Abstract
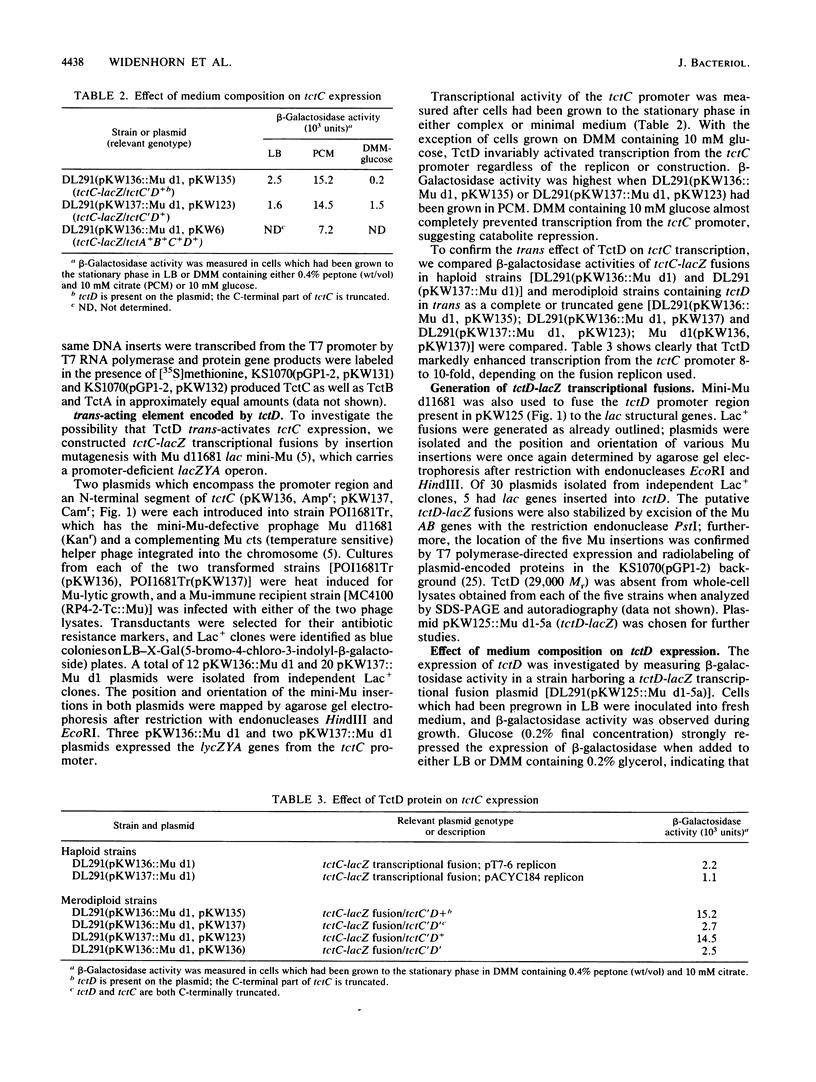
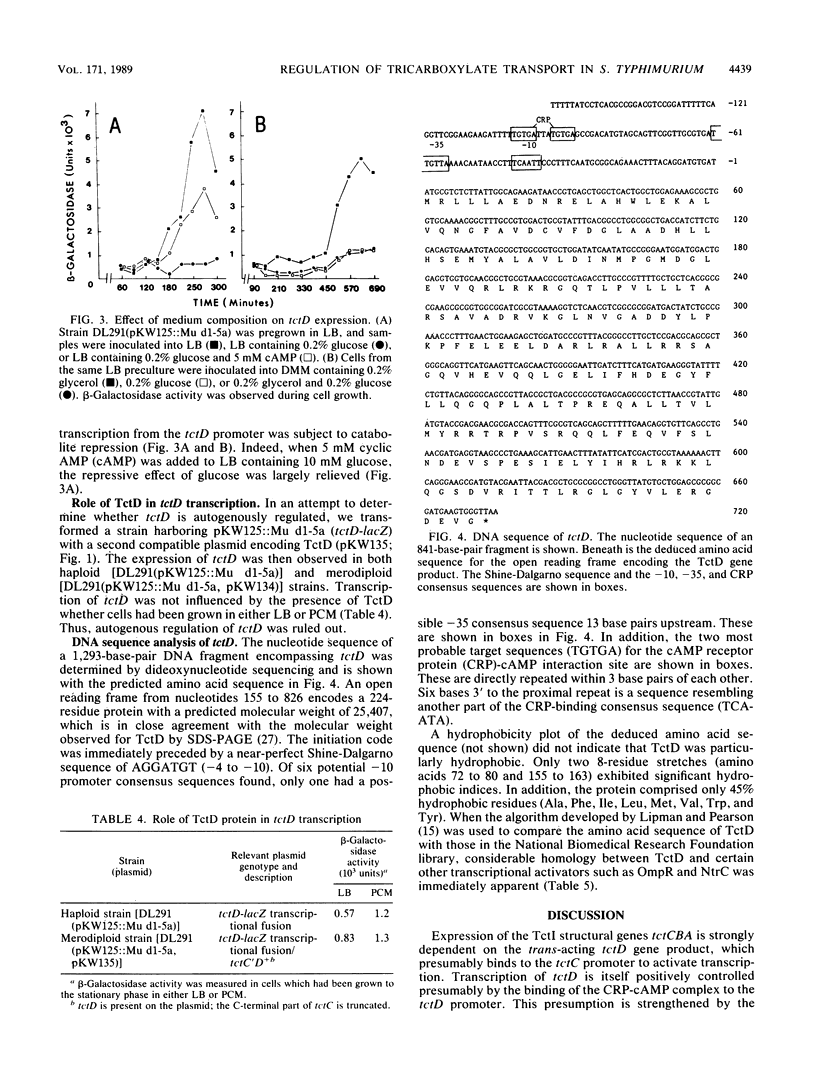
Tricarboxylates are transported into Salmonella typhimurium by a binding protein-dependent transport system known as TctI. Genetically, it comprises three structural genes, tctCBA, as well as a fourth gene of unknown function (tctD), which is transcribed divergently from tctC (K. A. Widenhorn, J. M. Somers, and W. W. Kay, J. Bacteriol. 170:3223-3227, 1988). Deletions in tctD strongly reduced expression of tctC or of tctC-lacZ transcriptional fusions; however, expression was restored when tctD was present in trans. Expression of tctD-lacZ transcriptional fusions was strongly repressed in the presence of D-glucose but could be alleviated by the addition of cyclic AMP. Furthermore, transcription of tctD was found not to be autogenously regulated. Thus, tctD is considered to be regulated by catabolite repression and encodes a transcriptional activator of tctCBA expression. From the DNA sequence of tctD, the predicted gene product was hydrophilic and shared distinct homologies with other globally regulated transcriptional activators such as OmpR and NtrC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Barnes W. M., Bevan M. Kilo-sequencing: an ordered strategy for rapid DNA sequence data acquisition. Nucleic Acids Res. 1983 Jan 25;11(2):349–368. doi: 10.1093/nar/11.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J Bacteriol. 1982 May;150(2):722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Chromosomal mutation for citrate utilization by Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):269–273. doi: 10.1128/jb.151.1.269-273.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A. C., Weatherwax R., Ames G. F. ATP-binding sites in the membrane components of histidine permease, a periplasmic transport system. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7333–7337. doi: 10.1073/pnas.81.23.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Cameron M. Citrate transport in Salmonella typhimurium. Arch Biochem Biophys. 1978 Sep;190(1):270–280. doi: 10.1016/0003-9861(78)90276-x. [DOI] [PubMed] [Google Scholar]
- Kustu S. G., Ames G. F. The hisP protein, a known histidine transport component in Salmonella typhimurium, is also an arginine transport component. J Bacteriol. 1973 Oct;116(1):107–113. doi: 10.1128/jb.116.1.107-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Albright L. M., Ausubel F. M. Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J Bacteriol. 1987 Jun;169(6):2424–2431. doi: 10.1128/jb.169.6.2424-2431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Scripture J. B., Voelker C., Miller S., O'Donnell R. T., Polgar L., Rade J., Horazdovsky B. F., Hogg R. W. High-affinity L-arabinose transport operon. Nucleotide sequence and analysis of gene products. J Mol Biol. 1987 Sep 5;197(1):37–46. doi: 10.1016/0022-2836(87)90607-3. [DOI] [PubMed] [Google Scholar]
- Somers J. M., Kay W. W. Genetic fine structure of the tricarboxylate transport (tct) locus of Salmonella typhimurium. Mol Gen Genet. 1983;190(1):20–26. doi: 10.1007/BF00330319. [DOI] [PubMed] [Google Scholar]
- Sweet G. D., Kay C. M., Kay W. W. Tricarboxylate-binding proteins of Salmonella typhimurium. Purification, crystallization, and physical properties. J Biol Chem. 1984 Feb 10;259(3):1586–1592. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenhorn K. A., Boos W., Somers J. M., Kay W. W. Cloning and properties of the Salmonella typhimurium tricarboxylate transport operon in Escherichia coli. J Bacteriol. 1988 Feb;170(2):883–888. doi: 10.1128/jb.170.2.883-888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenhorn K. A., Somers J. M., Kay W. W. Expression of the divergent tricarboxylate transport operon (tctI) of Salmonella typhimurium. J Bacteriol. 1988 Jul;170(7):3223–3227. doi: 10.1128/jb.170.7.3223-3227.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




