Abstract
P1 bacteriophage carries at least two replicons: a plasmid replicon and a viral lytic replicon. Since the isolated plasmid replicon can maintain itself stably at the low copy number characteristic of intact P1 prophage, it has been assumed that this replicon is responsible for driving prophage replication. We provide evidence that when replication from the plasmid replicon is prevented, prophage replication continues, albeit at a reduced rate. The residual plasmid replication is due to incomplete repression of the lytic replicon by the c1 immunity repressor. Incomplete repression was particularly evident in lysogens of the thermoinducible P1 c1.100 prophage, whose replication at 32 degrees C remained almost unaffected when use of the plasmid replicon was prevented. Moreover, the average plasmid copy number of P1 in a P1 c1.100 lysogen was elevated with respect to the copy number of P1 c1+. The capacity of the lytic replicon to act as an auxiliary in plasmid maintenance may contribute to the extraordinary stability of P1 plasmid prophage.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L. P1 plasmid replication. Purification and DNA-binding activity of the replication protein RepA. J Biol Chem. 1986 Mar 15;261(8):3548–3555. [PubMed] [Google Scholar]
- Abeles A. L., Snyder K. M., Chattoraj D. K. P1 plasmid replication: replicon structure. J Mol Biol. 1984 Mar 5;173(3):307–324. doi: 10.1016/0022-2836(84)90123-2. [DOI] [PubMed] [Google Scholar]
- Austin S. J., Mural R. J., Chattoraj D. K., Abeles A. L. Trans- and cis-acting elements for the replication of P1 miniplasmids. J Mol Biol. 1985 May 25;183(2):195–202. doi: 10.1016/0022-2836(85)90212-8. [DOI] [PubMed] [Google Scholar]
- Austin S., Abeles A. Partition of unit-copy miniplasmids to daughter cells. II. The partition region of miniplasmid P1 encodes an essential protein and a centromere-like site at which it acts. J Mol Biol. 1983 Sep 15;169(2):373–387. doi: 10.1016/s0022-2836(83)80056-4. [DOI] [PubMed] [Google Scholar]
- Austin S., Hart F., Abeles A., Sternberg N. Genetic and physical map of a P1 miniplasmid. J Bacteriol. 1982 Oct;152(1):63–71. doi: 10.1128/jb.152.1.63-71.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Sternberg N., Yarmolinsky M. Miniplasmids of bacteriophage P1. I. Stringent plasmid replication does not require elements that regulate the lytic cycle. J Mol Biol. 1978 Apr 5;120(2):297–309. doi: 10.1016/0022-2836(78)90069-4. [DOI] [PubMed] [Google Scholar]
- Austin S., Ziese M., Sternberg N. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981 Sep;25(3):729–736. doi: 10.1016/0092-8674(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bornhoeft J. W., Stodolsky M. Lytic cycle replicative forms of bacteriophages P1 and P1dl: concatemer forms. Virology. 1981 Jul 30;112(2):518–528. doi: 10.1016/0042-6822(81)90299-3. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Snyder K. M., Abeles A. L. P1 plasmid replication: multiple functions of RepA protein at the origin. Proc Natl Acad Sci U S A. 1985 May;82(9):2588–2592. doi: 10.1073/pnas.82.9.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D., Cordes K., Abeles A. Plasmid P1 replication: negative control by repeated DNA sequences. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6456–6460. doi: 10.1073/pnas.81.20.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. Electron microscopy study of early lytic replication forms of bacteriophage P1 DNA. Virology. 1983 Nov;131(1):159–170. doi: 10.1016/0042-6822(83)90542-1. [DOI] [PubMed] [Google Scholar]
- Cohen G., Sternberg N. Genetic analysis of the lytic replicon of bacteriophage P1. I. Isolation and partial characterization. J Mol Biol. 1989 May 5;207(1):99–109. doi: 10.1016/0022-2836(89)90443-9. [DOI] [PubMed] [Google Scholar]
- Couturier M., Bex F., Bergquist P. L., Maas W. K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988 Sep;52(3):375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin B. H., Baumstark B. R., Scott J. R. Superimmunity: characterization of a new gene in the immunity region of P1. Virology. 1982 Jul 30;120(2):360–375. doi: 10.1016/0042-6822(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Elespuru R. K., Yarmolinsky M. B. A colorimetric assay of lysogenic induction designed for screening potential carcinogenic and carcinostatic agents. Environ Mutagen. 1979;1(1):65–78. doi: 10.1002/em.2860010113. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Weisberg R. A. The red plaque test: a rapid method for identification of excision defective variants of bacteriophage lambda. Virology. 1976 Jul 1;72(1):147–153. doi: 10.1016/0042-6822(76)90319-6. [DOI] [PubMed] [Google Scholar]
- Froehlich B. J., Watkins C., Scott J. R. IS1-dependent generation of high-copy-number replicons from bacteriophage P1 Ap Cm as a mechanism of gene amplification. J Bacteriol. 1986 May;166(2):609–617. doi: 10.1128/jb.166.2.609-617.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Hansen E. B. Structure and regulation of the lytic replicon of phage P1. J Mol Biol. 1989 May 5;207(1):135–149. doi: 10.1016/0022-2836(89)90445-2. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Yarmolinsky M. B. Host participation in plasmid maintenance: dependence upon dnaA of replicons derived from P1 and F. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4423–4427. doi: 10.1073/pnas.83.12.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Arber W. On the role of IS1 in the formation of hybrids between the bacteriophage P1 and the R plasmid NR1. Mol Gen Genet. 1980 Jan;177(2):261–270. doi: 10.1007/BF00267437. [DOI] [PubMed] [Google Scholar]
- Jaffé A., Ogura T., Hiraga S. Effects of the ccd function of the F plasmid on bacterial growth. J Bacteriol. 1985 Sep;163(3):841–849. doi: 10.1128/jb.163.3.841-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Kaiser A. D. Lambda dv: an autonomously replicating DNA fragment. Cold Spring Harb Symp Quant Biol. 1968;33:769–775. doi: 10.1101/sqb.1968.033.01.088. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Ward D. F. A bacteriophage lambda vector for cloning with BamHI and Sau3A. Gene. 1982 Dec;20(3):317–322. doi: 10.1016/0378-1119(82)90200-1. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Oeschger N. S., Wiprud G. T., Woods S. L. High efficiency temperature-sensitive amber suppressor strains of Escherichia coli K12: isolation of strains with suppressor-enhancing mutations. Mol Gen Genet. 1980;177(4):545–552. doi: 10.1007/BF00272662. [DOI] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4784–4788. doi: 10.1073/pnas.80.15.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
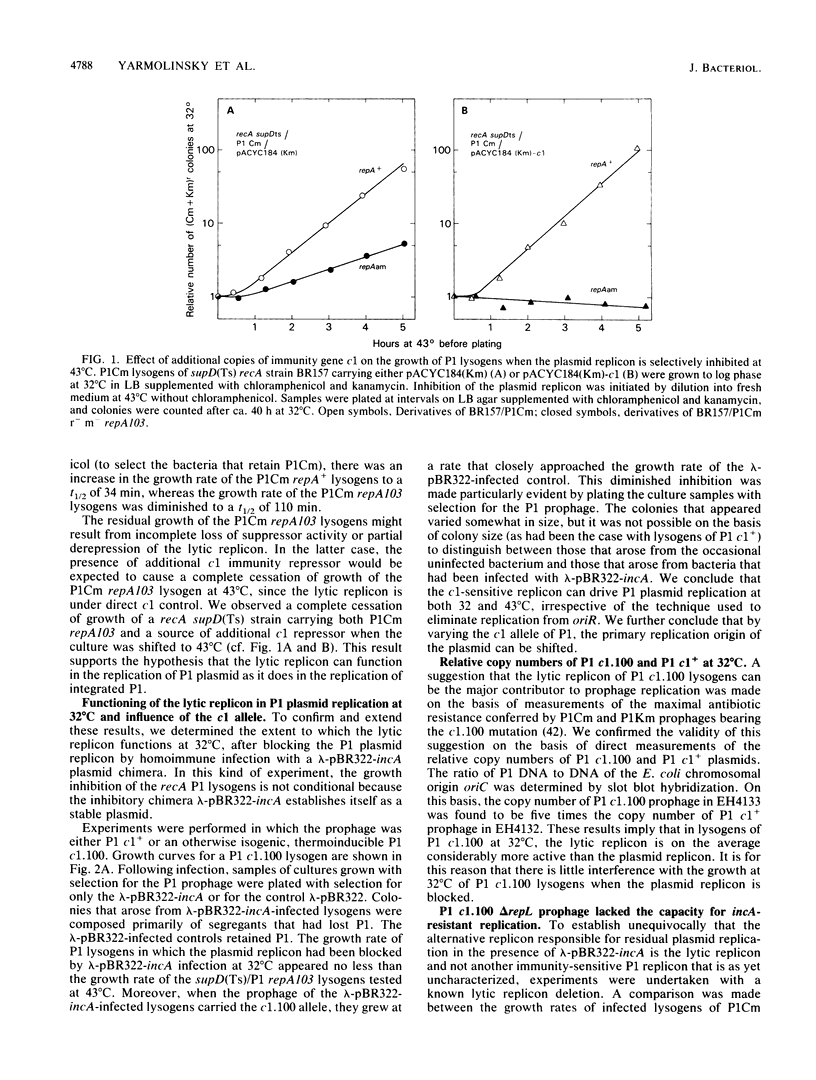
- Ow D. W., Ausubel F. M. Recombinant P4 bacteriophages propagate as viable lytic phages or as autonomous plasmids in Klebsiella pneumoniae. Mol Gen Genet. 1980;180(1):165–175. doi: 10.1007/BF00267366. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Scott J. R. Clear plaque mutants of phage P1. Virology. 1970 May;41(1):66–71. doi: 10.1016/0042-6822(70)90054-1. [DOI] [PubMed] [Google Scholar]
- Scott J. R. Phage Pl cryptic. II. Location and regulation of prophage genes. Virology. 1973 Jun;53(2):327–336. doi: 10.1016/0042-6822(73)90210-9. [DOI] [PubMed] [Google Scholar]
- Sternberg N. A characterization of bacteriophage P1 DNA fragments cloned in a lambda vector. Virology. 1979 Jul 15;96(1):129–142. doi: 10.1016/0042-6822(79)90179-x. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Austin S., Hamilton D., Yarmolinsky M. Analysis of bacteriophage P1 immunity by using lambda-P1 recombinants constructed in vitro. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5594–5598. doi: 10.1073/pnas.75.11.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Austin S. Isolation and characterization of P1 minireplicons, lambda-P1:5R and lambda-P1:5L. J Bacteriol. 1983 Feb;153(2):800–812. doi: 10.1128/jb.153.2.800-812.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Cohen G. Genetic analysis of the lytic replicon of bacteriophage P1. II. Organization of replicon elements. J Mol Biol. 1989 May 5;207(1):111–133. doi: 10.1016/0022-2836(89)90444-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981 Aug 25;150(4):467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D., Hoess R. Bacteriophage P1 site-specific recombination. II. Recombination between loxP and the bacterial chromosome. J Mol Biol. 1981 Aug 25;150(4):487–507. doi: 10.1016/0022-2836(81)90376-4. [DOI] [PubMed] [Google Scholar]
- Takano T. Bacterial mutants defective in plasmid formation: requirement for the lon + allele. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1469–1473. doi: 10.1073/pnas.68.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati-Schwartz D. A new pleiotropic bacteriophage P1 mutation, bof, affecting c1 repression activity, the expression of plasmid incompatibility and the expression of certain constitutive prophage genes. Mol Gen Genet. 1979 Jul 13;174(2):189–202. doi: 10.1007/BF00268355. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Matsubara K. Replication control and switch-off function as observed with a mini-F factor plasmid. J Bacteriol. 1981 Aug;147(2):509–516. doi: 10.1128/jb.147.2.509-516.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Jr, Walker J. T. Genetic studies of coliphage P1. III. Extended genetic map. J Virol. 1976 Oct;20(1):177–187. doi: 10.1128/jvi.20.1.177-187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. T., Walker D. H., Jr Coliphage P1 morphogenesis: analysis of mutants by electron microscopy. J Virol. 1983 Mar;45(3):1118–1139. doi: 10.1128/jvi.45.3.1118-1139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Riise E., Bergmans H. E., Meijer M., Messer W. Origin of replication, oriC, of the Escherichia coli K12 chromosome: genetic mapping and minichromosome replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):121–128. doi: 10.1101/sqb.1979.043.01.018. [DOI] [PubMed] [Google Scholar]



