Abstract
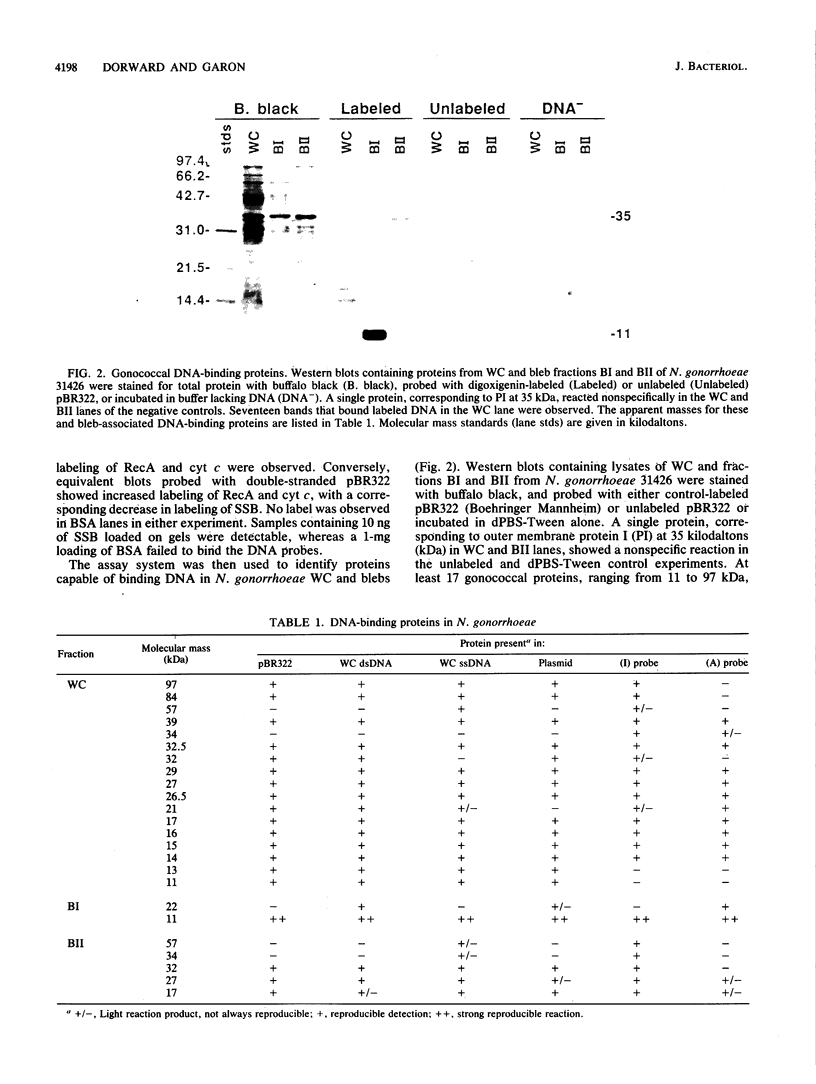
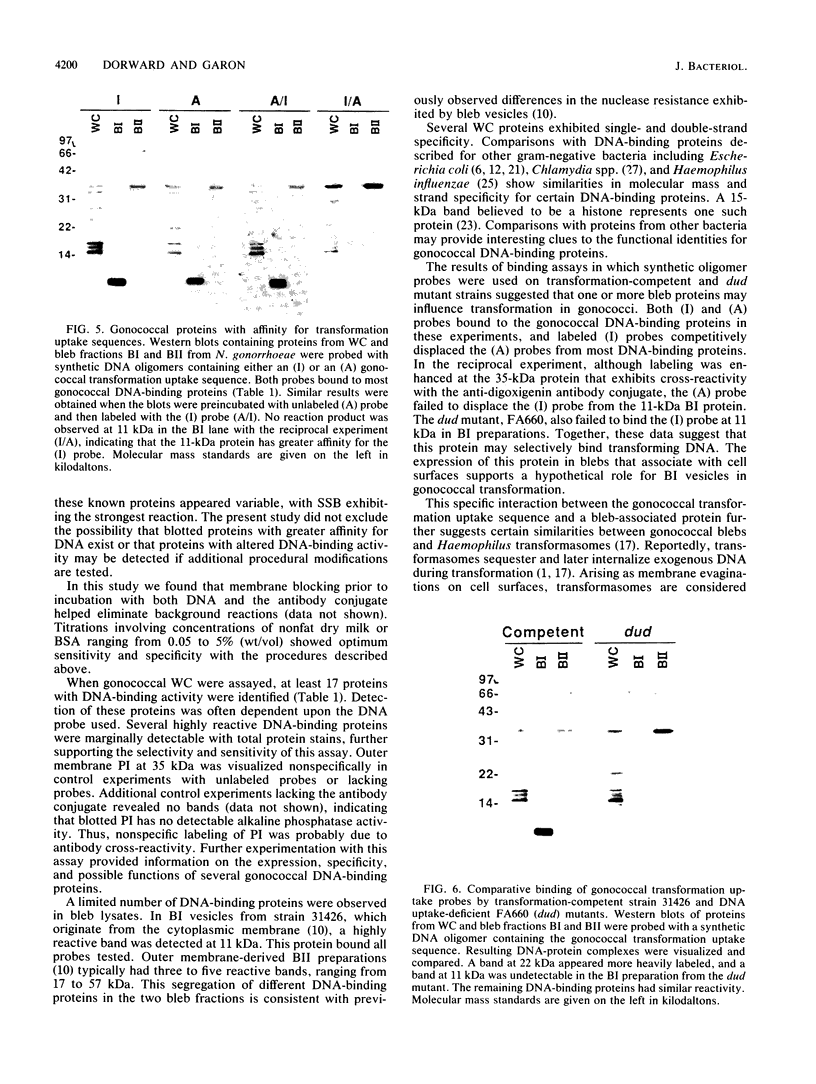
Naturally elaborated membrane bleb fractions BI and BII of Neisseria gonorrhoeae contain both linear and circular DNAs. Because little is known about the interactions between DNA and blebs, studies were initiated to identify specific proteins that bind DNA in elaborated membrane blebs. Western immunoblots of whole-cell and bleb proteins from transformation-competent and DNA-uptake-deficient (dud) mutants were probed with single- or double-stranded gonococcal DNA, pBR322, or synthetic DNA oligomers containing intact or altered gonococcal transformation uptake sequences. The specificity and sensitivity of a nonradioactive DNA-binding protein assay was evaluated, and the assay was used to visualize DNA-protein complexes on the blots. The complexes were then characterized by molecular mass, DNA-binding specificity, and expression in bleb fractions. The assay effectively detected blotted DNA-binding proteins. At least 17 gonococcal DNA-binding proteins were identified; unique subsets occurred in BI and BII. Certain DNA-binding proteins had varied affinities for single- and double-stranded DNA, and the intact transformation uptake sequence competitively displaced the altered sequence from a BI protein at 11 kilodaltons (kDa). A dud mutant, strain FA660, lacked DNA-binding activity at the 11-kDa protein in BI. The segregation of DNA-binding proteins within BI and BII correlates with their distinct protein profiles and suggests that these vesicles may play different roles. Although the DNA-binding proteins expressed in BII may influence the nuclease-resistant export of plasmids within BII vesicles, the BI 11-kDa protein may bind transforming DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barouki R., Smith H. O. Reexamination of phenotypic defects in rec-1 and rec-2 mutants of Haemophilus influenzae Rd. J Bacteriol. 1985 Aug;163(2):629–634. doi: 10.1128/jb.163.2.629-634.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Robbins K., Koomey J. M., Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Lacks S. A., Sparling P. F. Transformation-deficient mutants of piliated Neisseria gonorrhoeae. J Bacteriol. 1989 Feb;171(2):657–664. doi: 10.1128/jb.171.2.657-664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Sparling P. F. The genetics of the gonococcus. Annu Rev Microbiol. 1984;38:111–133. doi: 10.1146/annurev.mi.38.100184.000551. [DOI] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. DNA-binding vesicles released from the surface of a competence-deficient mutant of Haemophilus influenzae. J Bacteriol. 1982 Oct;152(1):441–450. doi: 10.1128/jb.152.1.441-450.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley S., Radtke J., Blin N., Unteregger G. Rapid detection of DNA-binding factors using protein-blotting and digoxigenin-dUTP marked probes. Nucleic Acids Res. 1988 Dec 23;16(24):11839–11839. doi: 10.1093/nar/16.24.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward D. W., Garon C. F., Judd R. C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989 May;171(5):2499–2505. doi: 10.1128/jb.171.5.2499-2505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. D., Scocca J. J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. F., Biswas G. D., Sparling P. F. Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J Bacteriol. 1982 Dec;152(3):1071–1077. doi: 10.1128/jb.152.3.1071-1077.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Barany F., Smith H. O. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6927–6931. doi: 10.1073/pnas.80.22.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C., Hagblom P., Ohman H., Göransson M., Normark S. Cryptic plasmid of Neisseria gonorrhoeae: complete nucleotide sequence and genetic organization. J Bacteriol. 1985 Aug;163(2):430–438. doi: 10.1128/jb.163.2.430-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., Ajioka R. S., Marchal C., Sparling P. F., So M. DNA transformation leads to pilin antigenic variation in Neisseria gonorrhoeae. Nature. 1988 Nov 24;336(6197):392–395. doi: 10.1038/336392a0. [DOI] [PubMed] [Google Scholar]
- Spassky A., Rimsky S., Garreau H., Buc H. H1a, an E. coli DNA-binding protein which accumulates in stationary phase, strongly compacts DNA in vitro. Nucleic Acids Res. 1984 Jul 11;12(13):5321–5340. doi: 10.1093/nar/12.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Sutrina S. L., Scocca J. J. DNA-binding proteins of Haemophilus influenzae: purification and characterization of a major intracellular binding protein. J Bacteriol. 1983 Jul;155(1):246–253. doi: 10.1128/jb.155.1.246-253.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Stephens R. S. Developmental-form-specific DNA-binding proteins in Chlamydia spp. Infect Immun. 1988 Jul;56(7):1678–1684. doi: 10.1128/iai.56.7.1678-1684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]