Abstract
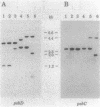
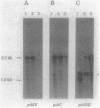
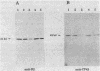
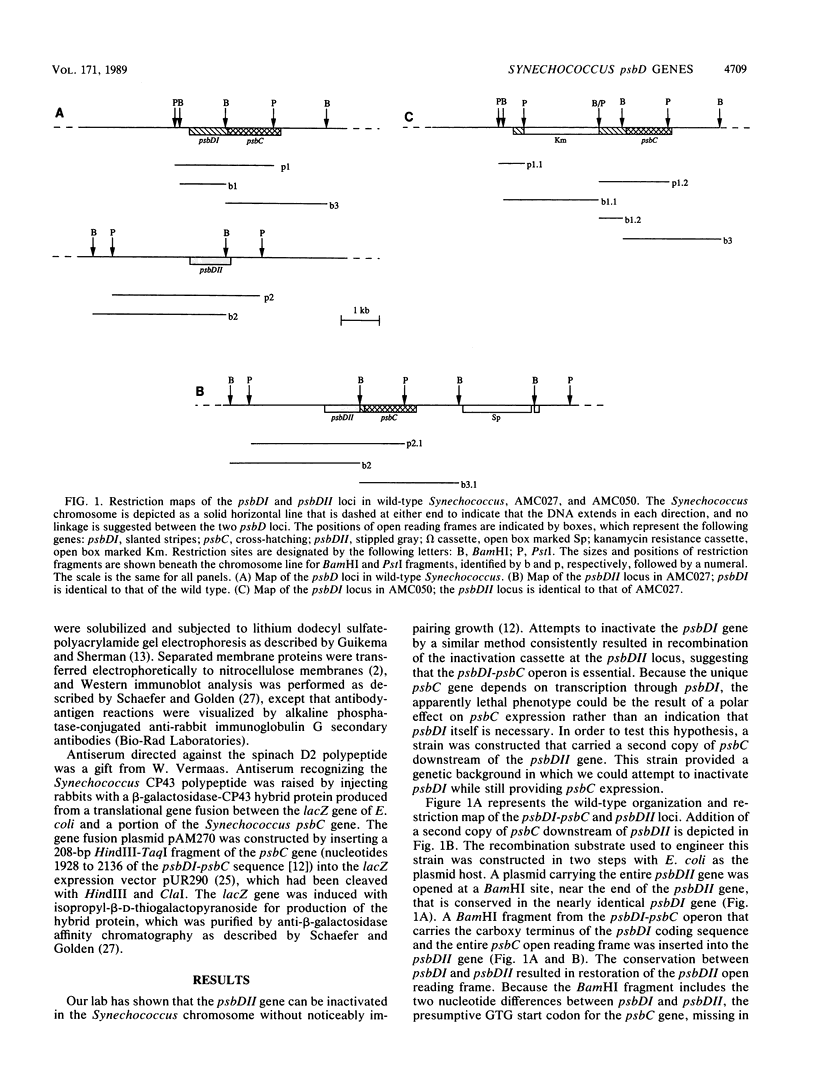
The cyanobacterium Synechococcus sp. strain PCC 7942 has two copies of the psbD gene which encodes the D2 polypeptide of the photosystem II (PSII) reaction center. One of the genes, psbDI, overlaps the open reading frame of another photosystem II gene, psbC; the psbDII gene is monocistronic. Gene inactivation experiments had previously shown that psbDII is dispensable under normal laboratory growth conditions. However, similar experiments with psbDI never produced viable psbDI-inactivated mutants, presumably because psbC expression depends on transcription through psbDI. The experiments described here were designed to assess the need for psbDI independent of the need for expression of psbC. A strain, AMC027, was engineered in which a second copy of psbC was expressed from the psbDII locus. Northern (RNA) blot analysis confirmed that both psbDI and psbDII gave rise to dicistronic messages containing psbC sequences in AMC027. In this genetic background, it was possible to inactivate psbDI, creating strain AMC050 and indicating that the psbDII gene is functional. Western immunoblot analysis showed that the products of psbD and psbC, the PSII proteins D2 and CP43, respectively, were present in thylakoids of AMC050, but at reduced levels relative to the wild type, the mutant AMC027, and two psbDII-inactivated mutants. AMC050 consistently formed small colonies on plates and competed poorly in mixed-culture experiments. This suggested that, although not essential for viability, expression from the psbDI locus is required to produce sufficient D2 and CP43 for optimal growth.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkan A. Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J. 1988 Sep;7(9):2637–2644. doi: 10.1002/j.1460-2075.1988.tb03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Sexton T. B., Mullet J. E. Light-dependent changes in psbD and psbC transcripts of barley chloroplasts: accumulation of two transcripts maintains psbD and psbC translation capability in mature chloroplasts. EMBO J. 1988 May;7(5):1289–1297. doi: 10.1002/j.1460-2075.1988.tb02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Nalty M. S., Cho D. S. Genetic relationship of two highly studied Synechococcus strains designated Anacystis nidulans. J Bacteriol. 1989 Jan;171(1):24–29. doi: 10.1128/jb.171.1.24-29.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1984 Apr;158(1):36–42. doi: 10.1128/jb.158.1.36-42.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Stearns G. W. Nucleotide sequence and transcript analysis of three photosystem II genes from the cyanobacterium Synechococcus sp. PCC7942. Gene. 1988 Jul 15;67(1):85–96. doi: 10.1016/0378-1119(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Structure of the spinach chloroplast genes for the D2 and 44 kd reaction-centre proteins of photosystem II and for tRNASer (UGA). Nucleic Acids Res. 1984 Dec 11;12(23):8819–8834. doi: 10.1093/nar/12.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson C., Debus R. J., Osiewacz H. D., Gurevitz M., McIntosh L. Construction of an Obligate Photoheterotrophic Mutant of the Cyanobacterium Synechocystis 6803 : Inactivation of the psbA Gene Family. Plant Physiol. 1987 Dec;85(4):1021–1025. doi: 10.1104/pp.85.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohchi T., Yoshida T., Komano T., Ohyama K. Divergent mRNA transcription in the chloroplast psbB operon. EMBO J. 1988 Apr;7(4):885–891. doi: 10.1002/j.1460-2075.1988.tb02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol. 1989 Jul;171(7):3973–3981. doi: 10.1128/jb.171.7.3973-3981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Light availability influences the ratio of two forms of D1 in cyanobacterial thylakoids. J Biol Chem. 1989 May 5;264(13):7412–7417. [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]