doi: 10.1007/BF00268375. [DOI] [PubMed] [Google Scholar]
- Defais M., Jeggo P., Samson L., Schendel P. F. Effect of the adaptive response on the induction of the SOS pathway in E. coli K-12. Mol Gen Genet. 1980;177(4):653–659. doi: 10.1007/BF00272676. [DOI] [PubMed] [Google Scholar]
- Demple B., Jacobsson A., Olsson M., Robins P., Lindahl T. Repair of alkylated DNA in Escherichia coli. Physical properties of O6-methylguanine-DNA methyltransferase. J Biol Chem. 1982 Nov 25;257(22):13776–13780. [PubMed] [Google Scholar]
- Drinkwater N. R., Miller E. C., Miller J. A. Estimation of apurinic/apyrimidinic sites and phosphotriesters in deoxyribonucleic acid treated with electrophilic carcinogens and mutagens. Biochemistry. 1980 Oct 28;19(22):5087–5092. doi: 10.1021/bi00563a023. [DOI] [PubMed] [Google Scholar]
- Eckert K. A., Drinkwater N. R. recA-dependent and recA-independent N-ethyl-N-nitrosourea mutagenesis at a plasmid-encoded herpes simplex virus thymidine kinase gene in Escherichia coli. Mutat Res. 1987 May;178(1):1–10. doi: 10.1016/0027-5107(87)90079-0. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E. Induction of transversion mutations in Escherichia coli by N-methyl-N'-nitro-N-nitrosoguanidine is SOS dependent. J Bacteriol. 1985 Jul;163(1):213–220. doi: 10.1128/jb.163.1.213-220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K. A cloning vehicle suitable for strand separation. Gene. 1980 Oct;11(1-2):109–115. doi: 10.1016/0378-1119(80)90091-8. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Verdier J. M., Bichara M., Freund A. M., Daune M. P., Fuchs R. P. Carcinogen-induced mutation spectrum in wild-type, uvrA and umuC strains of Escherichia coli. Strain specificity and mutation-prone sequences. J Mol Biol. 1984 Jul 25;177(1):33–51. doi: 10.1016/0022-2836(84)90056-1. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. Streptomycin resistance; a genetically recessive mutation. J Bacteriol. 1951 May;61(5):549–550. doi: 10.1128/jb.61.5.549-550.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Luisi-DeLuca C., Porter R. D., Taylor W. D. Stimulation of recombination between homologous sequences on plasmid DNA and chromosomal DNA in Escherichia coli by N-acetoxy-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1984 May;81(9):2831–2835. doi: 10.1073/pnas.81.9.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Apirion D., Schlessinger D. Polyribosome depletion and blockage of the ribosome cycle by streptomycin in Escherichia coli. J Mol Biol. 1969 Jun 14;42(2):315–335. doi: 10.1016/0022-2836(69)90046-1. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Marsh L., Walker G. C. Cold sensitivity induced by overproduction of UmuDC in Escherichia coli. J Bacteriol. 1985 Apr;162(1):155–161. doi: 10.1128/jb.162.1.155-161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T. V., Karran P., Lindahl T. Inducible repair of O-alkylated DNA pyrimidines in Escherichia coli. EMBO J. 1984 Mar;3(3):545–550. doi: 10.1002/j.1460-2075.1984.tb01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T. V., Lindahl T. Methyl phosphotriesters in alkylated DNA are repaired by the Ada regulatory protein of E. coli. Nucleic Acids Res. 1985 Apr 25;13(8):2683–2698. doi: 10.1093/nar/13.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa H., Lee C. H., Kakefuda T. Alteration of plasmid DNA-mediated transformation and mutation induced by covalent binding of benzo[alpha]pyrene-7,8-dihydrodiol-9,10-oxide in Escherichia coli. Mutat Res. 1981 Jun;82(1):47–57. doi: 10.1016/0027-5107(81)90137-8. [DOI] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Preston B. D., Singer B., Loeb L. A. Mutagenic potential of O4-methylthymine in vivo determined by an enzymatic approach to site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8501–8505. doi: 10.1073/pnas.83.22.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K. K., Richardson F. C., Crosby R. M., Swenberg J. A., Skopek T. R. DNA base changes and alkylation following in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1987 Jan;84(2):344–348. doi: 10.1073/pnas.84.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel P. F., Defais M. The role of umuC gene product in mutagenesis by simple alkylating agents. Mol Gen Genet. 1980;177(4):661–665. doi: 10.1007/BF00272677. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Robins P. E. Repair of O6-methylguanine in adapted Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6017–6020. doi: 10.1073/pnas.75.12.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Sági J., Kuśmierek J. T. Escherichia coli polymerase I can use O2-methyldeoxythymidine or O4-methyldeoxythymidine in place of deoxythymidine in primed poly(dA-dT).poly(dA-dT) synthesis. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4884–4888. doi: 10.1073/pnas.80.16.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Davis B. D. Bactericidal action of streptomycin and comparison with spectinomycin in heterozygotes of Escherichia coli. Antimicrob Agents Chemother. 1972 Mar;1(3):252–258. doi: 10.1128/aac.1.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo I., Sedgwick B., Demple B., Li B., Lindahl T. Induction of resistance to alkylating agents in E. coli: the ada+ gene product serves both as a regulatory protein and as an enzyme for repair of mutagenic damage. EMBO J. 1984 Sep;3(9):2151–2157. doi: 10.1002/j.1460-2075.1984.tb02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Davis B. D. Cyclic blockade of initiation sites by streptomycin-damaged ribosomes in Escherichia coli: an explanation for dominance of sensitivity. J Mol Biol. 1973 Apr 5;75(2):377–390. doi: 10.1016/0022-2836(73)90028-4. [DOI] [PubMed] [Google Scholar]


