Abstract
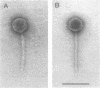
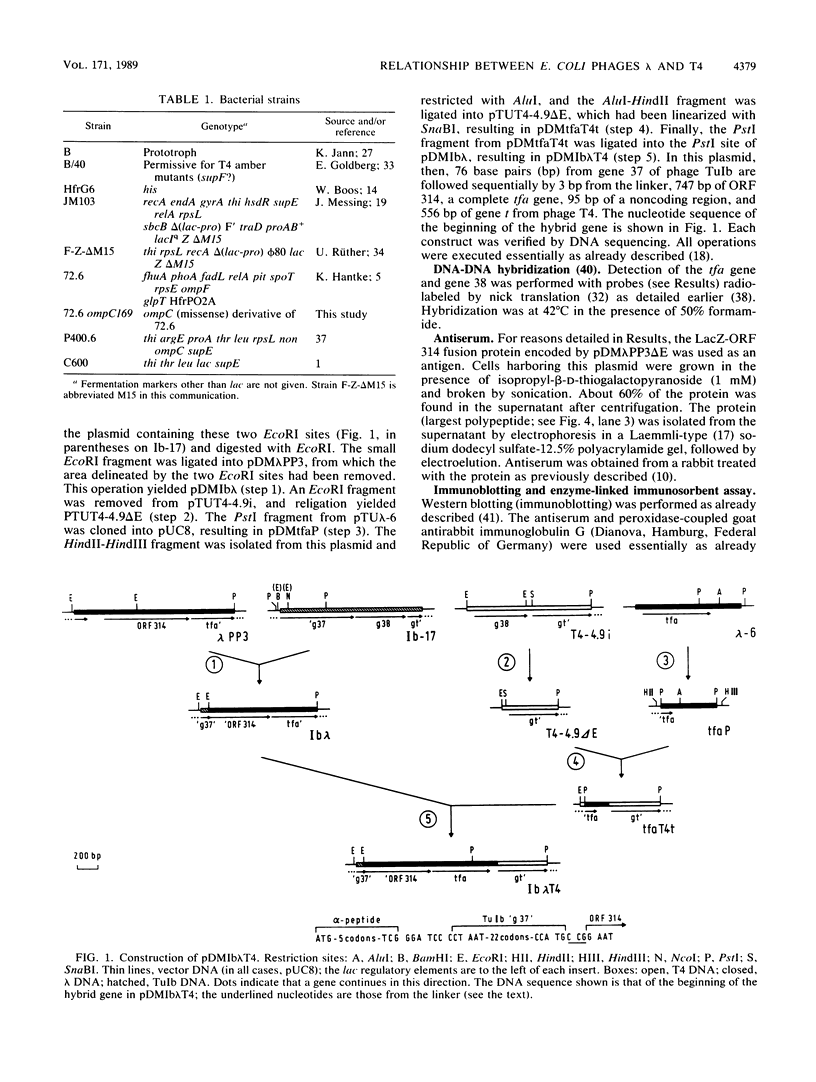
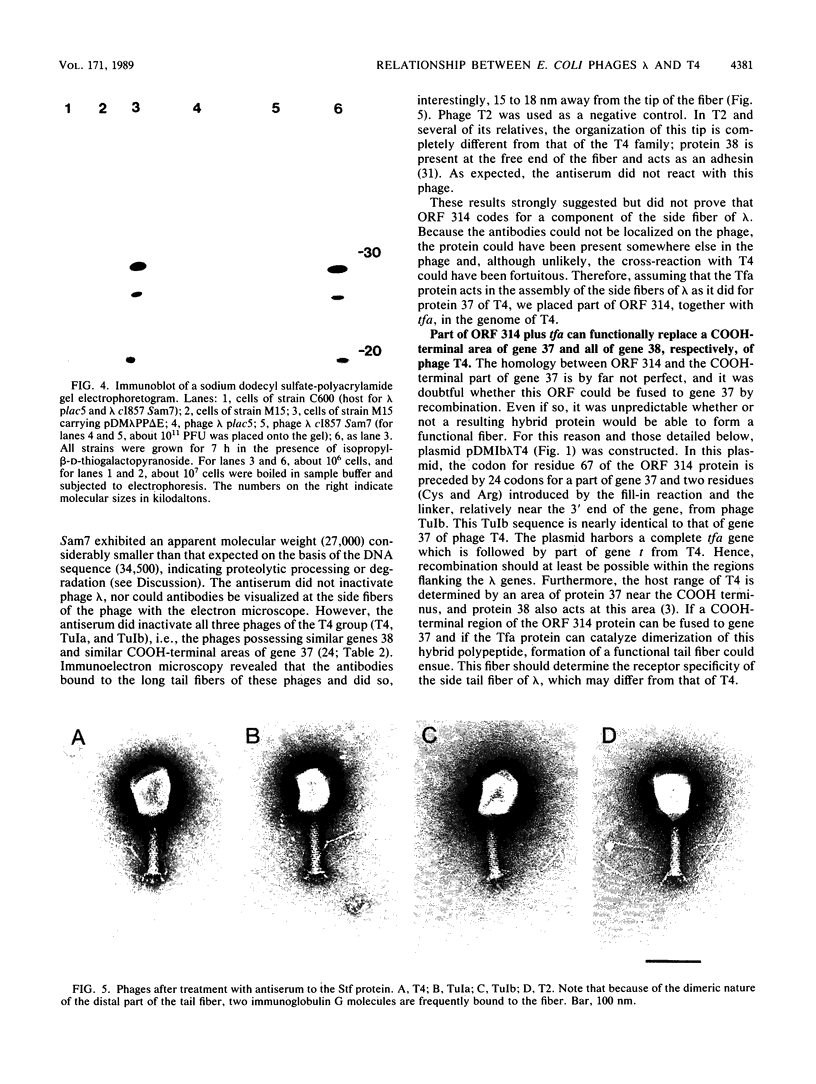
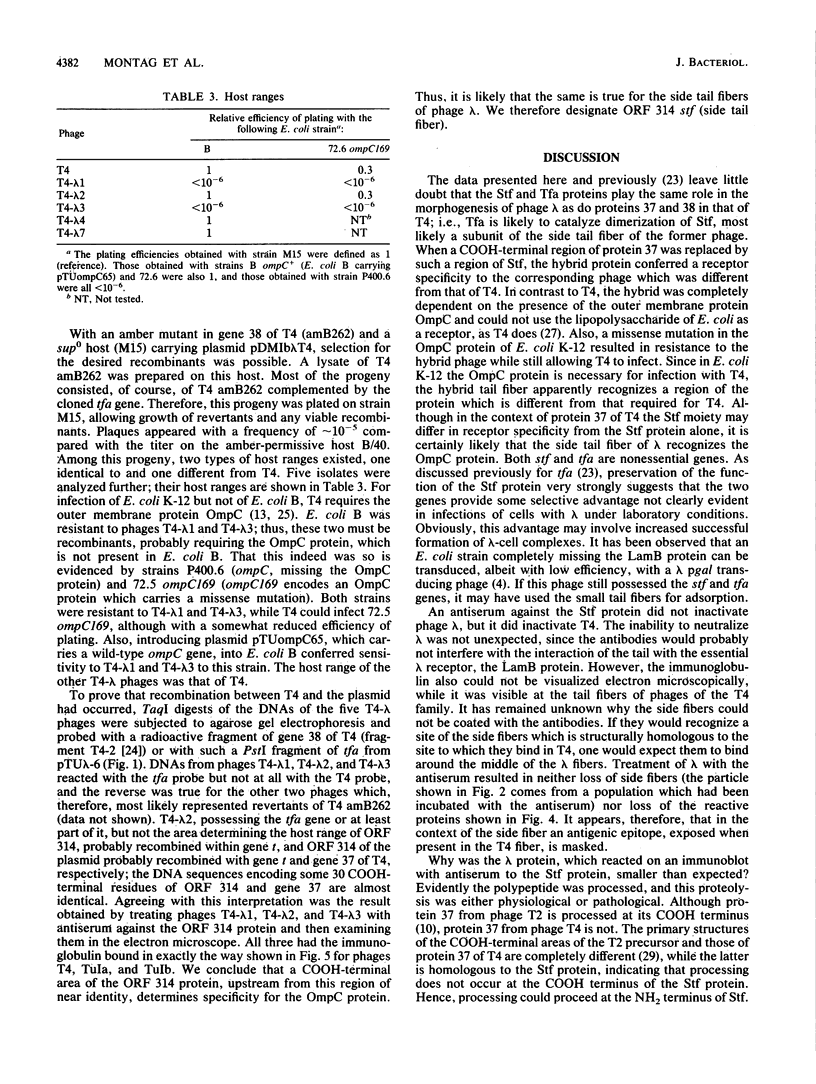
The distal part of the long tail fiber of Escherichia coli bacteriophage T4 consists of a dimer of protein 37. Dimerization requires the catalytic action of protein 38, which is encoded by T4 and is not present in the virion. It had previously been shown that gene tfa of the otherwise entirely unrelated phage lambda can functionally replace gene 38. Open reading frame (ORF) 314, which encodes a protein that exhibits homology to a COOH-terminal area of protein 37, is located immediately upstream of tfa. The gene was cloned and expressed in E. coli. An antiserum against the corresponding polypeptide showed that it was present in phage lambda. The serum also reacted with the long tail fibers of phage T4 near their free ends. An area of the gene encoding a COOH-terminal region of ORF 314 was recombined, together with tfa, into the genome of T4, thus replacing gene 38 and a part of gene 37 that codes for a COOH-terminal part of protein 37. Such T4-lambda hybrids, unlike T4, required the presence of outer membrane protein OmpC for infection of E. coli B. An ompC missense mutant of E. coli K-12, which was still sensitive to T4, was resistant to these hybrids. We conclude that the ORF 314 protein represents a subunit of the side tail fibers of phage lambda which probably recognize the OmpC protein. ORF 314 was designated stf (side tail fiber). The results also offer an explanation for the very unusual fact that, despite identical genomic organizations, T4 and T2 produce totally different proteins 38. An ancestor of T4 from the T2 lineage may have picked up tfa and stf from a lambdoid phase, thus possibly demonstrating horizontal gene transfer between unrelated phage species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckendorf S. K., Kim J. S., Lielausis I. Structure of bacteriophage T4 genes 37 and 38. J Mol Biol. 1973 Jan;73(1):17–35. doi: 10.1016/0022-2836(73)90156-3. [DOI] [PubMed] [Google Scholar]
- Beckendorf S. K. Structure of the distal half of the bacteriophage T4 tail fiber. J Mol Biol. 1973 Jan;73(1):37–53. doi: 10.1016/0022-2836(73)90157-5. [DOI] [PubMed] [Google Scholar]
- Braun-Breton C., Hofnung M. Explanations accounting for transduction by bacteriophage lambda in maltose negative bacteriophage lambda resistant mutants of Escherichia coli K-12. Mol Gen Genet. 1978 Feb 16;159(2):143–149. doi: 10.1007/BF00270887. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M, Fano U. Bacteriophage-Resistant Mutants in Escherichia Coli. Genetics. 1945 Mar;30(2):119–136. doi: 10.1093/genetics/30.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C. Assembly of bacteriophage T4 tail fibers. IV. Subunit composition of tail fibers and fiber precursors. J Mol Biol. 1973 Oct 5;79(4):633–647. doi: 10.1016/0022-2836(73)90068-5. [DOI] [PubMed] [Google Scholar]
- Drexler K., Riede I., Henning U. Morphogenesis of the long tail fibers of bacteriophage T2 involves proteolytic processing of the polypeptide (gene product 37) constituting the distal part of the fiber. J Mol Biol. 1986 Sep 20;191(2):267–272. doi: 10.1016/0022-2836(86)90263-9. [DOI] [PubMed] [Google Scholar]
- George D. G., Yeh L. S., Barker W. C. Unexpected relationships between bacteriophage lambda hypothetical proteins and bacteriophage T4 tail-fiber proteins. Biochem Biophys Res Commun. 1983 Sep 30;115(3):1061–1068. doi: 10.1016/s0006-291x(83)80043-6. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Henning U., Jann K. Two-component nature of bacteriophage T4 receptor activity in Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):664–666. doi: 10.1128/jb.137.1.664-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M. Divergent operons and the genetic structure of the maltose B region in Escherichia coli K12. Genetics. 1974 Feb;76(2):169–184. doi: 10.1093/genetics/76.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. J., Jacq B., Arquès D. G., Bickle T. A. A remarkable amino acid sequence homology between a phage T4 tail fibre protein and ORF314 of phage lambda located in the tail operon. Gene. 1986;44(1):147–150. doi: 10.1016/0378-1119(86)90055-7. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Montag D., Henning U. An open reading frame in the Escherichia coli bacteriophage lambda genome encodes a protein that functions in assembly of the long tail fibers of bacteriophage T4. J Bacteriol. 1987 Dec;169(12):5884–5886. doi: 10.1128/jb.169.12.5884-5886.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag D., Riede I., Eschbach M. L., Degen M., Henning U. Receptor-recognizing proteins of T-even type bacteriophages. Constant and hypervariable regions and an unusual case of evolution. J Mol Biol. 1987 Jul 5;196(1):165–174. doi: 10.1016/0022-2836(87)90519-5. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Furukawa H., Mizushima S. Role of lipopolysaccharide and outer membrane protein of Escherichia coli K-12 in the receptor activity for bacteriophage T4. J Bacteriol. 1978 Nov;136(2):693–699. doi: 10.1128/jb.136.2.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Crowther R. A. DNA sequence of the tail fibre genes 36 and 37 of bacteriophage T4. J Mol Biol. 1981 Dec 15;153(3):545–568. doi: 10.1016/0022-2836(81)90407-1. [DOI] [PubMed] [Google Scholar]
- Prehm P., Jann B., Jann K., Schmidt G., Stirm S. On a bacteriophage T3 and T4 receptor region within the cell wall lipopolysaccharide of Escherichia coli B. J Mol Biol. 1976 Feb 25;101(2):277–281. doi: 10.1016/0022-2836(76)90377-6. [DOI] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede I., Drexler K., Eschbach M. L., Henning U. DNA sequence of the tail fiber genes 37, encoding the receptor recognizing part of the fiber, of bacteriophages T2 and K3. J Mol Biol. 1986 Sep 20;191(2):255–266. doi: 10.1016/0022-2836(86)90262-7. [DOI] [PubMed] [Google Scholar]
- Riede I., Drexler K., Schwarz H., Henning U. T-even-type bacteriophages use an adhesin for recognition of cellular receptors. J Mol Biol. 1987 Mar 5;194(1):23–30. doi: 10.1016/0022-2836(87)90712-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Huskey R. J. Partial exclusion between T-even bacteriophages: an incipient genetic isolation mechanism. Genetics. 1974 Dec;78(4):989–1014. doi: 10.1093/genetics/78.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitges C. J., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Heterogeneity of protein I. Eur J Biochem. 1976 Mar 16;63(1):47–52. doi: 10.1111/j.1432-1033.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Schwarz H., Riede I., Sonntag I., Henning U. Degrees of relatedness of T-even type E. coli phages using different or the same receptors and topology of serologically cross-reacting sites. EMBO J. 1983;2(3):375–380. doi: 10.1002/j.1460-2075.1983.tb01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P. Dominance interactions in Escherichia coli cells mixedly infected with bacteriophage T4D wild-type and amber mutants and their possible implications as to type of gene-product function: catalytic vs. stoichiometric. Virology. 1968 Aug;35(4):550–563. doi: 10.1016/0042-6822(68)90285-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]