Abstract
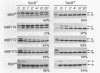
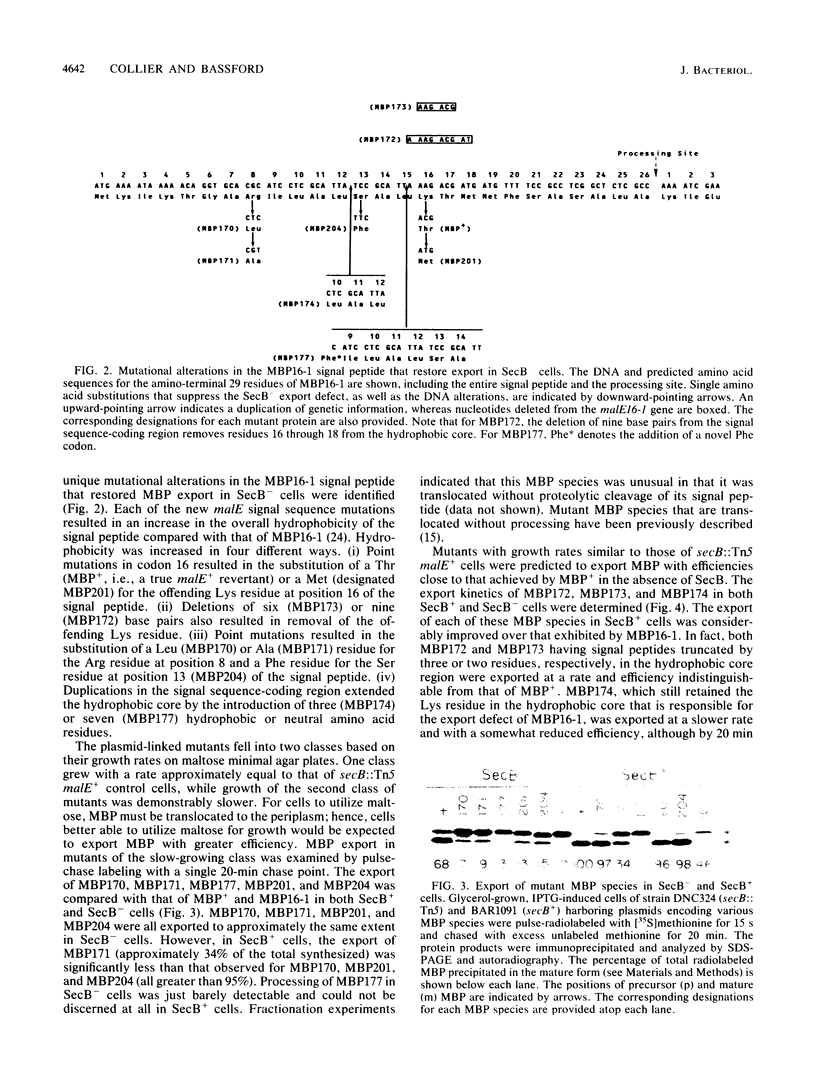
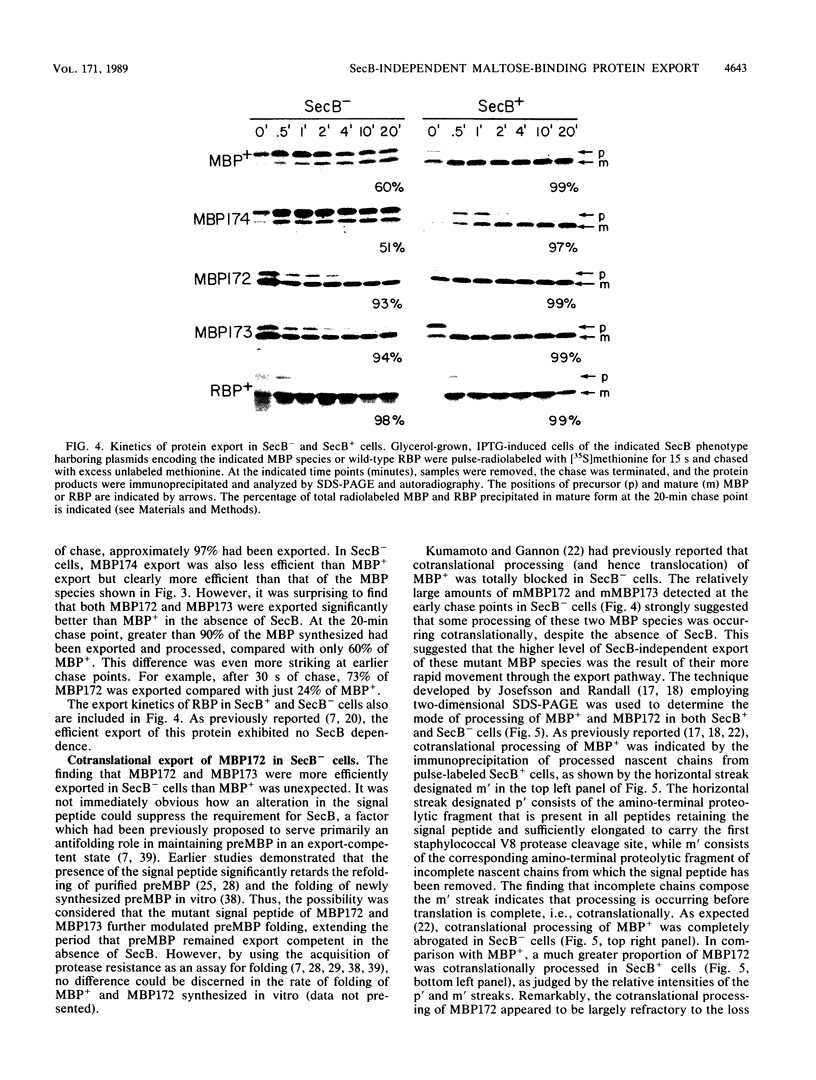
It previously has been proposed that the Escherichia coli SecB protein promotes the export of the maltose-binding protein (MBP) from the cytoplasm by preventing the folding of the precursor MBP (preMBP) into a translocation-incompetent conformation. The export of wild-type MBP is only partially blocked in SecB- cells. In contrast, the export of MBP16-1, an MBP species with a defective signal peptide, is totally dependent on SecB; hence, SecB- cells that synthesize MBP16-1 are unable to utilize maltose as a sole carbon source. The selection of Mal+ revertants primarily yielded mutants with alterations in the MBP16-1 signal peptide that permitted SecB-independent MBP export to the periplasm to various extents. Although each of these alterations increased the overall hydrophobicity of the signal peptide, it was not possible to strictly equate changes in hydrophobicity with the degree of SecB-independent export. Somewhat unexpectedly, two mutants were obtained in which MBP export in SecB- cells was markedly superior to that of the wild-type MBP. Although wild-type MBP is not cotranslationally translocated in SecB- cells, the two mutant proteins designated MBP172 and MBP173 exhibited significant cotranslational export in the absence of SecB. Thus, the role of SecB was partially supplanted by a signal peptide that promoted more rapid movement of MBP through the export pathway. When preMBP included the MBP172 signal peptide as well as an alteration in the mature moiety that slows folding, the SecB requirement for maximal MBP export efficiency was almost totally eliminated. These results provide additional strong support for the proposed antifolding role of SecB in MBP export.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankaitis V. A., Bassford P. J., Jr The synthesis of export-defective proteins can interfere with normal protein export in Escherichia coli. J Biol Chem. 1984 Oct 10;259(19):12193–12200. [PubMed] [Google Scholar]
- Bankaitis V. A., Rasmussen B. A., Bassford P. J., Jr Intragenic suppressor mutations that restore export of maltose binding protein with a truncated signal peptide. Cell. 1984 May;37(1):243–252. doi: 10.1016/0092-8674(84)90320-9. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Cover W. H., Ryan J. P., Bassford P. J., Jr, Walsh K. A., Bollinger J., Randall L. L. Suppression of a signal sequence mutation by an amino acid substitution in the mature portion of the maltose-binding protein. J Bacteriol. 1987 May;169(5):1794–1800. doi: 10.1128/jb.169.5.1794-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke E., Guthrie B., Lecker S., Lill R., Wickner W. ProOmpA is stabilized for membrane translocation by either purified E. coli trigger factor or canine signal recognition particle. Cell. 1988 Sep 23;54(7):1003–1011. doi: 10.1016/0092-8674(88)90115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke E., Wickner W. Trigger factor: a soluble protein that folds pro-OmpA into a membrane-assembly-competent form. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5216–5220. doi: 10.1073/pnas.84.15.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Bassford P. J., Jr Localization and processing of outer membrane and periplasmic proteins in Escherichia coli strains harboring export-specific suppressor mutations. J Biol Chem. 1982 May 25;257(10):5852–5860. [PubMed] [Google Scholar]
- Ferenci T., Silhavy T. J. Sequence information required for protein translocation from the cytoplasm. J Bacteriol. 1987 Dec;169(12):5339–5342. doi: 10.1128/jb.169.12.5339-5342.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes J. D., Bankaitis V. A., Ryan J. P., Bassford P. J., Jr Mutational alterations affecting the export competence of a truncated but fully functional maltose-binding protein signal peptide. J Bacteriol. 1987 Jun;169(6):2345–2351. doi: 10.1128/jb.169.6.2345-2351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes J. D., Bassford P. J., Jr Export of unprocessed precursor maltose-binding protein to the periplasm of Escherichia coli cells. J Bacteriol. 1987 Jun;169(6):2352–2359. doi: 10.1128/jb.169.6.2352-2359.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P. M., Li P., Kumamoto C. A. The mature portion of Escherichia coli maltose-binding protein (MBP) determines the dependence of MBP on SecB for export. J Bacteriol. 1989 Feb;171(2):813–818. doi: 10.1128/jb.171.2.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Analysis of cotranslational proteolytic processing of nascent chains using two-dimensional gel electrophoresis. Methods Enzymol. 1983;97:77–85. doi: 10.1016/0076-6879(83)97121-5. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Processing in vivo of precursor maltose-binding protein in Escherichia coli occurs post-translationally as well as co-translationally. J Biol Chem. 1981 Mar 10;256(5):2504–2507. [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985 Jul;163(1):267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Chen L., Fandl J., Tai P. C. Purification of the Escherichia coli secB gene product and demonstration of its activity in an in vitro protein translocation system. J Biol Chem. 1989 Feb 5;264(4):2242–2249. [PubMed] [Google Scholar]
- Kumamoto C. A., Gannon P. M. Effects of Escherichia coli secB mutations on pre-maltose binding protein conformation and export kinetics. J Biol Chem. 1988 Aug 15;263(23):11554–11558. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Liu G. P., Topping T. B., Cover W. H., Randall L. L. Retardation of folding as a possible means of suppression of a mutation in the leader sequence of an exported protein. J Biol Chem. 1988 Oct 15;263(29):14790–14793. [PubMed] [Google Scholar]
- Meyer D. I. Preprotein conformation: the year's major theme in translocation studies. Trends Biochem Sci. 1988 Dec;13(12):471–474. doi: 10.1016/0968-0004(88)90233-2. [DOI] [PubMed] [Google Scholar]
- Park S., Liu G., Topping T. B., Cover W. H., Randall L. L. Modulation of folding pathways of exported proteins by the leader sequence. Science. 1988 Feb 26;239(4843):1033–1035. doi: 10.1126/science.3278378. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986 Sep 12;46(6):921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen B. A., MacGregor C. H., Ray P. H., Bassford P. J., Jr In vivo and in vitro synthesis of Escherichia coli maltose-binding protein under regulatory control of the lacUV5 promoter-operator. J Bacteriol. 1985 Nov;164(2):665–673. doi: 10.1128/jb.164.2.665-673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. P., Bassford P. J., Jr Post-translational export of maltose-binding protein in Escherichia coli strains harboring malE signal sequence mutations and either prl+ or prl suppressor alleles. J Biol Chem. 1985 Nov 25;260(27):14832–14837. [PubMed] [Google Scholar]
- Ryan J. P., Duncan M. C., Bankaitis V. A., Bassford P. J., Jr Intragenic reversion mutations that improve export of maltose-binding protein in Escherichia coli malE signal sequence mutants. J Biol Chem. 1986 Mar 5;261(7):3389–3395. [PubMed] [Google Scholar]
- Shuman H. A. Active transport of maltose in Escherichia coli K12. Role of the periplasmic maltose-binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J Biol Chem. 1982 May 25;257(10):5455–5461. [PubMed] [Google Scholar]
- Stader J., Benson S. A., Silhavy T. J. Kinetic analysis of lamB mutants suggests the signal sequence plays multiple roles in protein export. J Biol Chem. 1986 Nov 15;261(32):15075–15080. [PubMed] [Google Scholar]
- Thom J. R., Randall L. L. Role of the leader peptide of maltose-binding protein in two steps of the export process. J Bacteriol. 1988 Dec;170(12):5654–5661. doi: 10.1128/jb.170.12.5654-5661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Weiss J. B., MacGregor C. H., Collier D. N., Fikes J. D., Ray P. H., Bassford P. J., Jr Factors influencing the in vitro translocation of the Escherichia coli maltose-binding protein. J Biol Chem. 1989 Feb 15;264(5):3021–3027. [PubMed] [Google Scholar]
- Weiss J. B., Ray P. H., Bassford P. J., Jr Purified secB protein of Escherichia coli retards folding and promotes membrane translocation of the maltose-binding protein in vitro. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8978–8982. doi: 10.1073/pnas.85.23.8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]
- Zagursky R. J., Berman M. L. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. doi: 10.1016/0378-1119(84)90139-2. [DOI] [PubMed] [Google Scholar]