Abstract
The synaptic membrane proteins synaptobrevin, syntaxin, and SNAP-25 form a ternary complex that can be disassembled by the ATPase N-ethylmaleimide-sensitive factor (NSF) in the presence of soluble cofactors (SNAP proteins). These steps are thought to represent molecular events involved in docking and subsequent exocytosis of synaptic vesicles. Using two independent and complementary approaches, we now report that such ternary complexes form in the membrane of highly purified and monodisperse synaptic vesicles in the absence of the plasma membrane. Furthermore, the complexes are reversibly dissociated by NSF and SNAP proteins. Thus, ternary complexes can be assembled and disassembled while all three proteins are anchored as neighbors in the same membrane, suggesting that NSF is involved in priming synaptic vesicles for exocytosis.
Synaptobrevin (also referred to as VAMP), SNAP-25, and syntaxin are crucial components of the exocytotic apparatus in neurons (1–4). Synaptobrevin is exclusively localized to synaptic vesicles whereas syntaxin and SNAP-25 are mainly localized to the neuronal plasma membrane. Any interference with the function of these proteins, e.g., proteolysis by clostridial neurotoxins (5, 6) or genetic deletion (7), inhibits exocytotic neurotransmitter release. Relatives of these proteins participate in many intracellular membrane traffic steps in all eukaryotic cells (3), suggesting that membrane fusion is mediated by a common and conserved mechanism.
Despite compelling evidence linking syntaxin, SNAP-25, and synaptobrevin to exocytosis, it is not understood how these proteins operate in the sequence of events that leads to vesicle docking and membrane fusion. In detergent extracts, the three proteins form a stable complex that binds the soluble proteins α/β/γ-SNAP and N-ethylmaleimide-sensitive factor (NSF), leading to their designation as v-SNAREs (synaptobrevin, vesicular SNAp-REceptors) and t-SNAREs (syntaxin and SNAP-25, target membrane SNAp REceptor) (8, 9). ATP-hydrolysis by NSF causes disassembly of the complex. A complex with very similar properties also can be formed from recombinant proteins lacking their transmembrane domains (10–12). Based on these, observations it has been suggested that v-SNARE–t-SNARE interactions are responsible for docking of vesicles at their target membrane and that the conformational change caused by NSF contributes to membrane fusion (1, 9). The specificity of v-SNARE–t-SNARE pairing would ensure that trafficking vesicles only dock at an appropriate target membrane. These proposals are referred to as the SNARE hypothesis (1) and have gained wide acceptance in the field (1–3).
Recently, we have observed that, unlike other residents of the presynaptic plasma membrane, significant amounts of syntaxin and SNAP-25 are also localized to synaptic vesicles (13). Using two independent and complementary approaches, we now report that a ternary complex containing syntaxin, synaptobrevin, and SNAP-25 can form in the membrane of synaptic vesicles in the absence of plasma membranes and that this complex is disassembled by NSF and SNAP proteins in an ATP-dependent manner.
MATERIALS AND METHODS
Materials.
cDNAs for α-SNAP, γ-SNAP, and NSF were kindly provided by S. Whiteheart and J. E. Rothman (New York). The proteins were expressed in Escherichia coli as His6-tagged fusion proteins and were purified on Ni2+–NTA–agarose columns as described earlier (12). Recombinant L chains of tetanus toxin (TeNT), botulinum neurotoxin (BoNT/A), and BoNT/C1 were generous gifts of H. Niemann (Tübingen, Germany).
Disassembly of the Ternary Complex.
Synaptic vesicles were prepared from synaptosomes using chromatography on controlled pore glass beads as the last purification step (14). For a typical disassembly reaction, synaptic vesicle protein (5–25 μg unless indicated otherwise) was incubated in a 50-μl volume containing Hepes–NaOH (pH 7.8; 20 mM), ouabain (1 μM), KCl (100 mM), glycerol (1% vol/vol), DTT (1 mM), phenylmethylsulfonyl fluoride (1 μM), α-SNAP (8 μM), γ-SNAP (1 μM), and NSF (0.6 μM) and an ATP regenerating system consisting of ATP (2.5 mM), MgCl2 (2 mM), creatine phosphate (20 mM), and creatine kinase (0.1 mg/ml). In control and reassembly reactions, 10 mM EDTA was added to chelate the Mg2+ and thus prevent ATP hydrolysis by NSF. Unless indicated otherwise, reactions were carried out for 30 min at 30°C and terminated by addition of SDS sample buffer containing 62.5 mM Tris⋅HCl, pH 6.8, 4% (wt/vol) SDS, 10% (wt/vol) sucrose, 5% (vol/vol) β-mercaptoethanol, and 0.01% (wt/vol) bromphenol blue. The samples were then incubated for an additional 30 min at 30°C before separation by SDS/PAGE and immunoblotting.
Electrophoretic Procedures.
SDS/PAGE and immunoblotting were carried out using standard protocols (15, 16). For reelectrophoresis in a second dimension, synaptic vesicle proteins were prepared for SDS/PAGE as described above. After completion of the first dimension, the lane containing the separated proteins was excised, soaked for 20 min in 10% (vol/vol) acetic acid and 25% (vol/vol) isopropanol, briefly washed with H2O, and incubated for 20 min in SDS sample buffer. After heating for 2 min to 100°C in a microwave oven, the strip was mounted on top of a 12.5% gel, reelectrophoresed, and analyzed by immunoblotting.
Proteolysis by Light Chains of Clostridial Neurotoxins.
Proteolysis of syntaxin, SNAP-25, or synaptobrevin was initiated by adding 2 μM of the appropriate toxin light chain to each disassembly reaction immediately after adding α/γ-SNAP and NSF and before starting the reaction with Mg-ATP. The samples then were treated as described for the disassembly reaction. Unless otherwise stated, samples were heated for 3 min to 100°C before SDS/PAGE and immunoblotting.
RESULTS
SDS-Resistant Ternary Complexes Are Present in Synaptic Vesicles and Are Disassembled by α-SNAP and NSF.
To study membrane protein complexes, it is customary to solubilize membranes in nondenaturing detergents that allow for biochemical analysis of protein complexes. However, in preliminary experiments, we found that stable ternary complexes of synaptobrevin, syntaxin, and SNAP-25 assemble after solubilization of brain membranes in nonionic detergent and thus do not report the status of the proteins before solubilization (unpublished observations). To avoid assembly after solubilization, we took advantage of the recent observation made by Niemann and coworkers that ternary SNARE complexes partially resist treatment with SDS (10). Large forms of these complexes appear as heat-sensitive, distinct bands of high molecular mass when the samples are separated by SDS/PAGE (10). These SDS-resistant forms cannot assemble after addition of SDS (see below) and thus represent ternary complex preexisting in the membrane before solubilization.
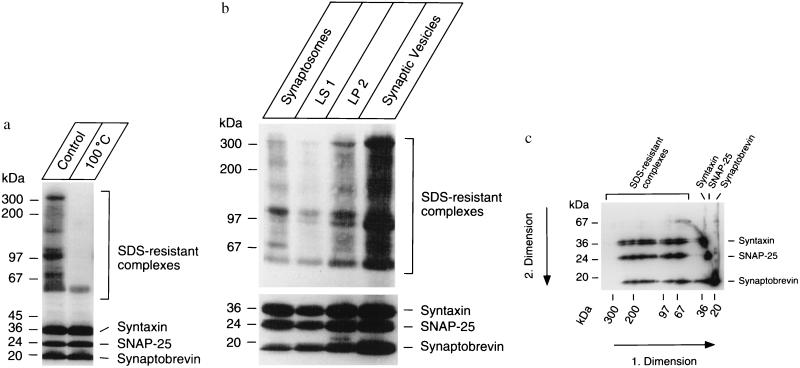
Synaptic vesicles were purified according to established procedures using chromatography on controlled pore glass beads as the last purification step. Vesicles purified by this procedure have been extensively characterized and are low in contamination by other membranes, including plasma membranes (13). Electrophoretic separation of vesicle proteins after solubilization in SDS resulted in the appearance of several distinct bands, with Mrs ranging from 60 to ≈300 that reacted with a mix of antibodies directed against synaptobrevin, syntaxin, and SNAP-25 (Fig. 1a). When the samples were incubated at 100°C before electrophoresis, these bands largely disappeared, and only the monomeric proteins were detected (Fig. 1a). To ensure that these complexes are associated with synaptic vesicles, we monitored their distribution in intermediate fractions obtained during vesicle purification. Fig. 1b shows that the complexes coenriched with synaptic vesicles. To examine whether each of the high Mr bands contained all three proteins, a gel lane was excised after electrophoresis, heated to 100°C to disrupt the complexes, and electrophoresed under identical conditions in a second dimension. Fig. 1c shows that all high Mr complexes contained comparable amounts of syntaxin, synaptobrevin, and SNAP-25, confirming observations by us and others that only ternary complexes containing all three proteins are resistant to SDS treatment. To ensure that the high Mr complexes did not form during or after SDS treatment, radioactively labeled syntaxin, generated by in vitro translation, was added together with SDS to the sample before electrophoresis. The radioactively labeled syntaxin was not incorporated into the high Mr complexes (not shown).
Figure 1.
Synaptic vesicles contain ternary complexes of synaptobrevin, SNAP-25, and syntaxin that are resistant to mild treatment by SDS. All complexes were visualized by SDS/PAGE and immunoblotting using a mixture of mAb specific for syntaxin (25), synaptobrevin (18), and SNAP-25 (13) and enhanced chemoluminescence for detection. To visualize monomers and high Mr complexes on the same gel, the separation gel was discontinuous, with the upper two-thirds and the lower one-third being adjusted to 7% and 12.5% acrylamide, respectively. (a) SDS-resistant complexes disappear when the sample is heated to 100°C before electrophoresis. (b) SDS-resistant complexes copurify with synaptic vesicles upon subfractionation of synaptosomes. Synaptosomes were lysed by hypotonic shock and heavy membranes were removed from the lysate. The resulting fraction (LS1) was used as a source for a light membrane fraction (LP2) from which synaptic vesicles were further purified by sucrose density gradient centrifugation and size-exclusion chromatography (14). (c) All SDS-resistant complexes contain comparable amounts of syntaxin, SNAP-25, and synaptobrevin. After completion of the first dimension, a lane was excised, heated to 100°C, and reelectrophoresed in the second dimension on a 12.5% gel.
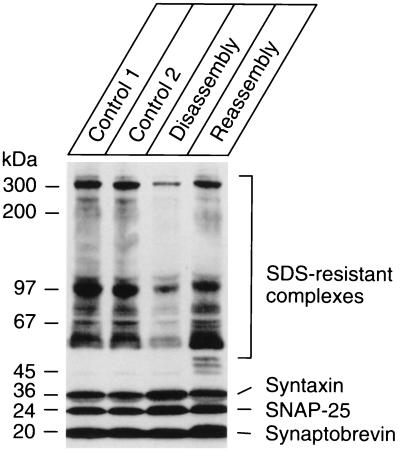
To examine whether NSF can disassemble the vesicular ternary complex, synaptic vesicles were incubated with recombinant NSF, α-SNAP, γ-SNAP, and ATP in the absence or presence of EDTA, which complexes Mg2+ and thus prevents ATP cleavage. As shown in Fig. 2 (control 2), NSF and α/γ-SNAP had no influence on the SDS-resistant complexes when ATP hydrolysis was prevented. In the presence of Mg2+, however, the amount of these complexes was markedly reduced (Fig. 2, disassembly). Subsequent addition of either EDTA (Fig. 2, reassembly) or of an ATP-depleting system (not shown) allowed reformation of the SDS-resistant complexes. NSF-driven disassembly was thus reversible, suggesting that a dynamic equilibrium exists between assembly and disassembly when NSF and Mg-ATP are present.
Figure 2.
NSF causes ATP-dependent disassembly of SDS-resistant complexes in the membrane of isolated synaptic vesicles. In control 1, NSF, α-, and γ-SNAP were added at the end of the incubation immediately before adding SDS sample buffer. In control 2, ATP hydrolysis was prevented by addition of 10 mM EDTA to complex Mg2+ ions. For reassembly, 10 mM EDTA was added at the end of the disassembly reaction, and the sample was incubated for an additional 60 min. See Fig. 1 for antibodies and detection procedures.
Several control experiments were performed to ensure that the vesicular complexes are formed in single vesicles and do not represent bridging complexes between vesicles in vesicle clusters. First, vesicles were filtered through Whatman Anotop filters with 100-nm pore size to remove any vesicle clusters and were immediately analyzed. There was no difference in the band pattern of SDS-resistant complexes seen or in the ability of NSF to disassemble these complexes. Electron microscopy (negative staining) of these fractions revealed that there were no clusters present (not shown). Second, highly dilute vesicles (≈2 μg protein/ml), obtained directly from the glass bead column, were passed through 100-nm filters and analyzed, again resulting in SDS-resistant, NSF-sensitive, high Mr complexes. Dynamic light scattering revealed that synaptic vesicles are monodisperse under these conditions (not shown; K. Stenius and R.J., manuscript in preparation). Third, vesicles were aggregated intentionally by adding 10 mM MgCl2. Despite the presence of large vesicle clusters, no change was observed in the composition or abundance of the SDS-resistant complexes or in the ability of NSF to disassemble these complexes.
The Constituents of Vesicular Ternary Complexes Become Susceptible to Cleavage by BoNT and TeNT After NSF-Driven Disassembly.
The results shown above clearly demonstrate that isolated synaptic vesicles contain complexes of synaptobrevin, syntaxin, and SNAP-25 that resemble those found in Triton X-100 extracts and that can be reversibly disassembled by NSF and α-SNAP in an ATP-dependent manner. However, the extent to which these complexes are present is difficult to estimate because resistance to SDS may not be complete, and thus the amount of detectable SDS-resistant complexes could be an underestimation of the complexes present in the intact membrane. To overcome this problem, we took advantage of the observation that ternary complexes, but none of the binary complexes, formed from recombinant synaptobrevin, SNAP-25, and syntaxin are resistant to proteolytic cleavage by clostridial neurotoxin light chains (ref. 17 and unpublished observations). These toxins can thus be used to probe for the presence of the ternary complex in an intact membrane.
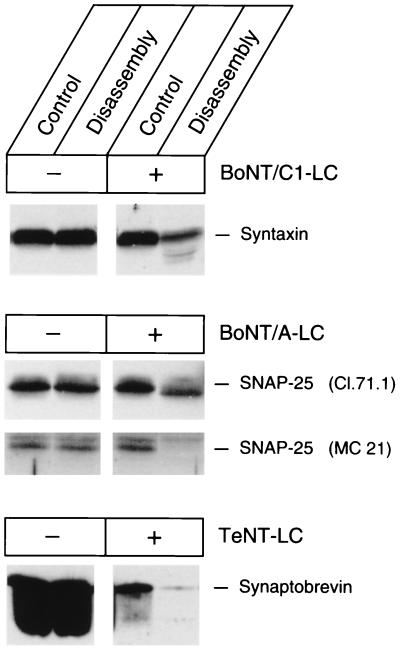
Synaptic vesicles were incubated separately with light chains of TeNT (specific for synaptobrevin), BoNT/A (specific for SNAP-25), or BoNT/C1 (largely specific for syntaxin) (5). In addition, recombinant α/γ-SNAP and NSF were present. As shown in Fig. 4, cleavage of vesicular SNAP-25 and syntaxin by BoNT/A-LC and BoNT/C-LC, respectively, is barely detectable under control conditions in which no ATP was added, and thus no disassembly occurs (compare control lanes in the absence and presence of toxin). Thus, most of the vesicular SNAP-25 and syntaxin are inaccessible to the toxins, suggesting that they are part of a ternary complex. In contrast, a large proportion of synaptobrevin is broken down by TeNT-LC, reflecting the presence of excess synaptobrevin that is not bound to syntaxin and SNAP-25 (18). When Mg-ATP was added to disassemble the ternary complex in the presence of toxin light chains, massive proteolytic cleavage of the respective substrate protein was observed in each case (Fig. 3, right lanes). These results confirm that NSF disassembles ternary complexes present in intact vesicle membranes, rendering the proteins susceptible to toxin cleavage. Furthermore, they show that the majority of vesicular syntaxin and SNAP-25 are assembled in a complex with synaptobrevin whereas large pools of synaptobrevin do not participate, in accordance with the approximately 6:1 molar ratio of synaptobrevin to syntaxin and SNAP-25, respectively, on purified synaptic vesicles (13).
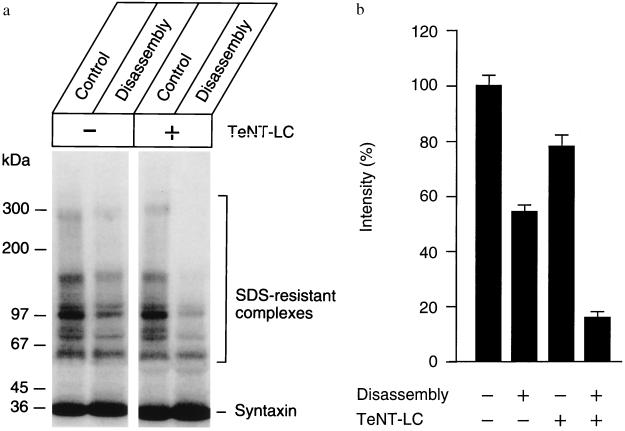
Figure 4.
TeNT enhances NSF-driven disassemby by preventing reassembly during the disassembly reaction. Experimental conditions are identical to those described in Fig. 2 except that only an antibody specific for syntaxin was used and that immunoreactive bands were detected by a 125I-Protein A procedure for quantitation (26). In b, each band was quantitated by PhosphorImaging (Molecular Dynamics). Shown is the average relative intensity for each of the experimental conditions. Toxin and control incubations were carried out as described in Fig. 3.
Figure 3.
NSF-driven disassembly renders syntaxin, SNAP-25, and synaptobrevin susceptible to cleavage by the light chains of BoNT/C1, BoNT/A, and TeNT, respectively. Disassembly was carried out in parallel in separate incubation that included, in addition to the toxin-free controls, BoNT/C1 L chain, BoNT/A L chain, and TeNT L chain, respectively, each at 2 μM. All samples were heated to 100°C before electrophoresis. Control incubations contained 10 mM EDTA. See Fig. 1 for antibodies and detection procedures.
Finally, we examined how the SDS-resistant complexes are affected when disassembly is carried out in the presence of toxin. Fig. 4 shows that, in the presence of TeNT-LC, NSF-treatment reduced SDS-resistant complexes by more than 80% whereas only ≈50% disassembly was observed in the absence of toxin. We conclude that NSF can dissociate the vesicular SNARE complex almost completely when reassembly is impaired by degradation of one of the components.
DISCUSSION
In the present study, we have provided direct evidence that a ternary complex containing syntaxin, SNAP-25, and synaptobrevin assembles in a single membrane and that such a complex can be disassembled by NSF and SNAP proteins. Both complex formation and disassembly occur while all components are anchored to the same membrane, demonstrating that docking of a vesicle at its target membrane is not required for these reactions.
No straightforward methods are available to monitor the assembly of membrane protein complexes in an intact membrane, particularly when, as in this case, nondenaturing detergents are unable to prevent association after membrane solubilization. Here we have exploited two unique biochemical properties of the ternary SNARE complex, resistance to SDS and resistance to cleavage by clostridial neurotoxins, to analyze the status of the SNAREs in synaptic vesicles. SDS treatment of synaptic vesicles yields several high Mr aggregates that contain all three components. This differs from the situation of recombinant proteins, with which only a single major complex of Mr 67 is obtained (ref. 10 and unpublished observations). The reason for this discrepancy is not clear. Apparently, the native proteins tend to form higher order aggregates because so far we have failed to detect any other proteins in these larger complexes (M. Margittai and R.J., unpublished observations). In any case, all of these complexes are reversibly disassembled by treating synaptic vesicles with ATP–NSF and SNAPs before detergent extraction, proving that NSF operates on protein complexes in an intact membrane.
It remains to be established to what extent the vesicular pool of these proteins is complexed in intact nerve endings. In our highly purified vesicle fraction, virtually all syntaxin and SNAP-25 was resistant to toxin, documenting that the majority is bound in ternary complexes. Similarly, SDS-resistant complexes recently have been observed in purified fractions of chromaffin granules (19). The proteins readily associate with each other as soon as NSF is removed (Fig. 2), so it is possible that, in a living neuron, most of the complexes are disassembled because of the continuous activity of NSF and that assembly occurs during purification. This could explain our previous observation that vesicular syntaxin is partially susceptible to BoNT/C1 cleavage when the vesicles are rapidly obtained after lysis of synaptosomes and immediately incubated in toxins (13).
In summary, our data suggest that NSF-driven disassembly of the complex reflects an early event in exocytosis that can precede docking. This view agrees well with recent experiments addressing the fusion mechanism of yeast vacuole precursor vesicles (20) and of mammalian endosomes in vitro (21). A “priming” role for NSF would be consistent with experiments studying exocytosis in permeabilized neuroendocrine cells that show that the final steps of exocytosis are independent of ATP (22–24). The physiological role of the vesicular complex, however, remains to be established. Our data do not exclude that synaptobrevin, syntaxin, and SNAP-25 also associate with each other when they reside in different membranes. In fact, the role of NSF may be to activate the proteins in one membrane for a subsequent intermembrane interaction.
Acknowledgments
We thank Drs. S. W. Whiteheart, J. E. Rothman, and H. Niemann for the supply of cDNAs and Drs. D. Bruns and D. Fasshauer for critical reading of the manuscript and many helpful discussions. P.I.H. was supported by a postdoctoral fellowship from the Helen Hay Whitney Foundation. This work was supported by a grant from the National Institute of Health to R.J.
ABBREVIATIONS
- BoNT
botulinum neurotoxin
- NSF
N-ethylmaleimide-sensitive factor
- SNAP
soluble NSF attachment protein
- SNAP-25
synaptosomal-associated protein of 25 kDa
- SNARE
SNAP receptor
- TeNT
tetanus toxin
- LC
light chain
References
- 1.Rothman J E. Nature (London) 1995;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 2.Scheller R H. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 3.Ferro-Novick S, Jahn R. Nature (London) 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- 4.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 5.Niemann H, Blasi J, Jahn R. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney S T, Broadie K, Keane J, Niemann H, O’Kane C J. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 7.Broadie K, Prokop A, Bellen H J, O’Kane C J, Schulze K L, Sweeney S T. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 8.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 9.Söllner T, Bennet M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof T C, Niemann H. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calakos N, Bennett M K, Peterson K E, Scheller R H. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- 12.Hanson P I, Otto H, Barton N, Jahn R. J Biol Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- 13.Walch-Solimena C, Blasi J, Edelmann L, Chapman E R, Fischer von Mollard G, Jahn R. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huttner W B, Schiebler W, Greengard P, De Camilli P. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Towbin H, Staehelin H, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H. EMBO J. 1995;14:2317–2325. doi: 10.1002/j.1460-2075.1995.tb07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelmann L, Hanson P I, Chapman E R, Jahn R. EMBO J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höhne-Zell B, Gratzl M. FEBS Lett. 1996;394:109–116. doi: 10.1016/0014-5793(96)00931-3. [DOI] [PubMed] [Google Scholar]
- 20.Mayer A, Wickner W, Haas A. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 21.Colombo M I, Taddese M, Whiteheart S W, Stahl P D. J Biol Chem. 1996;271:18810–18816. doi: 10.1074/jbc.271.31.18810. [DOI] [PubMed] [Google Scholar]
- 22.Bittner M A, Holz R W. J Biol Chem. 1992;267:16219–16225. [PubMed] [Google Scholar]
- 23.Parsons T D, Coorssen J R, Horstmann H, Almers W. Neuron. 1995;15:1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee A, Barry V A, DasGupta B R, Martin T F J. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- 25.Barnstable C J, Hofstein R, Akagawa K. Dev Brain Res. 1985;20:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- 26.Jahn R, Schiebler W, Ouimet C, Greengard P. Proc Natl Acad Sci USA. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]