Abstract
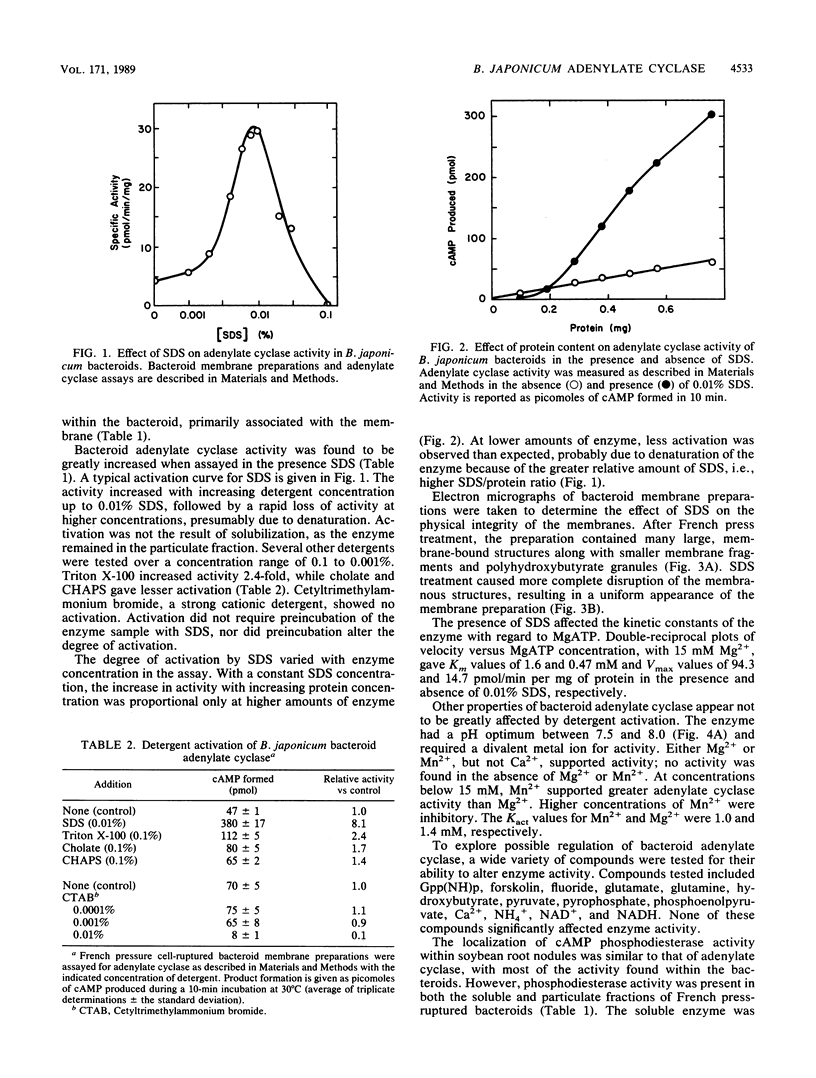
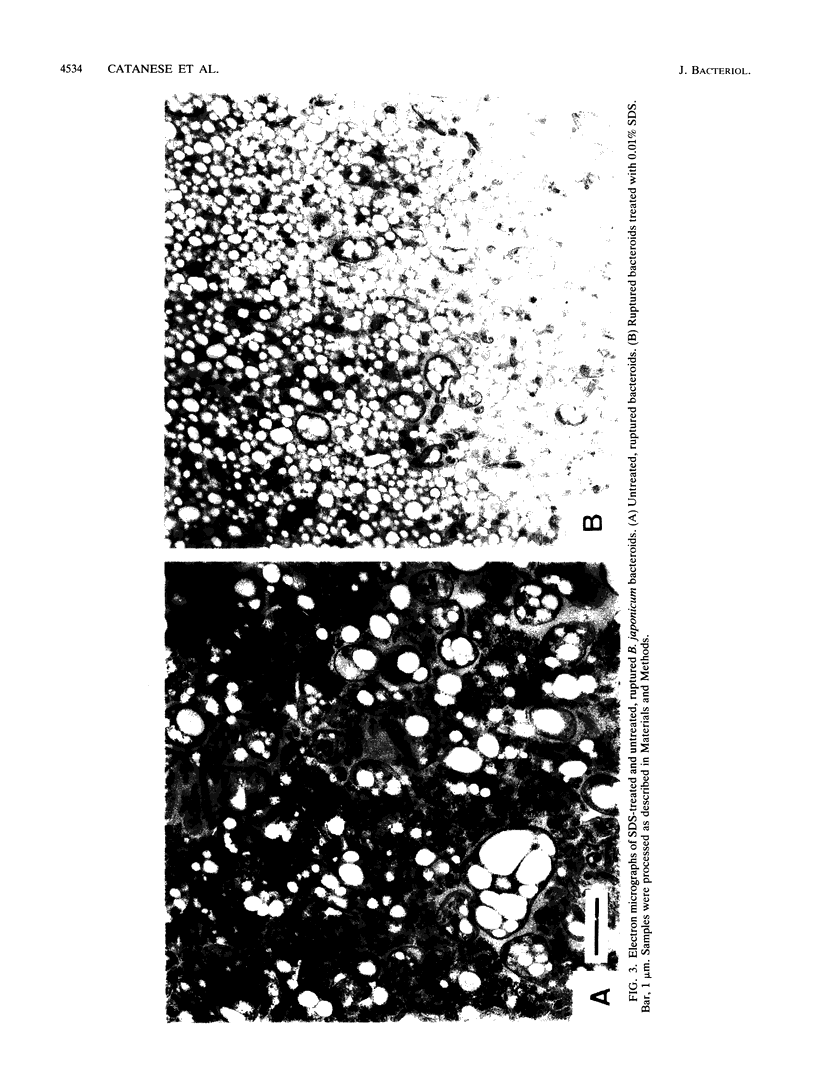
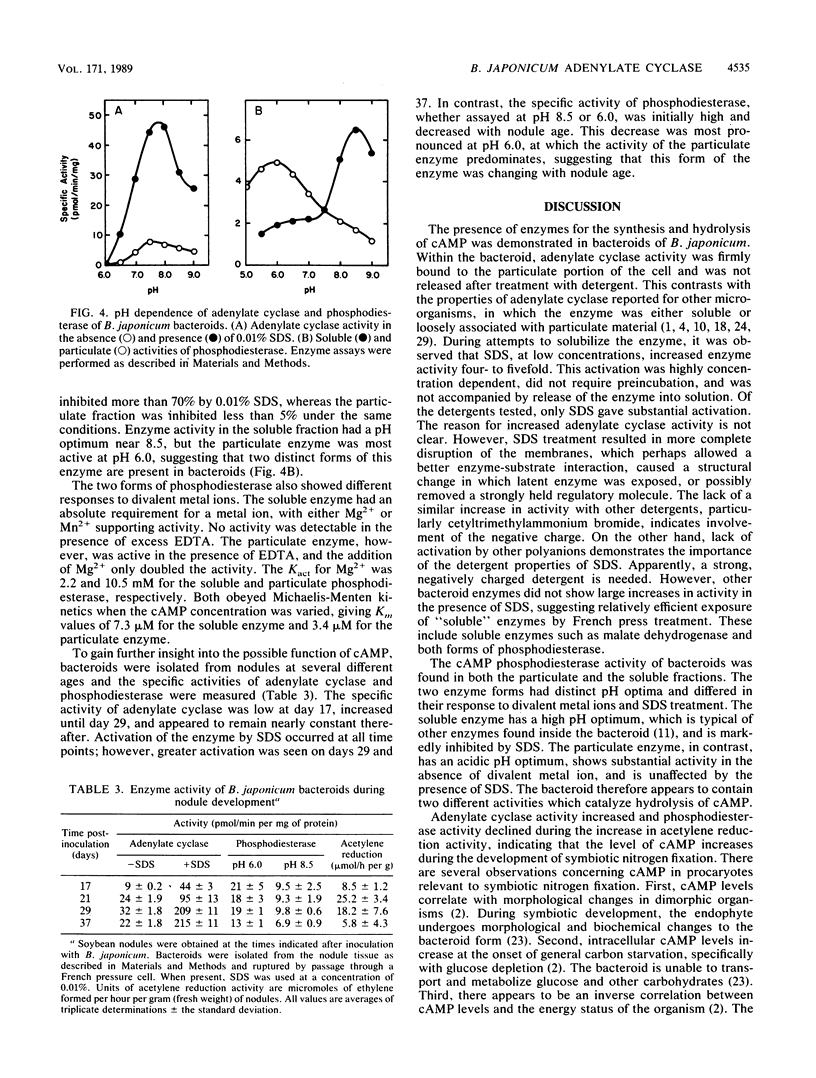
Adenylate cyclase and cyclic AMP (cAMP) phosphodiesterase have been identified and partially characterized in bacteroids of Bradyrhizobium japonicum 3I1b-143. Adenylate cyclase activity was found in the bacteroid membrane fraction, whereas cAMP phosphodiesterase activity was located in both the membrane and the cytosol. In contrast to other microorganisms, B. japonicum adenylate cyclase remained firmly bound to the membrane during treatment with detergents. Adenylate cyclase was activated four- to fivefold by 0.01% sodium dodecyl sulfate (SDS), whereas other detergents gave only slight activation. SDS had no effect on the membrane-bound cAMP phosphodiesterase but strongly inhibited the soluble enzyme, indicating that the two enzymes are different. All three enzymes were characterized by their kinetic constants, pH optima, and divalent metal ion requirements. With increasing nodule age, adenylate cyclase activity increased, the membrane-bound cAMP phosphodiesterase decreased, and the soluble cAMP phosphodiesterase remained largely unchanged. These results suggest that cAMP plays a role in symbiosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Cyclic 3', 5'-adenosine monophosphate phosphodiesterase mutants of Salmonella typhimurium. J Bacteriol. 1975 Jun;122(3):1081–1090. doi: 10.1128/jb.122.3.1081-1090.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M. H., Cheung W. Y. Adenylate cyclase of Brevibacterium liquefaciens. Inactivation by neuraminidase, phospholipase A and phospholipase C. Biochem Biophys Res Commun. 1973 Nov 1;55(1):187–192. doi: 10.1016/s0006-291x(73)80077-4. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Londos C. Evaluation of the effects of adenosine on hepatic and adipocyte adenylate cyclase under conditions where adenosine is not generated endogenously. J Cyclic Nucleotide Res. 1979;5(4):289–302. [PubMed] [Google Scholar]
- Davis C. W., Daly J. W. A simple direct assay of 3',5'-cyclic nucleotide phosphodiesterase activity based on the use of polyacrylamide-bononate affinity gel chromatography. J Cyclic Nucleotide Res. 1979;5(1):65–74. [PubMed] [Google Scholar]
- Guerinot M. L., Chelm B. K. Isolation and expression of the Bradyrhizobium japonicum adenylate cyclase gene (cya) in Escherichia coli. J Bacteriol. 1984 Sep;159(3):1068–1071. doi: 10.1128/jb.159.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Hayaishi O. Adenyl cyclase of Brevibacterium liquefaciens. Biochim Biophys Acta. 1967 Nov 21;149(1):1–11. doi: 10.1016/0005-2787(67)90685-5. [DOI] [PubMed] [Google Scholar]
- Karr D. B., Waters J. K., Suzuki F., Emerich D. W. Enzymes of the Poly-beta-Hydroxybutyrate and Citric Acid Cycles of Rhizobium japonicum Bacteroids. Plant Physiol. 1984 Aug;75(4):1158–1162. doi: 10.1104/pp.75.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Schleif R. Different cyclic AMP requirements for induction of the arabinose and lactose operons of Escherichia coli. J Mol Biol. 1973 Sep 5;79(1):149–162. doi: 10.1016/0022-2836(73)90276-3. [DOI] [PubMed] [Google Scholar]
- Martinez De Drets G., Arias A. Metabolism of some polyols by Rhizobium meliloti. J Bacteriol. 1970 Jul;103(1):97–103. doi: 10.1128/jb.103.1.97-103.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGetrick A. M., Goulding C. F., Manian S. S., O'Gara F. Catabolite repression and role of cyclic AMP in CO2 fixation and H2 metabolism in Rhizobium spp. J Bacteriol. 1985 Sep;163(3):1282–1284. doi: 10.1128/jb.163.3.1282-1284.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. D., Monard D., Rickenberg H. V. Cyclic 3',5'-adenosine monophosphate phosphodiesterase of Escherichia coli. J Bacteriol. 1973 Nov;116(2):857–866. doi: 10.1128/jb.116.2.857-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Primrose S. B. Effect of glucose on polyol metabolism by Rhizobium trifolii. J Bacteriol. 1979 Sep;139(3):1075–1078. doi: 10.1128/jb.139.3.1075-1078.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Signer E. R. Catabolite-repression-like phenomenon in Rhizobium meliloti. J Bacteriol. 1978 Dec;136(3):1197–1200. doi: 10.1128/jb.136.3.1197-1200.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch R. G., Elkan G. H. The role of ammonia, L-glutamate, and cyclic adenosine 3',5'-monophosphate in the regulation of ammonia assimilation in Rhizobium japonicum. Biochim Biophys Acta. 1978 Jan 18;538(2):244–248. doi: 10.1016/0304-4165(78)90352-5. [DOI] [PubMed] [Google Scholar]
- Upchurch R. G., Mortenson L. E. In vivo energetics and control of nitrogen fixation: changes in the adenylate energy charge and adenosine 5'-diphosphate/adenosine 5'-triphosphate ratio of cells during growth on dinitrogen versus growth on ammonia. J Bacteriol. 1980 Jul;143(1):274–284. doi: 10.1128/jb.143.1.274-284.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. K., Bloom R. W., Epstein W. Catabolite and transient repression in Escherichia coli do not require enzyme I of the phosphotransferase system. J Bacteriol. 1979 Apr;138(1):275–279. doi: 10.1128/jb.138.1.275-279.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]




