Abstract
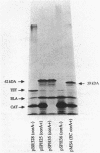
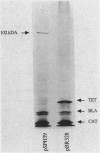
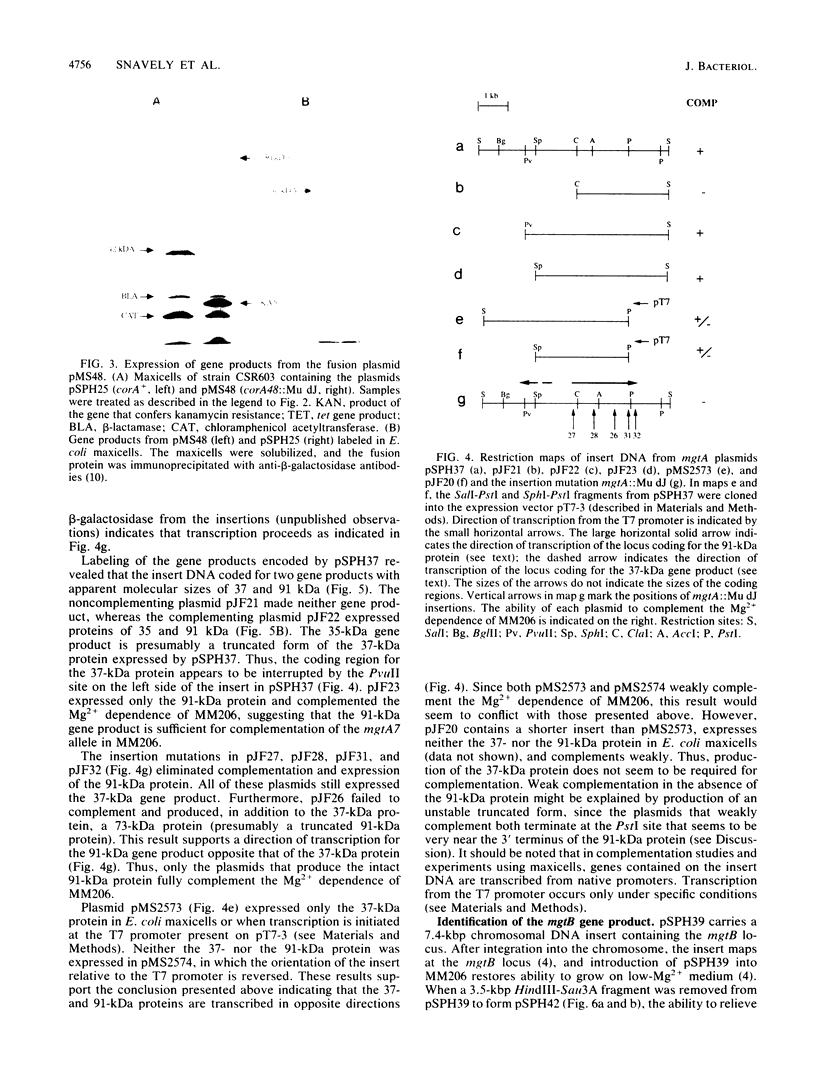
In Salmonella typhimurium, the corA, mgtA, and mgtB loci are involved in active transport of Mg2+ (S. P. Hmiel, M. D. Snavely, C. G. Miller, and M. E. Maguire, J. Bacteriol. 168:1444-1450, 1988; S. P. Hmiel, M. D. Snavely, J. B. Florer, M. E. Maguire, and C. G. Miller, J. Bacteriol. 171:4742-4751, 1989). In this study, the gene products coded for by the corA, mgtA, and mgtB genes were identified by using plasmid expression in Escherichia coli maxicells. Complementation was assessed by introducing plasmids into a Mg2+-dependent corA mgtA mgtB strain and determining the ability of the plasmid to restore growth on medium without a Mg2+ supplement. Complementing plasmids containing corA expressed a 42-kilodalton (kDa) protein. This protein was not expressed by plasmids containing insertions or deletions that eliminated complementation. A plasmid containing mgtA expressed 37- and 91-kDa gene products. Data obtained with subclones and insertions in this plasmid indicated that plasmids expressing only the 91-kDa polypeptide complemented; plasmids that did not express this protein did not complement regardless of whether they expressed the 37-kDa protein. Plasmids carrying mgtB expressed a single protein of 102 kDa whose presence or absence correlated with the ability of the plasmid to complement the Mg2+-dependent triple mutant. Fractionation of labeled maxicells demonstrated that the 42-kDa corA, the 91-kDa mgtA, and the 102-kDa mgtB gene products are all tightly associated with the membrane, a location consistent with involvement in a transport process. These data provide further support the for existence of three distinct systems for Mg2+ transport in S. typhimurium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs R. D., Maguire M. E. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6(3):113–127. [PubMed] [Google Scholar]
- Harayama S., Bollinger J., Iino T., Hazelbauer G. L. Characterization of the mgl operon of Escherichia coli by transposon mutagenesis and molecular cloning. J Bacteriol. 1983 Jan;153(1):408–415. doi: 10.1128/jb.153.1.408-415.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmiel S. P., Snavely M. D., Florer J. B., Maguire M. E., Miller C. G. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J Bacteriol. 1989 Sep;171(9):4742–4751. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmiel S. P., Snavely M. D., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986 Dec;168(3):1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky B. F., Hogg R. W. High-affinity L-arabinose transport operon. Gene product expression and mRNAs. J Mol Biol. 1987 Sep 5;197(1):27–35. doi: 10.1016/0022-2836(87)90606-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lusk J. E., Kennedy E. P. Magneisum transport in Escherichia coli. J Biol Chem. 1969 Mar 25;244(6):1653–1655. [PubMed] [Google Scholar]
- Maihle N. J., Raines M. A., Flickinger T. W., Kung H. J. Proviral insertional activation of c-erbB: differential processing of the protein products arising from two alternate transcripts. Mol Cell Biol. 1988 Nov;8(11):4868–4876. doi: 10.1128/mcb.8.11.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Kennedy E. P. Transport of magnesium by a repressible and a nonrepressible system in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1091–1093. doi: 10.1073/pnas.69.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran T. V., Frantz B., Shin M. K., Ralston D. M., Wright J. G. The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell. 1989 Jan 13;56(1):119–129. doi: 10.1016/0092-8674(89)90990-2. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Liljeström P., Harayama S. Cosmid cloning and transposon mutagenesis in Salmonella typhimurium using phage lambda vehicles. Mol Gen Genet. 1981;181(2):153–157. doi: 10.1007/BF00268420. [DOI] [PubMed] [Google Scholar]
- Park M. H., Wong B. B., Lusk J. E. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J Bacteriol. 1976 Jun;126(3):1096–1103. doi: 10.1128/jb.126.3.1096-1103.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Rupert C. S. Determination of plasmid molecular weights from ultraviolet sensitivities. Nature. 1978 Mar 30;272(5652):471–472. doi: 10.1038/272471a0. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Packman L. C., Burleigh B. D., Dell A., Morris H. R., Hartley B. S. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature. 1979 Dec 20;282(5741):870–872. doi: 10.1038/282870a0. [DOI] [PubMed] [Google Scholar]
- Silver S. Active transport of magnesium in escherichia coli. Proc Natl Acad Sci U S A. 1969 Mar;62(3):764–771. doi: 10.1073/pnas.62.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely M. D., Florer J. B., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol. 1989 Sep;171(9):4761–4766. doi: 10.1128/jb.171.9.4761-4766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., Hughes S. H. Polypurine tract adjacent to the U3 region of the Rous sarcoma virus genome provides a cis-acting function. J Virol. 1982 Aug;43(2):482–488. doi: 10.1128/jvi.43.2.482-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. M. Magnesium and cell cycle control: an update. Magnesium. 1986;5(1):9–23. [PubMed] [Google Scholar]
- Webb M. Interrelationships between the utilization of magnesium and the uptake of other bivalent cations by bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):428–439. doi: 10.1016/0304-4165(70)90133-9. [DOI] [PubMed] [Google Scholar]