Abstract
The rd28 gene of Arabidopsis thaliana encodes a water channel protein, or aquaporin, of the plasma membrane. A construct in which transcription of the rd28 cDNA is controlled by the Dictyostelium actin15 promoter was transformed into Dictyostelium discoideum cells. Transformants contained RD28 protein in their plasma membranes. When shifted to a low-osmotic-strength buffer, cells expressing rd28 swelled rapidly and burst, indicating that the plant aquaporin allowed rapid water entry in the amoebae. The rate of osmotic lysis was a function of the osmotic pressure of the buffer. We also selected transformants in which the expression of the rd28 cDNA is driven by the promoter of the prespore cotB gene. These transformants accumulated rd28 mRNA uniquely in prespore cells. In low-osmotic-strength buffer, the cotB::rd28 cells aggregated and formed normally proportioned slugs but failed to form normal fruiting bodies. The number of spores was reduced 20-fold, and the stalks of the fruiting bodies were abnormally short. The consequences of expressing RD28 in prespore cells could be partially overcome by increasing the osmolarity of the medium. Under these conditions, the cotB::rd28 cells formed fruiting bodies of more normal appearance, and the number of viable spores increased slightly. Because prespore cells have to shrink and dehydrate to form spores, it was not unexpected that expression of an aquaporin would disrupt this process, but it was surprising to find that stalk differentiation was also affected by expression of rd28 in prespore cells. It appears that osmotic stress on prespore cells alters their ability to signal terminal differentiation in prestalk cells. The results provide independent confirmation that plant aquaporins can function in the cells of other organisms, and that D. discoideum can be used to study the properties of these water channels.
Keywords: aquaporins, osmotic sensitivity
Morphogenesis in multicellular organisms requires that cells communicate with each other through diffusible substances or through interactions between the surfaces of adjacent cells. Disruption of intercellular communication by chemical, physical, or genetic means can provide valuable insights into morphogenetic events. The dependence of such signaling events on the maintenance of cellular volume or water balance remains largely unexplored. Cells can adjust their volume when changes in the internal or external solute concentration cause transmembrane water flows that result in swelling or shrinking. The existence of mechanosensitive channels indicates that cells perceive and respond actively to changes in turgor pressure. Normal responses and signaling pathways may be upset if the ability of cells to control water flux across the cell membrane is impaired. Although water can move through the lipid bilayer, water channel proteins, or aquaporins, play an important role in the regulation of water transport across cellular membranes (1, 2). Aquaporins are 25- to 30-kDa membrane proteins with similar primary sequences belonging to the major intrinsic protein (MIP) family of transmembrane proteins that form water channels (3). These proteins have six putative transmembrane domains with cytosolic amino and carboxyl termini; they have short, perfectly conserved amino acid motifs, including the signature sequence SGXHXNPA and the sequence NPA repeated in the second half of the protein. In mammals, aquaporins are most abundant in tissues or cell types with a high water permeability (2). In the mustard plant Arabidopsis thaliana, 10 cDNAs that encode aquaporins and 13 others that encode putative aquaporins (MIPs) have been identified (A. Weig, C. Deswarte, and M.J.C., unpublished work). Some aquaporins, called PIPs (plasma membrane integral proteins), are located in the plasma membrane, whereas others, called TIPs (tonoplast integral proteins), are found in the vacuolar membrane (4–7). The expression patterns of specific aquaporins are tissue- and cell-type-specific. The plasma membrane aquaporin RD28 is found in all plant organs but is absent from seeds (7). The tonoplast aquaporins γ-TIP and δ-TIP are preferentially expressed in elongating root cells and in the parenchymal cells of vascular tissues, respectively (8, 9).
The transport function of these membrane channels is usually assayed by injection of the pertinent cRNAs in Xenopus oocytes (10, 11). When shifted to a lower-osmotic-strength buffer several days later, the oocytes that have accumulated aquaporins in their plasma membranes swell and burst much faster than do control oocytes. With use of this assay, the dependence of water transport efficiency and selectivity on protein topology and posttranslational modification of aquaporins has been determined (10, 12–14). Recently, the activity of a human aquaporin (AQP1) was characterized after expression in the yeast Saccharomyces cerevisiae, by characterizing the osmotic properties of purified membrane vesicles (15). It appears, therefore, that aquaporins function quite well in heterologous systems.
In Dictyostelium discoideum, cellular interactions during development regulate the differentiation of various cell types. Interestingly, the cells lack a cell wall and yet can adapt to varying osmotic conditions in both the soil and the laboratory (16, 17). Constant water influx through the plasma membrane is counterbalanced by water efflux via a contractile vacuole. Because Dictyostelium can be efficiently transformed (18–20), we reasoned that it might serve as a suitable heterologous system to test the activity of aquaporins and allow us to determine the effects that aquaporins have on osmotic adaptation and signaling between cells. When the amoebae reach high cell densities and deplete the available nutrients, they aggregate and undergo multicellular development to produce fruiting bodies in which the majority of the cells form spores that are held up on a cellular stalk. Recent analyses of wild-type and mutant strains of D. discoideum have uncovered a signal transduction pathway by which prestalk cells communicate with prespore cells (21–24). During terminal differentiation, stalk cells expand in volume 3- to 4-fold by forming a large central vacuole, whereas prespore cells expel water and shrink before encapsulating. When spores germinate, the cells rapidly take up water and escape the spore coat (25). Thus, Dictyostelium cells carefully regulate water influx and efflux at different stages of their life cycle. The objective of our study was to determine if a plant plasma membrane aquaporin would interfere with any of these developmental processes. We generated osmotically sensitive strains by expressing the cDNA for a plant water channel and found that expression of this transgene uniquely in prespore cells results in aberrations not only of prespore cells but also in stalk formation. Moreover, the data show that the function of genes from highly diverse organisms can be accessed in Dictyostelium where cellular processes are amenable to convenient manipulations.
MATERIALS AND METHODS
Cells, Transformation, and Development.
D. discoideum cells of strain AX4 (26) and its ecmA::lacZ derivative TL6 (27, 28) were grown axenically in HL5 medium (29). Transformations were carried out as described (27). G418-resistant clones were selected by progressive addition of 2.5, 5, 10, and 20 μg/ml geneticin (G418). The G418-resistant clones were then plated on SM agar in association with Klebsiella aerogenes. Synchronous development on filters and detergent resistance of spores were carried out as described (27).
Plasmid Constructions.
The cDNA encoding RD28 (30) was amplified using Pfu polymerase (Stratagene) with two oligonucleotides containing HindIII and XhoI restriction sites, respectively. The resulting PCR product was digested with HindIII and XhoI and cloned downstream of the D. discoideum actin15 promoter (20) present in pact15::lacZ plasmid (23) previously digested with HindIII and XhoI to eliminate the lacZ gene (pact15::rd28).
D. discoideum cotB promoter (31) was amplified from the pSP70::ricinA plasmid (27) with use of two oligonucleotides containing XbaI and HindIII restriction sites, respectively. The resulting PCR product was digested with XbaI and HindIII and inserted in front of the rd28 cDNA in the pact15::rd28 plasmid, previously digested with XbaI and HindIII to eliminate the actin15 promoter (pcotB::rd28).
The resulting ORFs contain the first six codons of actin15 or cotB coding regions, six codons from the linker and the entire rd28 coding sequence. Both vectors contain the neomycin phosphotransferase II gene under the control of D. discoideum actin 6 promoter for selection of G418-resistant transformants.
In Situ Hybridization and 5-Bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) Staining.
Structures developed on filters were fixed for hybridization as described (32). Antisense rd28, ecmA, and cotB riboprobes labeled with digoxigenin–dUTP were used for hybridization as described (22). Cells carrying a lacZ reporter construct were detected as described (22).
Osmotic Sensitivity Assays.
Exponentially growing cells at 1–3 × 106 cells per ml were sedimented in Eppendorf tubes at 4,000 rpm for 5 min and resuspended at a cell density of 106 cells per ml in the buffer indicated. Intact cells were counted at different times with a hemocytometer cell. The hyperosmotic shock experiments were carried out as described (17) except that cells were washed and incubated in PDF buffer (20.1 mM KCl/5.3 mM MgCl2/10.5 mM potassium phosphate, pH 6.4) (29) with or without sorbitol.
A. thaliana Microsome Preparation, Dictyostelium Subcellular Fractionation, and Protein Analysis.
A. thaliana microsomes were prepared as described (7). Dictyostelium plasma membrane purification was carried out as described (33). Sedimented cells were resuspended in 0.5 mM MgCl2/0.5 mM CaCl2/5 mM glycine (pH 8.5) at room temperature, and cells were disrupted by passage through 5-μm Nucleopore filters (Nucleopore). A crude membrane pellet was collected by centrifugation at 5,900 × g for 20 min at 2°C and washed twice in cold lysis buffer. The pellet (P1) was layered onto a 10-ml linear sucrose gradient [34–62% (wt/vol) sucrose in 50 mM glycine, pH 8.5], and the gradient was centrifuged for 18 hr at 145,000 × g. The fraction enriched in plasma membranes [42–50% (wt/vol) sucrose] was collected and washed twice in 20 mM sodium phosphate (pH 6.8) at 38,000 × g for 30 min (P2) and once in 10 mM MgCl2/0.1 mM EDTA/10 mM Tris⋅HCl (pH 7.9). The membranes were resuspended to 0.75 ml in 50% (vol/vol) Renografin-76 (Squibb) in 1.2 mM EDTA/5 mM MgCl2/10.9 mM sodium citrate (pH 6.9). The membrane suspension was overlayered with a linear 20–50% (vol/vol) Renografin-76 gradient (9 ml), and the gradient was centrifuged at 145,000 × g for 18 hr. The band at 28–32% Renografin-76 was collected and washed twice at 38,000 × g for 30 min in 20 mM sodium buffer (PM). Protein content was determined using the Bio-Rad DC Protein Assay. RD28 protein antibody and immunodetection were as described (7).
RESULTS
Expression and Localization of RD28.
To express the plant plasma membrane aquaporin RD28 in Dictyostelium, we fused the rd28 cDNA of A. thaliana to the Dictyostelium actin15 regulatory region in the pact15::lacZ expression vector (23). The resulting construct contains the actin15 promoter, six codons from the actin15 ORF, six codons from the linker, and the entire rd28 coding sequence. This act15::rd28 construct was introduced into cells of Dictyostelium strain AX4, and transformants were selected for G418 resistance. The presence of the construct in transformants was confirmed by PCR amplification of the rd28 cDNA (data not shown).
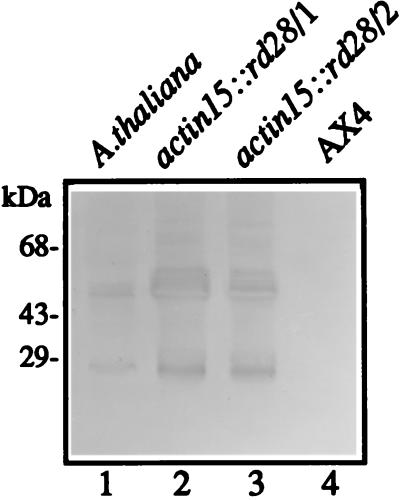
Growing cells of two independent act15::rd28 transformants were shown to have accumulated RD28 by immunoblot analysis with use of an anti-RD28 polyclonal serum raised against a carboxyl-terminal 12-aa peptide (7) (Fig. 1). Polypeptides at approximately 28 kDa and 56 kDa were found in both strains as well as in extracts from A. thaliana. MIP proteins are extremely hydrophobic and readily form aggregates even in the presence of SDS. The smaller band corresponds to the monomer form of RD28, and the larger band corresponds to the dimer aggregate also found in plant microsomes (7). The width of the 56-kDa band suggests that the polypeptide may be posttranslationally modified in Dictyostelium cells.
Figure 1.
Immunoblot of Dictyostelium extracts showing that RD28 is present in Dictyostelium transfected with the pact15::rd28 plasmid. Proteins (50 μg) were fractionated by SDS/PAGE, transferred to nitrocellulose membrane, and incubated with a polyclonal antibody raised against a carboxyl-terminal peptide of RD28. Lanes: 1, A. thaliana microsome fraction (positive control); 2 and 3, crude cellular extract of Dictyostelium transfected with the pact15::rd28 plasmid; 4, crude cellular extract of Dictyostelium parental strain (AX4; negative control). The band at 28 kDa represents monomeric RD28. This hydrophobic protein readily forms dimers under SDS/PAGE conditions (band at 56 kDa).
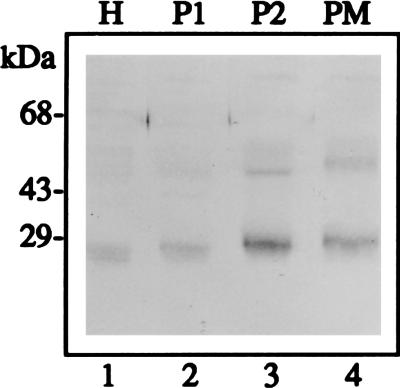
To determine the subcellular localization of RD28 in these transformants, we purified a plasma membrane fraction on a high-pH sucrose gradient followed by a Renografin gradient (33). According to Ingalls et al. (33), this method gives more than 20-fold purification of plasma membranes from D. discoideum with minimal contamination from the other membranes. However, in our study the degree of purification was not verified by the use of marker enzymes. Fig. 2 shows that RD28 is present in the plasma membrane fraction (lane 4). Because the enrichment appears less than expected, we cannot exclude that RD28 might also be associated with other membranes. This would be the case if the targeting of RD28 to the plasma membrane of the amoebae was not as complete as it is in the plant.
Figure 2.
Immunoblot of membrane fractions showing that RD28 is present in a plasma membrane-enriched fraction. Proteins (50 μg) were analyzed as in Fig. 1. Lanes: 1, H = total cellular extract; 2, P1 = crude membrane pellet (20 min at 5,900 × g); 3, P2 = plasma membrane-enriched fraction from a sucrose gradient; 4, PM = plasma membrane-enriched fraction from a Renografin gradient. As in Fig. 1, cross-reacting polypeptides at 28 and 56 kDa are visible.
RD28 Confers Osmotic Sensitivity.
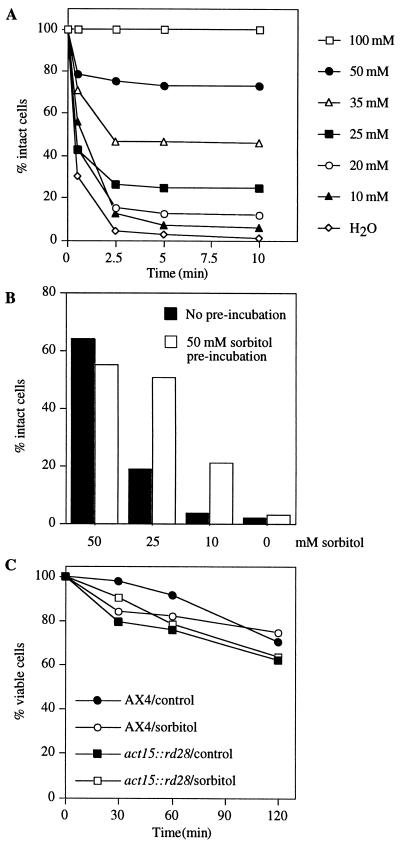
Unlike most other cells, wild-type Dictyostelium amoebae can survive being suspended in distilled water for over an hour by rapidly expelling water through the contraction of water vacuoles (34, 35). To find out if the expression of RD28 in plasma membranes affects this process, we suspended act15::rd28 cells in water and in media of differing sorbitol concentrations. Unlike wild-type cells, which were resistant to incubation in water for 10 min (data not shown), cells that express RD28 swelled and lysed within a few minutes (Fig. 3A). Such cells survived longer when the osmolarity of the medium was increased by adding sorbitol, and they were completely protected by 100 mM sorbitol (Fig. 3A). We interpret these results to show that RD28 functions as an active water channel in the plasma membrane of the Dictyostelium cells and that the influx of water caused by the osmotic imbalance can be prevented by raising the external solute concentration.
Figure 3.
Responses of act15::rd28 cells to different osmotic conditions. (A) Cells were incubated at 1 × 106 cells per ml with the indicated sorbitol concentration, and intact cells were counted after 1, 2.5, 5, and 10 min. Sorbitol is needed to stabilize the cells. (B) Cells were preincubated (open bars) or not (solid bars) with 50 mM sorbitol for 5 min, centrifuged, and exposed to the indicated sorbitol concentration. Intact cells were counted after 10 min. Incubation with sorbitol protects them against subsequent exposure to a low-osmotic-strength medium. (C) Effect of hyperosmotic shock on cell viability; act15::rd28 and AX4 cells were exposed at a concentration of 1 × 106 cells per ml to a medium (PDF) supplemented or not (control) with 400 mM sorbitol (sorbitol). At the times indicated, the cells were removed, and their viability was tested after 2–3 days of growth on agar in association with K. aerogenes.
For each sorbitol concentration, cells that survived the first 5 min did not lyse subsequently in the same medium, suggesting that they had adapted to the lower-osmotic-strength medium. To determine if this might indeed be the case, we preincubated the cells with 50 mM sorbitol for 5 min before challenging them with lower-osmotic-strength conditions. Preincubation with 50 mM sorbitol significantly protected RD28-expressing cells from lysis in 10 mM or 25 mM sorbitol but had no effect in buffer lacking sorbitol (Fig. 3B). Thus, during the 5 min of preincubation, the cells acquired some measure of protection against being cultured in a dilute medium.
When Dictyostelium cells are cultured for many hours under high-osmotic-strength conditions such as 300 mM glucose (36) or 400 mM sorbitol (17), they may gradually lose their viability to a greater or lesser extent. The wild-type cells used here also lost viability when cultured in 400 mM sorbitol, but the act15::rd28 cells did not appear to be any more sensitive to the osmotic strength of high concentrations of sorbitol than wild-type cells are because the viability of the cells declined at the same rate in the presence of 400 mM sorbitol as that in its absence (Fig. 3C).
Effects of RD28 on Development.
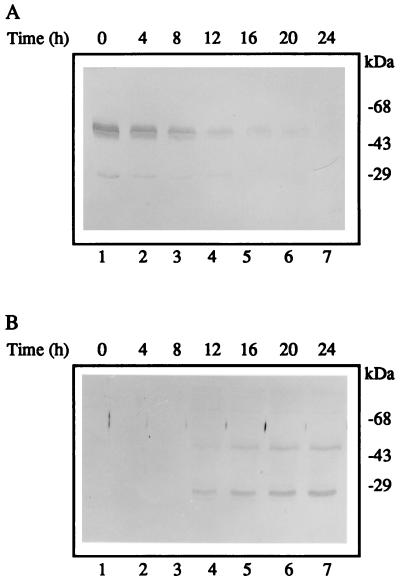
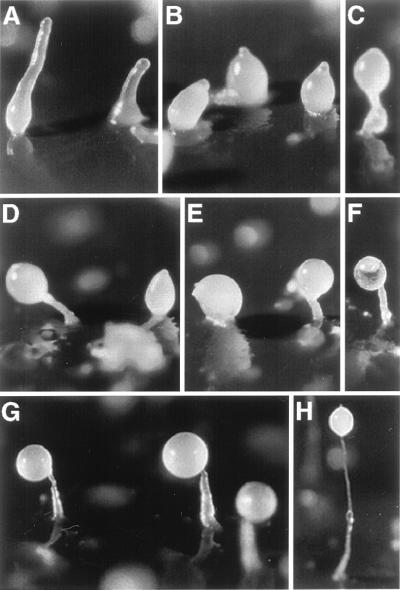
When act15::rd28 cells were induced to develop on filters saturated with buffer, the level of RD28 was initially high and then decreased about 10-fold during the first 12 hr of development (Fig. 4A). These cells aggregated normally and formed well proportioned fruiting bodies. This gradual decline in the level of RD28 may have resulted from a decreased expression of the actin promoter (27) and/or a decrease in the stability of the protein. To test the effect of expressing an aquaporin during the second half of the developmental cycle of D. discoideum, we selected transformants in which the expression of rd28 was driven by the promoter of the prespore gene cotB. These cells had no RD28 for the first 12 hr of development and accumulated it rapidly thereafter (Fig. 4B). Morphogenesis of cotB::rd28 cells became visibly aberrant after 16 hr of development (Fig. 5), whereas wild-type cells started to culminate at 18 hr; the transformed cells were still in the finger form at that time (Fig. 5A). A few hours later (24 hr of development), wild-type cells had formed fruiting bodies (Fig. 5H), but most of the cotB::rd28 aggregates remained in the finger stage of development, although a few culminating structures were observed (Fig. 5 B and C). At 40 hr, some sori were observed elevated on short and thick stalks (Fig. 5 D–F). These results indicate that the expression of rd28 driven by a prespore promoter altered the terminal differentiation of both the spore and the stalk cells. The absence of a normal stalk indicates that the prestalk cells, which do not have RD28, do not receive the correct signal from the prespore cells.
Figure 4.
Immunoblot of cellular extracts showing that the abundance of RD28 is differentially regulated by the two promoters (actin15 and cotB). Proteins (50 μg) were analyzed as in Fig. 1. (A) Crude extract of act15::rd28 cells developed on filter and collected at the times indicated. (B) Crude extracts of cotB::rd28 cells developed on filter and collected at the times indicated. With the actin15 promoter, the abundance of RD28 is greatest at the beginning of the developmental cycle. With the cotB promoter, the picture is reversed.
Figure 5.
Developmental morphology of cotB::rd28 strain and the effect of 100 mM sorbitol on this process. Cells were allowed to aggregate and form fruiting bodies synchronously on filters (PDF medium). (H) A typical fruiting body of the control line (AX4) after 24 hr of development. (A–F) Aggregates and fruiting bodies of the cotB::rd28 cells after 20 hr (A), 24 hr (B and C), and 40 hr (D–F). (G) The results shown are similar to those shown in D–F, but 100 mM sorbitol has been added to the PDF medium. This addition ameliorates the development of fruiting bodies in the RD28-expressing cells.
To find out how the expression of RD28 affected the viability of the cells during development, cells were collected from filters after different times of development of both wild-type and cotB::rd28 cells and were counted, and their viability was tested by plating them on a bacterial lawn (Table 1). The total number of wild-type cells recovered 24 and 40 hr after the initiation of development increased over the input number of cells, presumably because of the division of multinucleated cells. On the other hand, there was no increase in the total cell number in the cotB::rd28 strain. Whereas almost all of the wild-type cells collected at the different times were viable, the viability of the RD28-expressing cells decreased significantly between 24 and 40 hr of development (Table 1). In particular, there was a dramatic decrease (20-fold) in the number of viable spores.
Table 1.
Effect of cotB::rd28 expression during development
| Effects | AX4
|
cotB::rd28
|
||||
|---|---|---|---|---|---|---|
| 16 hr | 24 hr | 40 hr | 16 hr | 24 hr | 40 hr | |
| Total cells | 104 ± 5.5 | 161 ± 2.4 | 159 ± 3.9 | 105 ± 3 | 106 ± 3.3 | 87 ± 19 |
| Viability | 82 ± 18.3 | 124 ± 11.8 | 141 ± 27.4 | 77 ± 25 | 83 ± 15.8 | 39 ± 12.5 |
| Viable spores | ND | ND | 128 ± 11.6 | ND | ND | 6 ± 1.7 |
Cells were developed on filters saturated with 10 mM potassium phosphate buffer containing 20 mM KCl and 5 mM MgCl2. Results are the mean ± SD of three to seven independent experiments and given as percent of the original population (2.5 × 107 cells).
To determine if the osmotic pressure of the medium would modulate cell viability at 40 hr, spore formation, and spore viability, cotB::rd28 cells were induced to develop under both lower- and higher-osmotic-strength conditions (Table 2). A lower osmotic strength was created by allowing the cells to develop on 10 mM potassium phosphate without salts. The inclusion of 100 mM sorbitol was used to create the higher-osmotic-strength medium. When developed on filters saturated with 10 mM buffer without the addition of salts (low-osmotic-strength medium), the recovery of both wild-type and transformed cells was about half that of cells developed in the presence of 20 mM KCl and 5 mM MgCl2 (Table 2). When developed in the presence of 100 mM sorbitol (high-osmotic-strength medium), morphogenesis was improved in the cotB::rd28 cells (Fig. 5G), and cell viability was significantly increased (Table 2). In the same way, the number of viable spores formed by the cot::rd28 cells increased in the higher-osmotic-strength medium (Table 2), although spore formation decreased in the presence of 100 mM sorbitol in the control strain (AX4). These results suggest that the developmental defects observed with cotB::rd28 cells were caused by altered osmotic sensitivity.
Table 2.
Salt and sorbitol effect on cotB::rd28 cells after 40 hr of development
| Effects | AX4
|
cotB::rd28
|
||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| 10 mM | 35 mM | 135 mM | 10 mM | 35 mM | 135 mM | |
| Total Cells | 86 ± 14.3 | 162 ± 1.0 | 126 ± 1.5 | 47 ± 7.8 | 75 ± 9.4 | 88 ± 9.3 |
| Viability | 84 ± 12.5 | 153 ± 7.0 | 122 ± 6.0 | 16 ± 4.0 | 36 ± 0.2 | 62 ± 12.0 |
| Viable spores | 28 ± 11 | 137 ± 13 | 57 ± 1.0 | 0.4 ± 0.6 | 6 ± 2.1 | 11 ± 4.0 |
Results are the mean ± SD of three to four independent experiments and are given as percent of the original population (2.5 × 107 cells). Cells were developed on filters saturated with 10 mM potassium phosphate buffer, pH 6.4 (A); 10 mM potassium phosphate buffer containing 20 mM KCl and 5 mM MgCl2 (B); 10 mM potassium phosphate buffer containing 20 mM KCl, 5 mM MgCl2, and 100 mM sorbitol (C).
Lack of Cell Type Interconversion.
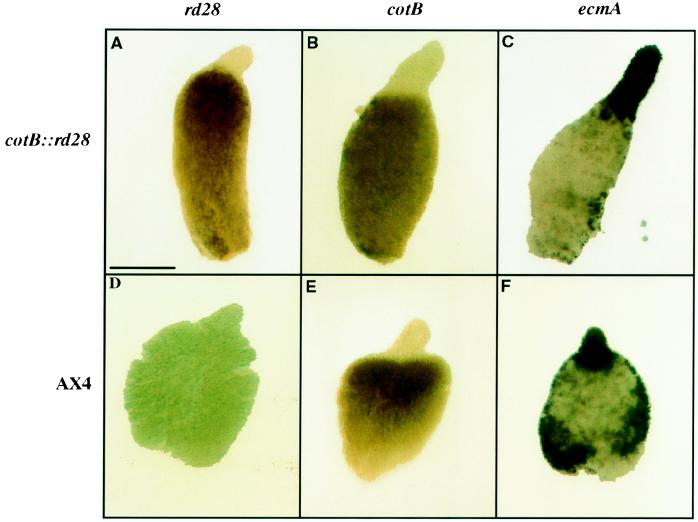
It was somewhat surprising that terminal differentiation of both prespore and prestalk cells was affected in cotB::rd28 strain because the latter should not have been osmotically altered. It is known that prestalk cells will convert to prespore cells if the existing prespore cells are removed or poisoned as a result of expressing the protein synthesis inhibitor ricin A (27, 37). We, therefore, used in situ hybridization with whole mount preparations to confirm that the cotB::rd28 construct was indeed expressed only in prespore cells (Fig. 6A). There was no cross-hybridization with control cells that did not have this construct (Fig. 6D). The cotB gene itself was correctly expressed in the prespore cells in the control (Fig. 6E) and in the cotB::rd28 transformed cells (Fig. 6B). To determine how the expression of a prestalk mRNA might be affected by the presence of RD28, we stained whole mounts for the prestalk specific mRNA encoded by the prestalk ecmA gene in both control and cotB::rd28 cells (Fig. 6 C and F). Prestalk cells at the anterior of slugs expressed this gene at 16 hr (Fig. 6C) and 24 hr (data not shown) of development in the cotB::rd28 transformed strain, and the proportion of prestalk cells was not measurably different than what was seen in wild-type strains (Fig. 6, compare C with F). Likewise, the proportion of cells that stained for the prespore specific mRNA from the cotB gene was identical in the two strains (Fig. 6 B and E). Thus, we see no evidence for cell type interconversion resulting from the accumulation of RD28 in prespore cells.
Figure 6.
Whole mount in situ hybridization of rd28, cotB, and ecmA mRNA in cellular aggregates. AX4 and cotB::rd28 cells were allowed to develop for 16 hr (AX4 and cotB::rd28), fixed, permeabilized, and probed with antisense mRNA for rd28 (A and D), cotB (B and E), or ecmA (C and F). rd28 is not expressed in AX4 cells (D) and is only expressed in prespore cells in the transformed line (A); cotB is only expressed in prespore cells (B and E); and ecmA is only expressed in prestalk cells (C and F).
Stalk Formation in Chimeric Aggregates.
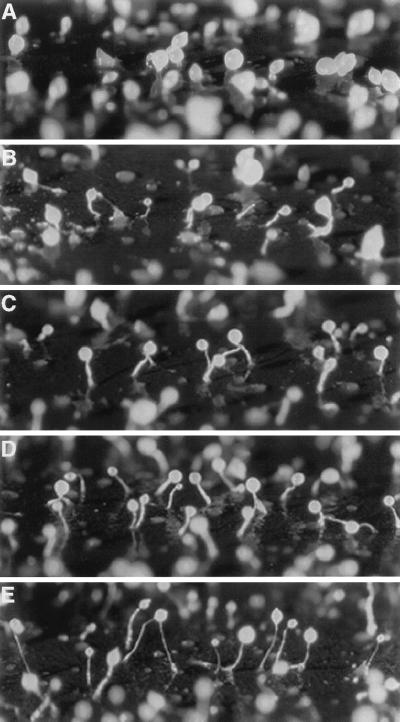
In D. discoideum, fruiting body formation requires an exchange of signals between prestalk and prespore cells. Fruiting bodies can develop from a homogeneous population of amoebae or from mixed populations of cells that differ in their genotype. Incubating two populations of cells together can give insights into the influence that specific gene products exert on these signaling events. By using a cytochemical marker such as β-galactosidase driven by a prespore or a prestalk promoter, it is possible to determine where specific cells are in the aggregate. The differentiation of stalk cells was investigated by following the development of cotB::rd28 and TL6 cells mixed in various ratios. The TL6 strain is derived from the control AX4 strain by transformation with p63NeoGal, resulting in a strain that expresses lacZ under the control of the prestalk specific ecmA promoter (27, 28). Allowing the cotB::rd28 cells to develop in association with TL6 cells substantially improved stalk formation in the developing fruiting bodies. For example, with a mixture of 10% wild-type cells and 90% cotB::rd28 cells, some stalks were made during the first 24 hr of development (Fig. 7B), and more were made during the next day. This improvement increased with the amount of wild-type cells present in the mixed aggregates (Fig. 7 C and D).
Figure 7.
Effect of mixing control (TL6) and cotB::rd28 cells on fruiting body formation. TL6 cells express lacZ gene under the ecmA promoter. Morphology after 24 hr of development of 100% cotB::rd28 cells (A), 90% cotB::rd28 and 10% TL6 cells (B), 80% cotB::rd28 and 20% TL6 cells (C), 50% cotB::rd28 and 50% TL6 cells (D), and 100% TL6 cells (E).
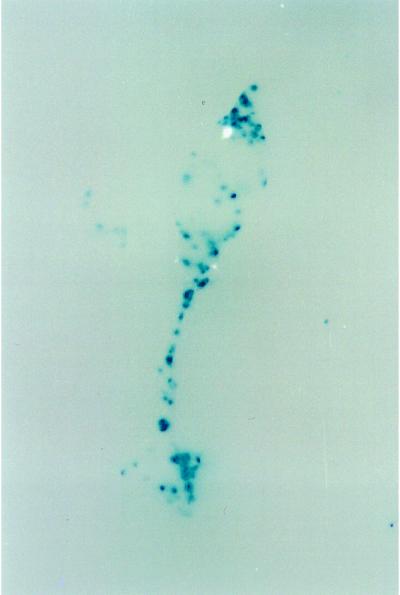
By staining the aggregates with X-Gal, it is possible to identify the location of the TL6 cells in the aggregate. Fig. 8 shows such a mixed aggregate after 24 hr of development. This aggregate is culminating and the prespore cells are nearly at the top of the stalk. The stalk has both stained and unstained regions, indicating that both cotB::rd28 and wild-type cells were recruited into the stalks. The presumed presence of TL6 cells (these cells are not visible because they are not stained) in the mass of prespore cells apparently improved the signal going to the prestalk cells because better stalks were formed.
Figure 8.
β-Galactosidase staining of aggregates formed by a mixture of 90% cotB::rd28 and 10% TL6 cells (ecmA::lacZ). Cells were developed on filters for 24 hr, and whole mounts were fixed, permeabilized, and stained with X-Gal (blue staining indicates enzymatic activity). The lack of solid staining of the stalk suggests that cotB::rd28 cells participate in stalk formation.
DISCUSSION
Aquaporins greatly increase the osmotic water permeability of cellular membranes, and the expression of the Arabidopsis plasma membrane aquaporin RD28 can cause a 10- to 15-fold increase in the rate of water uptake by Xenopus oocytes (7). Likewise, expression of RD28 in vegetative cells of D. discoideum can result in rapid uptake of water when they are suspended in distilled water. Wild-type cells protect themselves from lysis under these conditions by expelling water rapidly through the contractile vacuole (35). The rate of entry of water into act15::rd28 transformed cells appears to overwhelm this system such that the cells lyse within a few minutes. RD28 accumulates to a high level in these cells and is present in the plasma membrane. Thus, stable transformants of Dictyostelium can be used as an efficient tool for the analysis of the function of new plasma membrane aquaporins. Mammalian membrane proteins such as the m2 muscarinic receptor and the GLUT1 glucose transporter have also been successfully expressed in Dictyostelium (38, 39), and the system has been proposed as a general expression system for the production and secretion of recombinant proteins (40).
Increasing the osmolarity of the solution resulted in a higher proportion of intact act::rd28 cells as compared with those resuspended in distilled water. This suggests that the osmotic imbalance caused by the distilled water was indeed the cause of the rapid loss of viability. Interestingly, preincubation of cells with 50 mM sorbitol conferred a higher resistance to subsequent incubation in dilute media. These observations indicate that Dictyostelium cells are able to respond to a hypoosmotic condition quite rapidly, thereby protecting themselves. A rapid reaction of mammalian cells transferred into a medium of lower tonicity is the loss of cell electrolytes (K+ and Cl−) and/or loss of previously accumulated organic osmolytes (41). The cytoskeleton also plays an important role in osmotic regulation. Rapid changes in cell size require assembly and disassembly of actin and myosin filaments. In mammals, cell swelling disrupts F-actin, which activates Cl− channels (42). The involvement of actin binding proteins in volume regulation and activation of K+ channels has been also demonstrated in melanoma cells (43). Osmotic mutants impaired in cytoskeletal function have been described in Dictyostelium (36). A redistribution of myosin molecules allowed cells to adopt a spherical shape and to resist high external osmolarities. Interestingly, this function involves an osmotically induced activation of guanyl cyclase and phosphorylation of myosin (36). Signaling pathways coordinating this process could also involve the histidine kinase dokA, whose mutation affects the resistance of Dictyostelium to hyperosmotic stress (17). The observation that act15::rd28 cells are not killed under high osmotic stress indicates that the myosin redistribution process is still functional in these cells. Whether it plays a role in the acquired osmotic resistance observed after preincubation of cells with 50 mM sorbitol remains to be determined.
Expression of RD28 during development of prespore cells resulted in abnormalities in both stalk and spore formation as well as a reduction in total cell viability. The fact that stalk and cell viability defects were more pronounced when development was carried out in the presence of low salt concentrations and partly overcome when carried out in the presence of 100 mM sorbitol suggests that these developmental defects are the consequence of osmotic imbalances. Sporulation, which requires expulsion of water, was dramatically reduced in cells that had accumulated RD28. This result suggests that RD28 functions to continuously allow water influx into the spores, thereby negating the result of any water extrusion mechanism. Interestingly, a Dictyostelium gene encoding a 30-kDa protein with significant identity (34%) to a Cicadella viridis aquaporin (44) has been found to accumulate uniquely in prespore cells, but gene disruption did not measurably affect either sporulation or germination (45). However, we do not know if this Dictyostelium gene encodes a water channel protein.
The effects of RD28 on development and sporulation present some similarities with those described when prespore cells are poisoned by ricin A (27). Expression of this potent inhibitor of protein synthesis in prespore cells results in the ablation of the prespore cells and the conversion of prestalk to prespore cells. However, observations on the expression of ecmA and cotB mRNA after 24 hr of development of the RD28-expressing cells do not indicate that a similar conversion is taking place in osmotically stressed cells as a result of the presence of RD28.
The observation that cotB::rd28 stalk cells can participate in stalk formation when mixed with wild-type cells indicates that cotB::rd28 prestalk cells keep their identity but are unable to differentiate further and form normal stalk. The culmination process requires interaction between prespore and prestalk cells (46). The osmotic stress on prespore cells could modify cellular communication by modifying cell surface properties and altering signaling pathway components.
Finally, these observations provide an independent confirmation that plant aquaporins can function in cells of other organisms. Until now, plant aquaporins have been expressed exclusively in Xenopus oocytes mostly for the purpose of measuring their activities by shifting the oocytes to a hypotonic medium.
Acknowledgments
We thank Negin Iranfar for nucleic acid sequencing. This work was supported by a grant from the National Science Foundation (Cell Biology Program; to M.J.C.) and funds from a European Molecular Biology Organization fellowship (to F.C.).
ABBREVIATION
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Chrispeels M J, Maurel C. Plant Physiol. 1994;105:9–15. doi: 10.1104/pp.105.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King L S, Agre P. Annu Rev Physiol. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- 3.Reizer J, Reizer A, Saier M H., Jr Crit Rev Biochem Mol Biol. 1993;28:235–257. doi: 10.3109/10409239309086796. [DOI] [PubMed] [Google Scholar]
- 4.Kammerloher W, Fischer U, Piechottka G P, Schäffner A R. Plant J. 1994;6:187–199. doi: 10.1046/j.1365-313x.1994.6020187.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaldenhoff R, Kolling A, Meyers J, Karmann U, Ruppel G, Richter G. Plant J. 1995;7:87–95. doi: 10.1046/j.1365-313x.1995.07010087.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson K D, Herman E M, Chrispeels M J. Plant Physiol. 1989;91:1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels M J, Mirkov T E, Schroeder J I, Chrispeels M J. Plant Physiol. 1994;106:1325–1333. doi: 10.1104/pp.106.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels M J, Chaumont F, Mirkov T E, Chrispeels M J. Plant Cell. 1996;8:587–599. doi: 10.1105/tpc.8.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludevid D, Höfte H, Himelblau E, Chrispeels M J. Plant Physiol. 1992;100:1633–1639. doi: 10.1104/pp.100.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurel C, Reizer J, Schroeder J I, Chrispeels M J. EMBO J. 1993;12:2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preston G M, Carroll T P, Guggino W B, Agre P. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 12.Preston G M, Jung J S, Guggino W B, Agre P. J Biol Chem. 1994;269:1668–1673. [PubMed] [Google Scholar]
- 13.Yool A J, Stamer W D, Regan J W. Science. 1996;273:1216–1218. doi: 10.1126/science.273.5279.1216. [DOI] [PubMed] [Google Scholar]
- 14.Kuwahara M, Fushimi K, Terada Y, Bai L, Marumo F, Sasaki S. J Biol Chem. 1995;270:10384–10387. doi: 10.1074/jbc.270.18.10384. [DOI] [PubMed] [Google Scholar]
- 15.Laizé V, Rousselet G, Verbavatz J M, Berthonaud V, Gobin R, Roudier N, Abrami L, Ripoche P, Tacnet F. FEBS Lett. 1995;373:269–274. doi: 10.1016/0014-5793(95)01060-r. [DOI] [PubMed] [Google Scholar]
- 16.Bonner J T. The Cellular Slime Molds. Princeton: Princeton Univ. Press; 1967. [Google Scholar]
- 17.Schuster S C, Noegel A A, Oehme F, Gerisch G, Simon M I. EMBO J. 1996;15:3880–3889. [PMC free article] [PubMed] [Google Scholar]
- 18.Nellen W, Silan C, Firtel R A. Mol Cell Biol. 1984;4:2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuspa A, Loomis W F. Methods Mol Genet. 1994;3:3–21. [Google Scholar]
- 20.Cohen N R, Knecht D A, Lodish H F, Loomis W F. EMBO J. 1986;5:3361–3366. doi: 10.1002/j.1460-2075.1986.tb04651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson D, Loomis W F, Kimmel A. Development (Cambridge, UK) 1994;120:2891–2900. doi: 10.1242/dev.120.10.2891. [DOI] [PubMed] [Google Scholar]
- 22.Wang N, Shaulsky G, Escalante R, Loomis W F. EMBO J. 1996;15:3890–3898. [PMC free article] [PubMed] [Google Scholar]
- 23.Shaulsky G, Kuspa A, Loomis W F. Genes Dev. 1995;9:1111–1122. doi: 10.1101/gad.9.9.1111. [DOI] [PubMed] [Google Scholar]
- 24.Shaulsky G, Escalante R, Loomis W F. Proc Natl Acad Sci USA. 1996;93:15260–15265. doi: 10.1073/pnas.93.26.15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotter D A, Raper K B. J Bacteriol. 1968;96:1680–1689. doi: 10.1128/jb.96.5.1680-1689.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knecht D, Cohen S, Loomis W, Lodish H. Mol Cell Biol. 1986;6:3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaulsky G, Loomis W F. Dev Biol. 1993;160:85–95. doi: 10.1006/dbio.1993.1288. [DOI] [PubMed] [Google Scholar]
- 28.Jermyn K A, Williams J G. Development (Cambridge, UK) 1991;111:779–787. doi: 10.1242/dev.111.3.779. [DOI] [PubMed] [Google Scholar]
- 29.Sussman M. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi-Shinozaki K, Koizumi M, Urao S, Shinozaki K. Plant Cell Physiol. 1992;33:217–224. [Google Scholar]
- 31.Fosnaugh K L, Loomis W F. Dev Biol. 1993;157:38–48. doi: 10.1006/dbio.1993.1110. [DOI] [PubMed] [Google Scholar]
- 32.Escalante R, Loomis W F. Dev Biol. 1995;171:262–266. doi: 10.1006/dbio.1995.1278. [DOI] [PubMed] [Google Scholar]
- 33.Ingalls H M, Barcelo G, Wuestehube L J, Luna E J. Differentiation (Berlin) 1989;41:87–98. doi: 10.1111/j.1432-0436.1989.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 34.Nolta K V, Steck T L. J Biol Chem. 1994;269:2225–2233. [PubMed] [Google Scholar]
- 35.Patterson D J. Biol Rev. 1980;55:1–46. [Google Scholar]
- 36.Kuwayama H, Ecke M, Gerisch G, Van Haastert P J M. Science. 1996;271:207–209. doi: 10.1126/science.271.5246.207. [DOI] [PubMed] [Google Scholar]
- 37.Raper K. J Elisha Mitchell Sci Soc. 1940;59:241–282. [Google Scholar]
- 38.Cohen N R, Knecht D A, Lodish H F. Biochem J. 1996;315:971–975. doi: 10.1042/bj3150971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voith G, Dingermann T. Bio/Technology. 1995;13:1225–1229. doi: 10.1038/nbt1195-1225. [DOI] [PubMed] [Google Scholar]
- 40.Dittrich W, Williams K L, Slade M B. Bio/Technology. 1994;12:614–618. doi: 10.1038/nbt0694-614. [DOI] [PubMed] [Google Scholar]
- 41.Kwon H M, Handler J S. Curr Opin Cell Biol. 1995;7:465–471. doi: 10.1016/0955-0674(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 42.Schwiebert E M, Mills J W, Stanton B A. J Biol Chem. 1994;269:7081–7089. [PubMed] [Google Scholar]
- 43.Cantiello H F, Prat A G, Bonventre J V, Cunningham C C, Hartwig J H, Ausiello D A. J Biol Chem. 1993;268:4596–4599. [PubMed] [Google Scholar]
- 44.Le Caherec F, Bron P, Verbavatz J M, Garret A, Morel G, Cavalier A, Bonnec G, Thomas D, Gourenton J, Hubert J F. J Cell Sci. 1996;109:1285–1295. doi: 10.1242/jcs.109.6.1285. [DOI] [PubMed] [Google Scholar]
- 45.Flick, K. M., Shaulsky, G. & Loomis, W. F. (1997) Gene, in press. [DOI] [PubMed]
- 46.Loomis W F. Microbiol Rev. 1996;60:135–150. doi: 10.1128/mr.60.1.135-150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]