Abstract
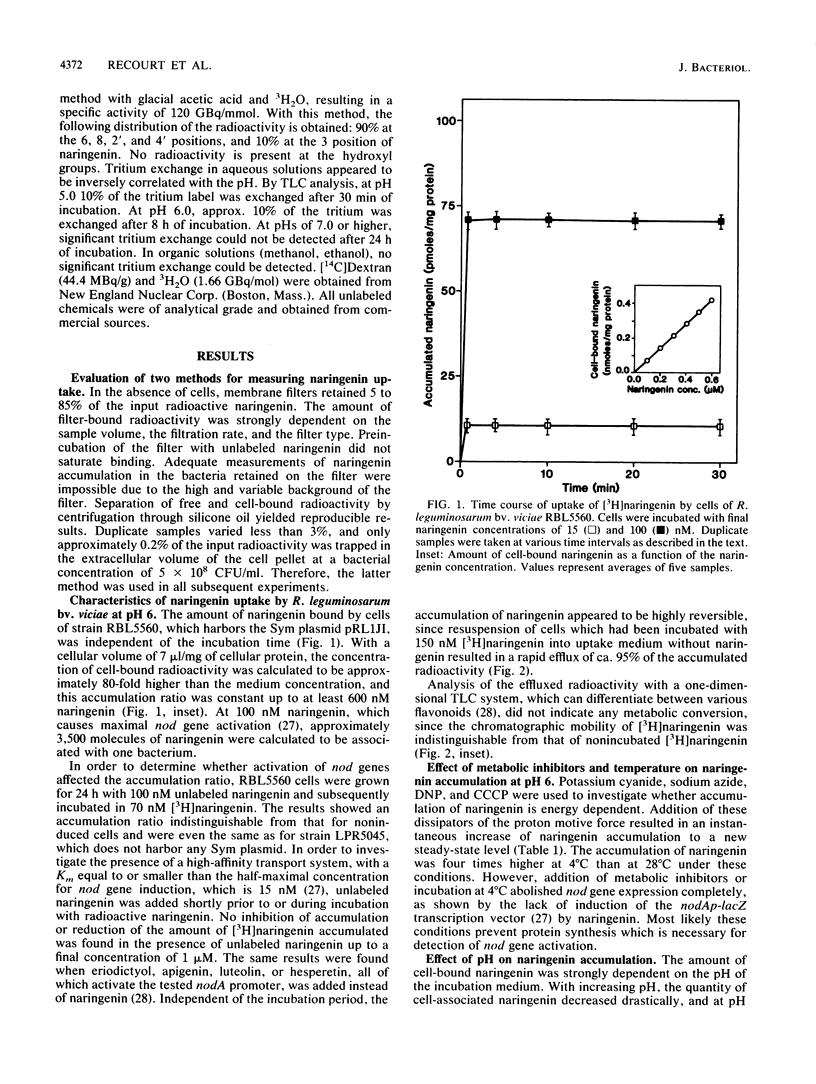
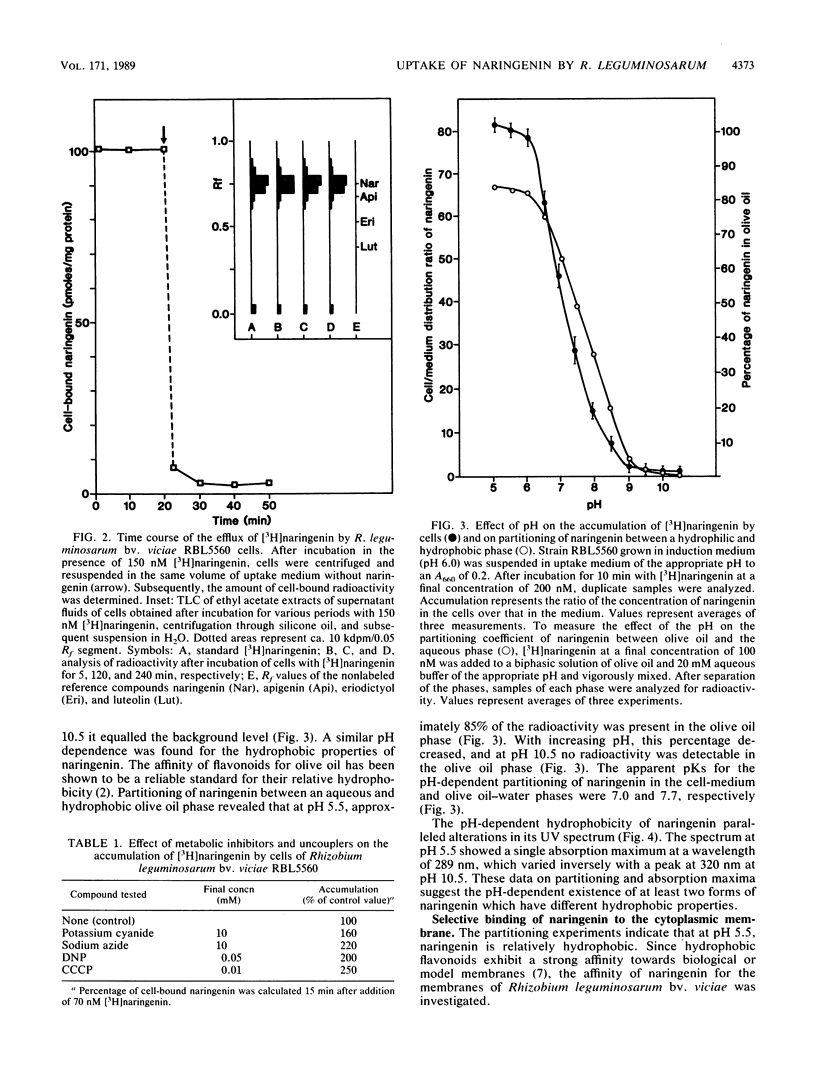
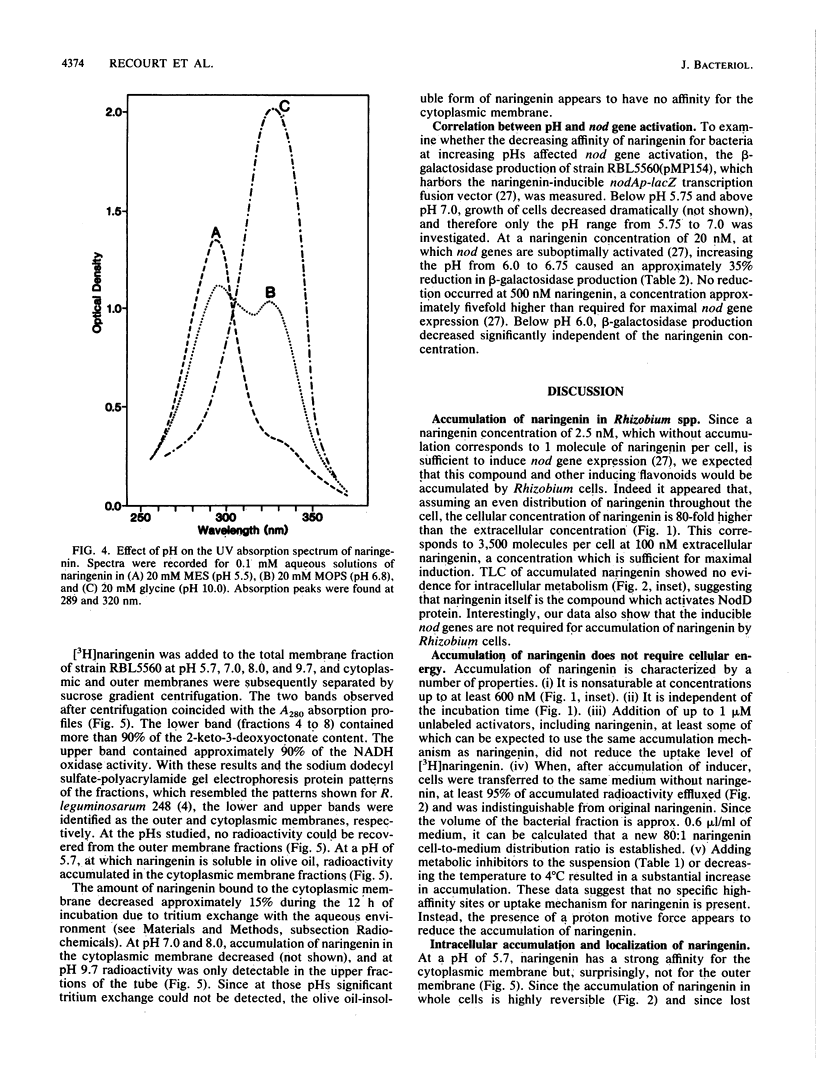
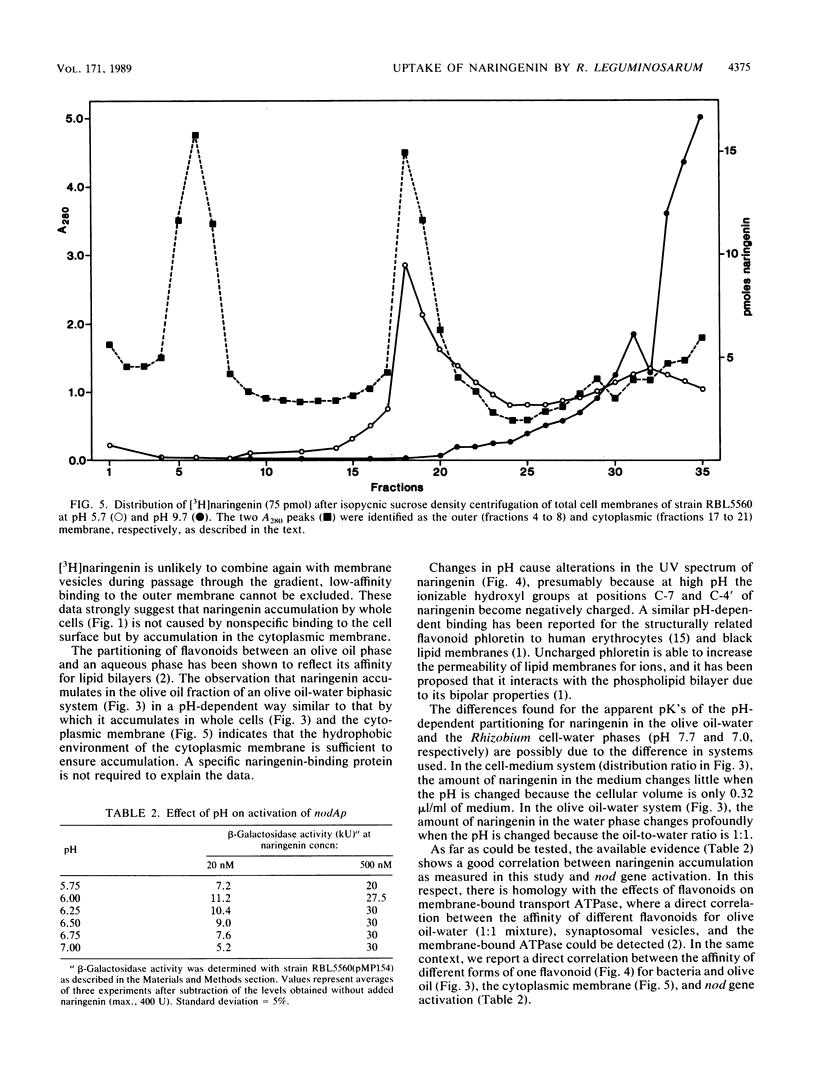
Most Sym plasmid-localized nodulation genes of Rhizobium leguminosarum bv. viciae are only expressed upon activation of the NodD protein by plant flavonoids, e.g., naringenin (S. A. J. Zaat, C. A. Wijffelman, H. P. Spaink, A. A. N. van Brussel, and B. J. J. Lugtenberg, J. Bacteriol, 169:198-204, 1987). As part of a study on the mechanism of NodD protein activation, the mechanism of uptake and the intracellular fate of [3H]naringenin were studied. Naringenin was accumulated by Rhizobium cells without apparent metabolic conversion to an 80-fold-higher concentration in a process which did not require any of the other Sym plasmid-localized nod genes. Naringenin accumulation was nonsaturable, highly reversible, and not inhibited by the presence of other flavonoids or the metabolic inhibitors potassium cyanide, sodium azide, 2,4-dinitrophenol, and carbonyl cyanide m-chlorophenylhydrazone. These data indicate an accumulation mechanism without high affinity sites which does not use cellular energy. In vitro, naringenin has high affinity for the cytoplasmic membrane. This binding was pH dependent, very high at pH 5.7 and not present anymore at pH 9.7. A similar pH dependency was found for the affinity of naringenin for the olive oil fraction of a biphasic olive oil-water system. pH-dependent changes in the UV spectrum indicate ionization of naringenin at high pH to a negatively charged form. Since it has recently been shown that the nodD gene product is located in the cytoplasmic membrane (H. R. M. Schlaman, H. P. Spaink, R. J. H. Okker, and B. J. J. Lugtenberg, J. Bacteriol., in press), our data are consistent with a model in which the un-ionized form of naringenin accumulates in the cytoplasmic membrane and activates, in a metabolically unaltered form, the NodD protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai A., Rahamimoff H. Inhibition of Ca2+-transport ATPase from synaptosomal vesicles by flavonoids. Biochim Biophys Acta. 1983 May 5;730(2):245–254. doi: 10.1016/0005-2736(83)90340-1. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Egelhoff T. T., Mulligan J. T., Long S. R. Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 1988 Mar;2(3):282–293. doi: 10.1101/gad.2.3.282. [DOI] [PubMed] [Google Scholar]
- Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983 Apr 1;32(7):1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Hong G. F., Burn J. E., Johnston A. W. Evidence that DNA involved in the expression of nodulation (nod) genes in Rhizobium binds to the product of the regulatory gene nodD. Nucleic Acids Res. 1987 Dec 10;15(23):9677–9690. doi: 10.1093/nar/15.23.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M. A., Ausubel F. M. Rhizobium meliloti has three functional copies of the nodD symbiotic regulatory gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8558–8562. doi: 10.1073/pnas.84.23.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Bachem C. W., Schell J., Kondorosi A. Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 1987 Apr;6(4):841–848. doi: 10.1002/j.1460-2075.1987.tb04829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. The atachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. J Biol Chem. 1959 Nov;234:3022–3026. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Correlation between extracellular fibrils and attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1986 Nov;168(2):821–827. doi: 10.1128/jb.168.2.821-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brussel A. A., Zaat S. A., Cremers H. C., Wijffelman C. A., Pees E., Tak T., Lugtenberg B. J. Role of plant root exudate and Sym plasmid-localized nodulation genes in the synthesis by Rhizobium leguminosarum of Tsr factor, which causes thick and short roots on common vetch. J Bacteriol. 1986 Feb;165(2):517–522. doi: 10.1128/jb.165.2.517-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat S. A., Wijffelman C. A., Mulders I. H., van Brussel A. A., Lugtenberg B. J. Root Exudates of Various Host Plants of Rhizobium leguminosarum Contain Different Sets of Inducers of Rhizobium Nodulation Genes. Plant Physiol. 1988 Apr;86(4):1298–1303. doi: 10.1104/pp.86.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat S. A., Wijffelman C. A., Spaink H. P., van Brussel A. A., Okker R. J., Lugtenberg B. J. Induction of the nodA promoter of Rhizobium leguminosarum Sym plasmid pRL1JI by plant flavanones and flavones. J Bacteriol. 1987 Jan;169(1):198–204. doi: 10.1128/jb.169.1.198-204.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., Lugtenberg B. Fractionation of Rhizobium leguminosarum cells into outer membrane, cytoplasmic membrane, periplasmic, and cytoplasmic components. J Bacteriol. 1986 Sep;167(3):1083–1085. doi: 10.1128/jb.167.3.1083-1085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



